Abstract
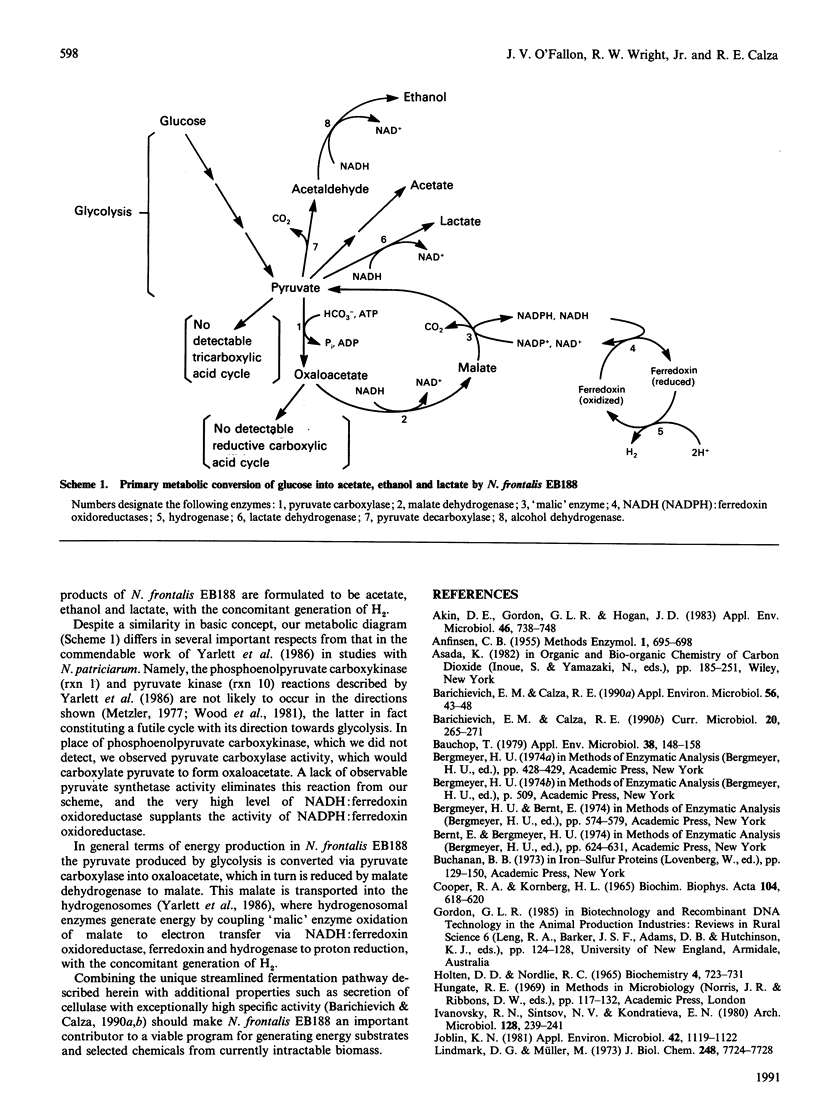
Primary pathways for glucose metabolism were established in the anaerobic rumen fungus Neocallimastix frontalis EB188. This highly capable cellulolytic organism demonstrated a strict anaerobic integration of metabolic pathways. Glycolysis in N. frontalis EB188 was coupled to malate dehydrogenase, 'malic' enzyme and specified hydrogenosome reactions. Pyruvate, as in most life forms, was a pivotal compound. The major fermentation products of N. frontalis EB188 were acetate, ethanol and lactate, with the concomitant generation of H2. On the basis of its unique characteristics and streamlined fermentation pathways, it was concluded that N. frontalis EB188 should be an important contributor to programs generating energy and selected chemicals from currently intractable biomass.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichievich E. M., Calza R. E. Supernatant protein and cellulase activities of the anaerobic ruminal fungus Neocallimastix frontalis EB188. Appl Environ Microbiol. 1990 Jan;56(1):43–48. doi: 10.1128/aem.56.1.43-48.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. Net formation of phosphoenolpyruvate from pyruvate by Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):618–620. doi: 10.1016/0304-4165(65)90374-0. [DOI] [PubMed] [Google Scholar]
- HOLTEN D. D., NORDLIE R. C. COMPARATIVE STUDIES OF CATALYTIC PROPERTIES OF GUINEA PIG LIVER INTRA- AND EXTRAMITOCHONDRIAL PHOSPHOENOLPYRUVATE CARBOXYKINASES. Biochemistry. 1965 Apr;4:723–731. doi: 10.1021/bi00880a018. [DOI] [PubMed] [Google Scholar]
- Joblin K. N. Isolation, enumeration, and maintenance of rumen anaerobic fungi in roll tubes. Appl Environ Microbiol. 1981 Dec;42(6):1119–1122. doi: 10.1128/aem.42.6.1119-1122.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Biochemical cytology of trichomonad flagellates. II. Subcellular distribution of oxidoreductases and hydrolases in Monocercomonas sp. J Protozool. 1974 May;21(2):374–378. doi: 10.1111/j.1550-7408.1974.tb03673.x. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973 Nov 25;248(22):7724–7728. [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G., Hamilton I. D., Fewson C. A., Wilkins M. B. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986 Oct 1;239(1):213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Fallon J. V., Wright R. W., Jr Calculation of the pentose phosphate and Embden-Myerhoff pathways from a single incubation with [U-14C]- and [5-3H]glucose. Anal Biochem. 1987 Apr;162(1):33–38. doi: 10.1016/0003-2697(87)90007-8. [DOI] [PubMed] [Google Scholar]
- O'Fallon J. V., Wright R. W., Jr Quantitative determination of the pentose phosphate pathway in preimplantation mouse embryos. Biol Reprod. 1986 Feb;34(1):58–64. doi: 10.1095/biolreprod34.1.58. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Nutrition and biochemistry of anaerobic Chytridiomycetes. Biosystems. 1988;21(3-4):365–370. doi: 10.1016/0303-2647(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Sphaeromonas communis. J Gen Microbiol. 1976 Jun;94(2):270–280. doi: 10.1099/00221287-94-2-270. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Walt D. R. Substrate specificity of acetyl coenzyme A synthetase. J Biol Chem. 1987 May 25;262(15):7132–7134. [PubMed] [Google Scholar]
- Phillips M. W., Gordon G. L. Sugar and polysaccharide fermentation by rumen anaerobic fungi from Australia, Britain and New Zealand. Biosystems. 1988;21(3-4):377–383. doi: 10.1016/0303-2647(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Stopak S. S., Pazoles C. J., Creutz C. E. A simple and novel method for radiometric analysis of glucose utilization by adrenal chromaffin cells. Anal Biochem. 1981 Jan 15;110(2):424–430. doi: 10.1016/0003-2697(81)90214-1. [DOI] [PubMed] [Google Scholar]
- Theodorou M. K., Lowe S. E., Trinci A. P. The fermentative characteristics of anaerobic rumen fungi. Biosystems. 1988;21(3-4):371–376. doi: 10.1016/0303-2647(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Orpin C. G., Munn E. A., Yarlett N. C., Greenwood C. A. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem J. 1986 Jun 15;236(3):729–739. doi: 10.1042/bj2360729. [DOI] [PMC free article] [PubMed] [Google Scholar]


