Abstract
Brown adipose tissue (BAT) plays an important role in energy metabolism because it uses fatty acids for thermogenesis during cold exposure. Preclinical studies found that boysenberry anthocyanins (BoyACs) activate BAT. Therefore, the aim of this preliminary study was to evaluate how BoyAC intake affects BAT in humans. We performed an open-label single-arm nonrandomized study in healthy volunteers. Before and after 4 weeks of daily consumption of 100 ml boysenberry juice (BoyJ) containing 61 mg of BoyACs, participants were assessed at 24 °C and then after 1 h of mild cold exposure (18 °C). An infrared thermography camera was used to measure skin surface temperatures in the supraclavicular BAT region (Tscv) and the non-BAT region of the upper chest (Tch). Energy metabolism was measured by indirect calorimetry. For each endpoint, we calculated Δ as the difference between values before and after cold exposure and compared the values before and after BoyJ intake. 10 volunteers participated (age: 36.1 ± 4.1, body mass index (BMI): 20.9 ± 0.6). After BoyJ intake, ΔTscv-ch was significantly higher (p = 0.029), but Δ energy expenditure, Δ fat oxidation, and Δ carbohydrate oxidation were not significantly different. We found a significant positive correlation between BMI and Δfat oxidation with BoyJ intake. The results indicate that 4 weeks of BoyJ intake activates cold-induced thermogenesis in the scv-BAT but does not have a significant effect on energy metabolism. BoyJ intake may increase fat oxidation during cold exposure in individuals with higher BMI.
Trial registry number: UMIN000043476, 05/03/2021.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76452-4.
Keywords: Brown adipose tissue, Anthocyanin, Boysenberry, Thermogenesis, Fat oxidation
Subject terms: Nutrition, Nutritional supplements, Translational research
Introduction
The primary function of brown adipose tissue (BAT) is non-shivering thermogenesis. In cold conditions, mitochondria in BAT produce heat by oxidizing fatty acids, preventing an attenuation of body temperature1. This process is especially important in neonates2and hibernating animals3, but studies have recently reported that it contributes to the maintenance of body temperature even in human adults4,5. BAT consumes energy for thermogenesis and thus increases the energy metabolic rate, so it may potentially play an important role in weight management and obesity prevention6. Several studies have shown that BAT activation improves insulin sensitivity and has a positive effect on glucose metabolism7. Activated BAT tissue is expected to consume excess lipids and reduce low-density lipoprotein cholesterol in the blood, and those effects may help to reduce the risk of cardiovascular disease8. BAT activity is known to decrease with aging and obesity9, and activation of BAT is thought to be important for the prevention and treatment of metabolic syndrome10.
In recent years, interest has grown in the influence of foods on BAT activation. For example, capsinoids11, omega-3 fatty acids12, and some polyphenols13have been reported to enhance energy metabolism by activating BAT. Furthermore, in preclinical studies, we showed that boysenberry anthocyanins (BoyACs), the major component of the juice from boysenberries (a hybrid Rubus berry of Rubus baileyanus and Rubus loganobaccus that contains multiple polyphenols)14, protect BAT from metabolic stress15. Other studies have also reported that anthocyanins activate BAT16,17. Most of the studies on the relationship between food and BAT were preclinical, and an insufficient number of studies have evaluated the effects of foods on human BAT. In particular, to our knowledge no study has investigated whether anthocyanins affect BAT in humans. Therefore, we think that to evaluate the relationship between anthocyanins intake and human BAT is necessary for real-world applications. We hypothesized that BoyACs intake would activate BAT and alter energy metabolism during cold exposure and performed a preliminary human intervention study to evaluate the effects of BoyACs on human BAT.
Results
Baseline characteristics
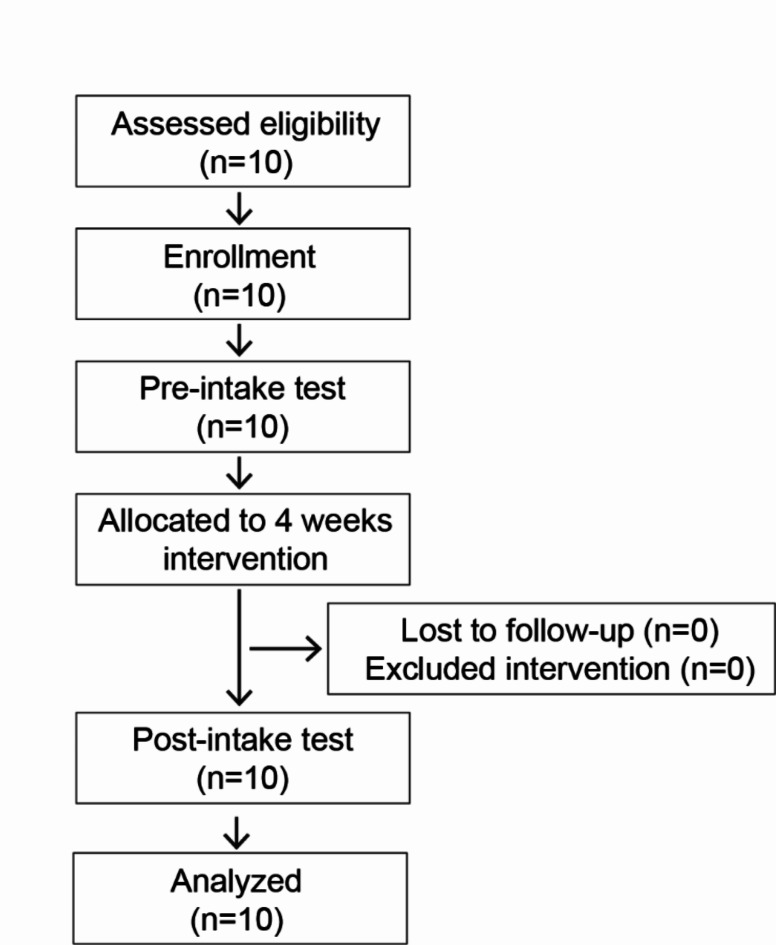
Ten volunteers, aged between 20 and 57 years, agreed to participate in the study, and at screening, all 10 were eligible for the study. Participant baseline characteristics are shown in Table 1. None of the participants discontinued or dropped out of the study, and all 10 were included in the analysis. The study flow diagram is shown in Fig. 1. There were no significant changes in height, body weight, or body mass index (BMI) from before to after intake of BoyJ.
Table 1.
Participant characteristics.
| Pre-intake | Post-intake | p value | |
|---|---|---|---|
| Age | 36.1 ± 4.1 | ||
| Male/Female | 4/6 | ||
| Height (cm) | 163.8 ± 2.8 | 163.8 ± 2.8 | 0.622 |
| Body weight (kg) | 56.5 ± 3.0 | 56.2 ± 2.9 | 0.332 |
| BMI (kg/m2)a | 20.9 ± 0.6 | 20.8 ± 0.6 | 0.365 |
Data represent the mean ± SEM. The two groups were compared by a paired t test. The raw data are shown in Supplementary Data S1.
a Body mass index (BMI) is weight (kilograms) divided by height (meters) squared.
Fig. 1.
Study flow diagram. Ten subjects participated in the study. All subjects completed the study and all data were used for analysis.
Skin surface temperature
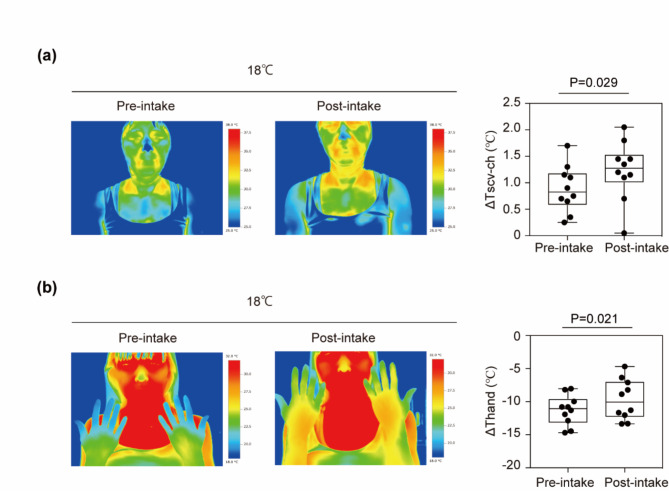
Skin surface temperature measurements are shown in Table 2. ΔTscv-ch, i.e., the activity of the BAT region, increased significantly from 0.89 °C ± 0.14 °C before BoyJ intake to 1.23 °C ± 0.18 °C after 4 weeks of intake (p = 0.029, Fig. 2a). On the other hand, no significant difference was found for Tscv or Tch (p = 0.329 and p = 0.958, respectively). Thand increased significantly from − 9.56 ± 0.49 °C before intake of BoyJ to -7.88 ± 0.67 °C after intake (p = 0.021, Fig. 2b).
Table 2.
Change in skin surface temperature before and after 4 weeks’ intake of boysenberry juice.
| Pre-intake | Post-intake | P value | ||
|---|---|---|---|---|
| Tscv (℃)a | 24 °C | 34.6 ± 0.1 | 34.7 ± 0.3 | 0.329 |
| 18 °C | 32.9 ± 0.4 | 33.3 ± 0.4 | ||
| Δ | -1.68 ± 0.33 | -1.35 ± 0.32 | ||
| Tch (℃)b | 24 °C | 33.3 ± 0.1 | 33.4 ± 0.3 | 0.958 |
| 18 °C | 30.7 ± 0.4 | 30.8 ± 0.5 | ||
| Δ | -2.56 ± 0.36 | -2.58 ± 0.40 | ||
| Tscv-ch (℃)c | 24 °C | 1.29 ± 0.09 | 1.27 ± 0.14 | 0.029 |
| 18 °C | 2.17 ± 0.19 | 2.50 ± 0.22 | ||
| Δ | 0.89 ± 0.14 | 1.23 ± 0.18 | ||
| Thand (℃)d | 24 °C | 31.4 ± 0.5 | 31.7 ± 0.6 | 0.021 |
| 18 °C | 21.8 ± 0.4 | 23.8 ± 0.6 | ||
| Δ | -9.56 ± 0.49 | -7.88 ± 0.67 |
Data represent the mean ± SEM. Δ represented by subtracting the value at 24 °C from the value at 18 °C. The two groups were compared by a paired t test. The raw data are shown in Supplementary Data S2.
aSkin surface temperature of the supraclavicular. bSkin surface temperature of the upper chest. cDifference between Tscv and Tch. dSkin surface temperature of the hand.
Fig. 2.
Change in skin surface temperature. (a) Boysenberry juice (BoyJ) intake increased the temperature of the supraclavicular brown adipose tissue (BAT) region (Tscv) and hand skin surface after cold exposure (18 °C) for 1 h. The data show the difference (Δ) in skin surface temperature when participants were exposed to 24 °C and 18 °C. Representative infrared thermography images of Tscv and the non-BAT region in the upper chest (Tch) during cold exposure. The graph shows the difference between Tscv and Tch (ΔTscv-ch). (b) Representative infrared thermography images of the hand skin surface. The graph shows the difference in hand temperature when participants were exposed to 24 °C and 18 °C. A paired t test was used to compare hand temperature before and after intake of BoyJ; p values are shown in the figure. The raw data are shown in Supplementary Data S2.
Energy metabolism
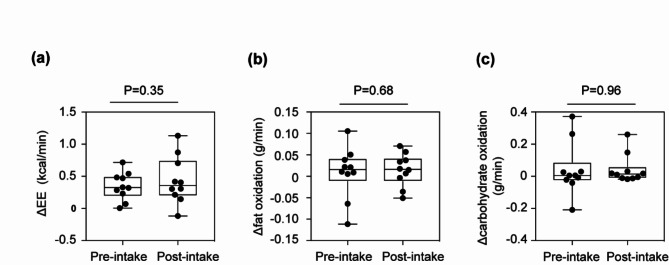
The results of the respiratory gas analysis are shown in Table 3, and the values of Δ EE, Δ fat oxidation, and Δ carbohydrate oxidation, which were analyzed to estimate the effects of cold exposure on BAT activation, are shown in Fig. 3. No significant changes were found in Δ EE (pre-intake, 0.35 ± 0.07 kcal/min; post-intake, 0.44 ± 0.12 kcal/min; p = 0.350, Fig. 3a), Δ fat oxidation (pre-intake, 0.0087 ± 0.0189 g/min; post-intake, 0.0148 ± 0.0120 g/min; p = 0.677, Fig. 3b), or Δ carbohydrate oxidation (pre-intake, 0.042 ± 0.051 g/min; post-intake, 0.044 ± 0.028 g/min; p = 0.959, Fig. 3c).
Table 3.
Change in energy metabolism from before to after 4 weeks’ intake of boysenberry juice.
| Pre-intake | Post-intake | P value | ||
|---|---|---|---|---|
|
EE (kcal/min) |
24 °C | 1.31 ± 0.11 | 1.21 ± 0.08 | 0.350 |
| 18 °C | 1.65 ± 0.16 | 1.65 ± 0.18 | ||
| Δ | 0.35 ± 0.07 | 0.44 ± 0.12 | ||
|
Fat oxidaition (g/min) |
24 °C | 0.0419 ± 0.0087 | 0.0379 ± 0.0047 | 0.677 |
| 18 °C | 0.0506 ± 0.0190 | 0.0526 ± 0.0154 | ||
| Δ | 0.0087 ± 0.0189 | 0.0148 ± 0.0120 | ||
|
Carbohydrate oxidation (g/min) |
24 °C | 0.137 ± 0.023 | 0.129 ± 0.014 | 0.959 |
| 18 °C | 0.179 ± 0.053 | 0.173 ± 0.041 | ||
| Δ | 0.042 ± 0.051 | 0.044 ± 0.028 |
Data represent the mean ± SEM. Δ represented by subtracting the value at 24 °C from the value at 18 °C. The two groups were compared by a paired t test. The raw data are shown in Supplementary Data S3. EE, energy expenditure.
Fig. 3.
Changes in energy metabolism. Boysenberry juice (BoyJ) had no significant effect on energy metabolism. The data show the difference (Δ) in energy metabolism when participants were exposed to 24 °C and 18 °C. Change (Δ) in energy expenditure (Δ EE, a), Δ fat oxidation (b), and Δ carbohydrate oxidation (c) were determined by indirect calorimetry measurements. A paired t test was used to compare hand temperature before and after intake of BoyJ; p values are shown in the figure. The raw data are shown in Supplementary Data S3.
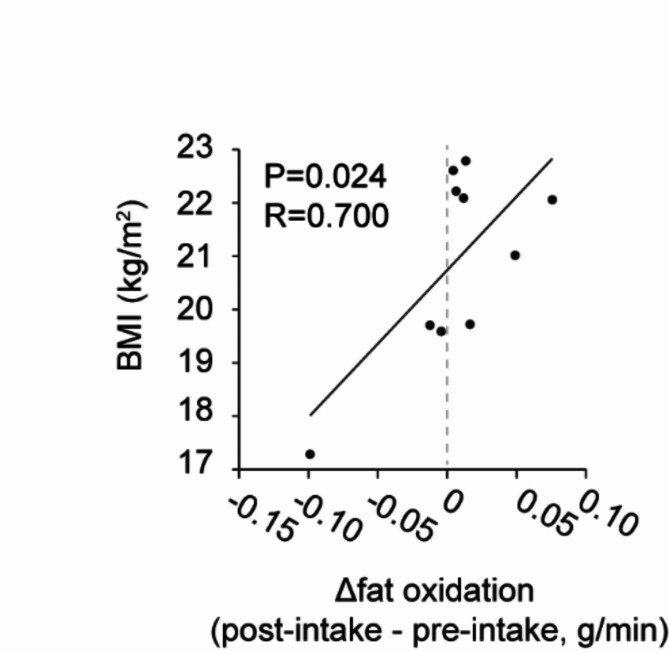
An exploratory evaluation of the correlation between each data revealed a positive correlation between Δ fat oxidation (post-intake – pre-intake) and BMI (Fig. 4, r = 0.700, p = 0.024). No significant correlations were observed between the other data.
Fig. 4.
Correlation between body mass index (BMI) and fat oxidation. BMI was positively correlated with increased cold-induced fat oxidation after 4 weeks of Boysenberry juice (BoyJ) consumption. The change in fat oxidation from before to after cold exposure (18 °C) was compared before and after 4 weeks’ intake of BoyJ. A Pearson correlation test was used to calculate the r and p values.
A post hoc power calculation for fat oxidation was performed with paired and two-sided t tests and a significance level of 5%. When all participants were included (N = 10; mean difference, 0.00105 [SD, 0.0370]), the power was calculated to be 0.051 and the effect size 0.028, indicating that the power was insufficient. On the other hand, when only participants with a BMI of 20 or more were included (N = 6; mean difference, 0.0173 [SD, 0.0392]), the power increased to 0.144 and the effect size to 0.442.
Cold sensation
Changes in the cold sensation as assessed by the VAS are shown in Table 4. The VAS score for Δ cold sensation did not change significantly from before consumption of BoyJ (50.6 ± 10.7 mm) to after (50.5 ± 8.7 mm; p = 0.994).
Table 4.
Change in cold sensation assessed by a visual analogue scale from before to after 4 weeks’ intake of boysenberry juice.
| Pre-intake | Post-intake | P value | ||
|---|---|---|---|---|
|
Cold sensation (mm) |
24 °C | 37.6 ± 10.8 | 31.5 ± 9.7 | 0.994 |
| 18 °C | 88.2 ± 4.1 | 82.0 ± 7.2 | ||
| Δ | 50.6 ± 10.7 | 50.5 ± 8.7 |
Data represent the mean ± SEM. Δ represented by subtracting the value at 24 °C from the value at 18 °C. The two groups were compared by a paired t test. The raw data are shown in Supplementary Data S4.
Discussion
This open-label nonrandomized single-arm before-and-after study investigated the effects of intake of BoyJ, which contains BoyACs, on BAT in healthy adults over 20 years old. To our knowledge, this is the first study to examine the association between BoyACs and human skin surface temperature of BAT activity indicator. The results suggest that intake of BoyJ for 4 weeks may activate BAT in the scv area.
In humans, BAT is known to exist in scv, interscapular, perinephric, and lower axillary areas, and this study evaluated BAT activity by measuring thermogenesis in the scv region. The gold standard for measuring BAT activity is to observe glucose uptake with fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography (FDG-PET/CT), but this method is invasive because it exposes participants to radiation4,18. Recently, a method that assesses skin surface temperature with an infrared thermography camera was reported as a simple and less invasive method for measuring BAT activity that shows similar results to FDG-PET/CT19,20. Therefore, to minimize the burden on study participants we chose to use an infrared thermography camera to measure BAT activity.
After 4 weeks of BoyJ consumption, skin surface temperatures, Tscv, and Tch did not change significantly, but Tscv-ch increased significantly. Subtracting the skin temperature of the non-BAT region in the upper chest from the scv BAT region has been reported to increase the sensitivity and specificity of BAT detection, so we think that significant differences were obtained only Tscv-ch20,21. On the other hand, skin temperature in the scv area is affected by blood flow. The decrease in hand temperature induced by cold exposure was suppressed after ingestion of BoyJ. The peripheral skin temperature is affected by skin blood flow22, we do not know whether this result was due to a change in skin blood flow or to BAT activation. BoyACs have also been reported to improve vascular function23,24. In addition, the effect of BoyACs on BAT function is assumed to be mediated through changes in vascular function15. Therefore, future research needs to evaluate the relationship between BAT function and vascular function. Some researchers have negative opinions about the use of skin surface temperature measurement as a method to evaluate BAT activation in individuals with a thick layer of subcutaneous fat25. Therefore, BAT activity should be evaluated in various ways, and future studies should consider using FDG-PET/CT.
We observed a positive correlation between Δ fat oxidation (post-intake – pre-intake) and BMI, but BoyJ intake did not significantly change EE, fat oxidation, or carbohydrate oxidation. BMI is known to be negatively correlated with BAT activity26. BAT produces heat predominantly through fat oxidation upon cold stimulation27. Blackcurrant anthocyanins only increased fat oxidation in human with a high BMI during exercise, suggesting that the effect was on adipocyte in the legs28. Therefore, we hypothesize that BoyP may contribute to increased fat oxidation in humans with higher BMI via BAT. In this study, the participants had a mean BMI of 20.9 ± 0.6, which is lower than the reference range defined by the Japan Society for the Study of Obesity29. When all participants were included, the effect size of fat oxidation was very small, suggesting that it would be difficult to show a significant difference. On the other hand, power calculations indicated that the effect size would increase by including only participants with a higher BMI. To achieve a statistical power of 0.8 with a two-sided significance level of 5% for this subgroup, the necessary sample size was estimated to be N = 42. These calculations indicate that the difference would be significant if sufficient participants were included. These results indicate the need to perform studies in individuals with higher BMIs.
Previous reports have pointed out the importance of carbohydrate loading before assessing BAT function30,31. BAT generates heat by taking up large amounts of glucose from the bloodstream and utilizing BAT free fatty acids as substrates. However, blood glucose levels are low in a fasting state, which may mean that changes in substrate utilization due to thermogenesis cannot be quantified. Our study was performed under fasting conditions, and consequently, the measurements of substrate oxidation of BAT may not have been accurate. Therefore, future research should conduct assessments under carbohydrate-loaded conditions.
The activity of BAT may be regulated by sex hormones32, and the positivity rate of BAT detected by FDG-PET/CT is reported to be higher in women than in men33. The present study included women, but it did not consider their menstrual cycle, which may have influenced the results. Therefore, future study designs should take menstrual cycle status into consideration.
BoyJ consumption had no significant effect on VAS assessments of the cold sensation. The result suggests that ingestion of BoyACs may not affect cold sensation. The measurements scores were 88.2 ± 4.1 mm before cold exposure and 82.0 ± 7.2 mm afterwards, indicating that the cold sensation may have reached a plateau after 1 h of cold exposure. Therefore, the results are difficult to interpret, and future studies should evaluate the cold sensation over time.
Conclusion
The study suggests that consumption of BoyAC-containing beverages for 4 weeks by healthy adults over 20 years of age increases skin surface temperature in the scv-BAT region. Although we did not observe any effect of cold exposure on systemic energy metabolism, we found a positive correlation between BMI and increased fat oxidation. Therefore, a placebo-controlled study in individuals with higher BMI is warranted to evaluate the effects of BoyACs on BAT.
Limitations
This study has some limitations. First, because it was an open-label nonrandomized single-arm before-and-after study, the possibility of investigator bias or a placebo effect in the participants cannot be excluded. Second, the results may have been affected by sample size because only 10 individuals were included. Third, we used skin surface temperature as an indicator of BAT activity, which may not be as accurate as FDG-PET/CT measurements. And last, BAT is known to be affected by seasons, and seasonal effects cannot be excluded as the protocol34. This study may have been influenced by the menstrual cycle of the female participants. This study was conducted under fasting conditions, so it is possible that substrate oxidation in BAT may not have been accurately evaluated.
Methods
Study design
We used an open-label nonrandomized single-arm before-and-after study design. The study was approved by the Ethical Review Committee of Niigata University (approval number: 2020 − 0332) and performed in accordance with the Declaration of Helsinki and the Japanese Ministry of Health, Labor and Welfare’s Ethical Guidelines for Medical and Biological Research Involving Human Subjects. Prior to the start of the study, the study was registered in the UMIN Clinical Trials Registry (registration number: UMIN000043476).
Participants
Participants were healthy adult males and females over 20 years old. We informed participants about the purpose, methods, expected effects, and possible adverse effects of the study and obtained their voluntary written consent to participate. Healthy men and women older than 20 years were eligible to participate if they were free from chronic diseases; gave written informed consent to participate; did not consume any drugs, Health foods that are approved as Specific Health Foods, Nutritional functional foods or Foods with Function Claim in Japan, or supplements; did not have allergies; and were not pregnant.
Test food
The test food was boysenberry juice (BoyJ, Bourbon Corporation). Participants consumed BoyJ every day for 4 weeks at 100 ml per dose. Participants consumed 100 ml/day of the test food, i.e., boysenberry juice (BoyJ, Bourbon Corporation), for four weeks; the consumption time was not specified.
The BoyACs in the BoyJ were analyzed by high-performance liquid chromatography (HPLC)35. For the HPLC analysis, BoyJ was dissolved in 25 ml of 2% hydrochloric acid methanol and centrifuged (9000 rpm, 10 min). Then, 10 ml of the supernatant was scaled up to 20 ml with 10% phosphoric acid solution to obtain the sample for HPLC. The samples were injected into an HPLC instrument (Shimadzu Corporation) connected to an HPLC column (ZORBAX Extend-C18, 4.6 mm × 250 mm, 5 μm, Agilent Technologies). Eluents A (water and formic acid in a ratio of 10:90) and B (water, methanol, acetonitrile, and formic acid in a ratio of 40:22.5:22.5:10) were added, eluted with a gradient (0 min, 14% B; 20 min, 14% B; 25 min, 100% B; 30 min, 100% B), and measured at 535 nm. Cyanidin-3-glucoside (Nagara Science Corporation, code: 639-43451) was used as the standard. The four detected BoyACs (cyanidin-3-2-glucosylglucoside, cyanidin-3-2-glucosylrhamnosylglucoside, cyanidin-3-glucoside, and cyanidin-3-6- rhamnosylglucoside) were calculated as cyanidin-3-glucoside equivalents14. The composition of BoyJ is shown in Table 5; the juice was found to contain 61 mg of BoyACs in 100 ml.
Table 5.
Nutritional values and boysenberry anthocyanin content of boysenberry juice.
| 100 ml BoyJ | |
|---|---|
| Energy content, kcal | 32.0 |
| Protein, g | 0.5 |
| Fat, g | < 0.01 |
| Carbohydrate, g | 7.2 |
| Sodium, mg | 2.0 |
| Anthocyaninsa | |
| Cyanidin-3-2-glucosylglucoside, mg | 27.6 |
| Cyanidin-3-2-glucosylrhamnosylglucoside, mg | 18.5 |
| Cyanidin-3-glucoside, mg | 12.8 |
| Cyanidin-3-6- rhamnosylglucoside, mg | 2.1 |
| Total anthocyanins (BoyACs), mg | 61.0 |
aQuantitative values were calculated as cyanidin-3-glucoside equivalents.
Assessments
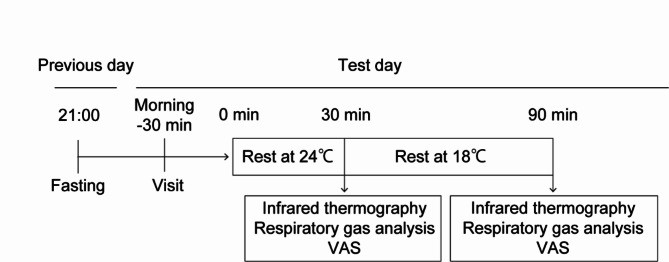
Assessments were performed at Niigata University, Niigata, Japan, in June and July 2021. Compliance was confirmed by asking participants to record their boysenberry juice intake, special activities, and any physical symptoms in a diary. Participants visited the University before and after 4 weeks of BoyJ consumption and were instructed not eat or drink anything except water after 9 p.m. on the day before the study visit. At each visit, the participants changed into a tank top with an open neck, shorts, and socks. Then, they rested for 30 min in a room set at 24 °C ± 1 °C, and their skin surface temperature was captured by an infrared thermographic camera. Subsequently, respiratory gas analysis was performed and participants rated their cold sensation on a visual analogue scale (VAS). Afterwards, participants were subjected to mild cold exposure for 1 h in a climate chamber set at 18 °C ± 1 °C. At the end of the hour, while the participants were still in the chamber, infrared thermographic imaging and respiratory gas analysis were repeated and participants completed another VAS. An overview of the test schedule is shown in Fig. 5.
Fig. 5.
Test schedule for the study. The subjects were fasted from 21:00 on the day before the test, and infrared thermographic cameras, respiratory gas analysis, and visual analogue scale (VAS) were measured before and after cold exposure at 18 °C.
Infrared thermographic camera
An infrared thermal camera (Testo 885, Testo K.K. Co. Ltd.) was used to measure the skin surface temperatures on the upper body and back of the hands, and the temperatures were averaged for the supraclavicular (Tscv), upper chest (Tch), and wrist to fingertip (Thand) areas. To determine the activity of BAT, the non-BAT region (Tch) was subtracted from the BAT region (Tscv) to obtain Tscv-ch. To evaluate thermogenesis by cold stimulation, ΔTscv, ΔTch, ΔTscv-ch, and ΔThand were calculated by subtracting the value before cold exposure from values after cold exposure.
Respiratory gas analysis
To evaluate energy metabolism, respiratory gas analysis was performed with the Douglas bag method, and energy expenditure (EE), fat oxidation, and carbohydrate oxidation were calculated with the following formulas36,37: energy expenditure (kcal/min) = (3.9 × VO2 + 1.1 × VCO2) × 1.44; carbohydrate oxidation (g/min) = 4.55 × VCO2 − 3.21 × VO2; and fat oxidation (g/min) = 1.67 × VO2 − 1.67 × VCO2.
Δ EE, Δ fat oxidation, and Δ carbohydrate oxidation were calculated by subtracting the value before cold exposure from values after cold exposure.
VAS
The VAS was used to evaluate the cold sensation. Participants were shown a straight line on which not feeling cold was the reference point (0 mm) and feeling very cold was the maximum value (100 mm) and were asked to draw a line that corresponded to their cold sensation; the length of the line was defined as the cold sensation. Δ cold sensation was calculated as the change in the length of the line from after to before cold exposure.
Statistical analysis
The study is a preliminarily clinical trial, the required sample size was not estimated, but the number of subjects was set at 10 from similar study38. Data are shown as mean ± SE. A box-and-whisker plot was used to display the maximum and minimum values (range of whiskers), 25th and 75th percentiles (boxes), and median (solid line), and a dot plot was used to display the data of individual participants. A paired t test was used to analyze the difference in values before and after BoyJ intake. In order to evaluate correlations between data in an exploratory manner, correlations were determined by the Pearson correlation test. A post-hoc power analysis was performed. The significance level was set at 5% two-tailed. SPSS 28.01 (IBM) was used for statistical analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
R.F. contributed to establishing the study concept and design and to data analysis and wrote the manuscript. S.K. contributed to preparing BoyJ and analyzing BoyACs. D.M. contributed to preparing BoyJ, establishing the study concept, and managing participants. I.S. contributed to establishing the research concept and design. T.A. contributed to performing measurements and managing participants. S.F. and T.M. contributed to supervising all experiments.
Data availability
All data analyzed in this study are included in Supplementary Data.
Declarations
Competing interests
R.F., S.K., and D.M. are researchers at Bourbon Corporation. BoyJ was provided by Bourbon Corporation. This study was conducted with joint research funds provided by Bourbon Corporation. Bourbon Corporation and Juntendo University have established a collaborative research program funded by Bourbon Corporation, and the program is organized by T.M. The other authors have no conflicts of interest to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ricquier, D. & Bouillaud, F. Mitochondrial uncoupling proteins: From mitochondria to the regulation of energy balance. J. Physiol.529 Pt 1, 3–10. 10.1111/j.1469-7793.2000.00003.x (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lidell, M. E. Brown adipose tissue in human infants. Handb. Exp. Pharmacol.251, 107–123. 10.1007/164_2018_118 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ballinger, M. A. & Andrews, M. T. Nature’s fat-burning machine: brown adipose tissue in a hibernating mammal. J. Exp. Biol.10.1242/jeb.162586 (2018). [DOI] [PMC free article] [PubMed]
- 4.Cypess, A. M. et al. Identification and importance of Brown adipose tissue in adult humans. N. Engl. J. Med.360, 1509–1517. 10.1056/NEJMoa0810780 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee, P., Swarbrick, M. M. & Ho, K. K. Brown adipose tissue in adult humans: A metabolic renaissance. Endocr. Rev.34, 413–438. 10.1210/er.2012-1081 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Liu, X., Zhang, Z., Song, Y., Xie, H. & Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. (Lausanne). 13, 1065263. 10.3389/fendo.2022.1065263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hankir, M. K. & Klingenspor, M. Brown adipocyte glucose metabolism: a heated subject. EMBO Rep.19, e46404. 10.15252/embr.201846404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berbée, J. F. et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun.6, 6356. 10.1038/ncomms7356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneshiro, T. et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obes. (Silver Spring). 19, 1755–1760. 10.1038/oby.2011.125 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Saito, M. & Okamatsu-Ogura, Y. Thermogenic brown fat in humans: Implications in energy homeostasis, obesity and metabolic disorders. World J. Mens Health. 41, 489–507. 10.5534/wjmh.220224 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun, L. et al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan1. Am. J. Clin. Nutr.107, 62–70. 10.1093/ajcn/nqx025 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Kalupahana, N. S., Goonapienuwala, B. L. & Moustaid-Moussa, N. Omega-3 fatty acids and adipose tissue: Inflammation and browning. Annu. Rev. Nutr.40, 25–49. 10.1146/annurev-nutr-122319-034142 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Silvester, A. J., Aseer, K. R. & Yun, J. W. Dietary polyphenols and their roles in fat browning. J. Nutr. Biochem.64, 1–12. 10.1016/j.jnutbio.2018.09.028 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Furuuchi, R., Yokoyama, T., Watanabe, Y. & Hirayama, M. Identification and quantification of short oligomeric proanthocyanidins and other polyphenols in boysenberry seeds and juice. J. Agric. Food Chem.59, 3738–3746. 10.1021/jf104976n (2011). [DOI] [PubMed] [Google Scholar]
- 15.Furuuchi, R. et al. Endothelial SIRT-1 has a critical role in the maintenance of capillarization in brown adipose tissue. iScience. 25, 105424. 10.1016/j.isci.2022.105424 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivamaruthi, B. S., Kesika, P. & Chaiyasut, C. The influence of supplementation of anthocyanins on obesity-Associated comorbidities: Aconcise review. Foods. 10.3390/foods9060687 (2020). [DOI] [PMC free article] [PubMed]
- 17.Han, S. et al. Cyanidin-3-O-glucoside regulates the expression of Ucp1 in Brown Adipose tissue by activating Prdm16 gene. Antioxidants. 10, 1986 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cypess, A. M., Haft, C. R., Laughlin, M. R. & Hu, H. H. Brown fat in humans: Consensus points and experimental guidelines. Cell. Metab.20, 408–415. 10.1016/j.cmet.2014.07.025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law, J. et al. Thermal imaging is a noninvasive alternative to PET/CT for measurement of brown adipose tissue activity in humans. J. Nucl. Med.59, 516–522. 10.2967/jnumed.117.190546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nirengi, S. et al. An optimal condition for the evaluation of human brown adipose tissue by infrared thermography. PLoS One. 14, e0220574. 10.1371/journal.pone.0220574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang, C. et al. Infrared thermography in the detection of brown adipose tissue in humans. Physiol. Rep.10.14814/phy2.12167 (2014). [DOI] [PMC free article] [PubMed]
- 22.Cheung, S. S. Responses of the hands and feet to cold exposure. Temp. (Austin). 2, 105–120. 10.1080/23328940.2015.1008890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuuchi, R. et al. Boysenberry polyphenol inhibits endothelial dysfunction and improves vascular health. PLoS One. 13, e0202051. 10.1371/journal.pone.0202051 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsusima, A. et al. Acute and chronic flow-mediated dilation and blood pressure responses to daily intake of boysenberry juice: Apreliminary study. Int. J. Food Sci. Nutr.64, 988–992. 10.3109/09637486.2013.812617 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Gatidis, S. et al. Is it possible to detect activated brown adipose tissue in humans using single-time-point Infrared thermography under thermoneutral conditions? Impact of BMI and subcutaneous adipose tissue thickness. PLoS One. 11, e0151152. 10.1371/journal.pone.0151152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijgen, G. H. et al. Brown adipose tissue in morbidly obese subjects. PLoS One. 6, e17247. 10.1371/journal.pone.0017247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M, U. D. et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell. Metab.28, 207–216e203 (2018). 10.1016/j.cmet.2018.05.020 [DOI] [PubMed]
- 28.Willems, M. E. T., Banic, M., Cadden, R. & Barnett, L. Enhanced walking-induced fat oxidation by New Zealand blackcurrant extract is body composition-dependent in recreationally active adult females. Nutrients. 10.3390/nu14071475 (2022). [DOI] [PMC free article] [PubMed]
- 29.Miyazaki, S. Himansyou shinryou guideline 2016. Nippon Naika Gakkai Zasshi. 107, 262–268. 10.2169/naika.107.262 (2018). [Google Scholar]
- 30.Van Schaik, L. et al. Both caffeine and Capsicum annuum fruit powder lower blood glucose levels and increase brown adipose tissue temperature in healthy adult males. Front. Physiol.13, 870154. 10.3389/fphys.2022.870154 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Schaik, L., Kettle, C., Green, R. A., Irving, H. R. & Rathner, J. A. Using a combination of indirect calorimetry, infrared thermography, and blood glucose levels to measure brown adipose tissue thermogenesis in humans. J. Vis. Exp.10.3791/64451 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Kaikaew, K., Grefhorst, A. & Visser, J. A. Sex differences in brown adipose tissue function: Sex hormones, glucocorticoids, and their crosstalk. Front. Endocrinol. (Lausanne). 12, 652444. 10.3389/fendo.2021.652444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouellet, V. et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab.96, 192–199. 10.1210/jc.2010-0989 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Au-Yong, I. T., Thorn, N., Ganatra, R., Perkins, A. C. & Symonds, M. E. Brown adipose tissue and seasonal variation in humans. Diabetes. 58, 2583–2587. 10.2337/db09-0833 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassinese, C. et al. New liquid chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparations. J. AOAC Int.90, 911–919 (2007). [PubMed] [Google Scholar]
- 36.Weir, J. B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol.109, 1–9. 10.1113/jphysiol.1949.sp004363 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frayn, K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir Environ. Exerc. Physiol.55, 628–634. 10.1152/jappl.1983.55.2.628 (1983). [DOI] [PubMed] [Google Scholar]
- 38.Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest.123, 3404–3408. 10.1172/jci67803 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this study are included in Supplementary Data.