Abstract
Background:
After menopause, reductions in ovarian hormones increase the risk of cardiovascular disease. Aerobic exercise training has been shown to reduce cardiovascular risk in older adults, but its effectiveness in postmenopausal females is less definitive.
Objectives:
The objectives of this study were to examine the: (1) effects of aerobic training, and (2) association between aerobic training intensity and cardiometabolic health outcomes in postmenopausal females.
Design:
Systematic review and meta-analysis of randomized controlled trials.
Data Sources and Methods:
Six electronic databases were searched from inception to July 21, 2023 for aerobic training interventions reporting cardiometabolic outcomes in postmenopausal females. Data were synthesized qualitatively and random-effects meta-analyses and subgroup analyses (light, moderate, and vigorous intensity) were performed. Grading of Recommendations, Assessment, Development and Evaluation was used to assess the certainty of evidence.
Results:
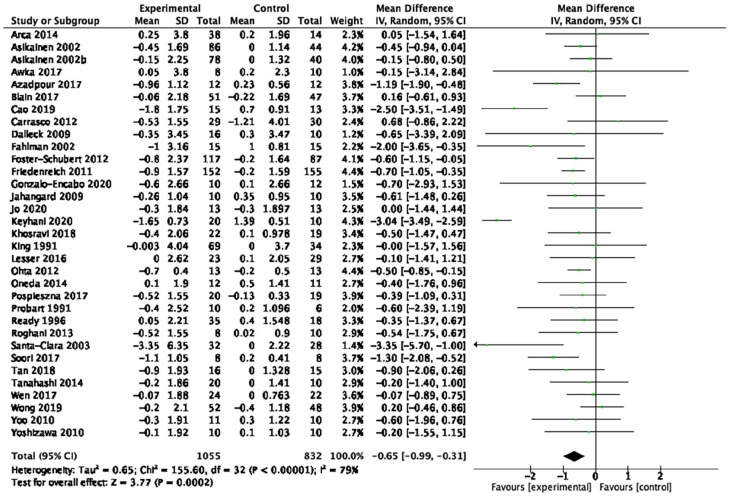
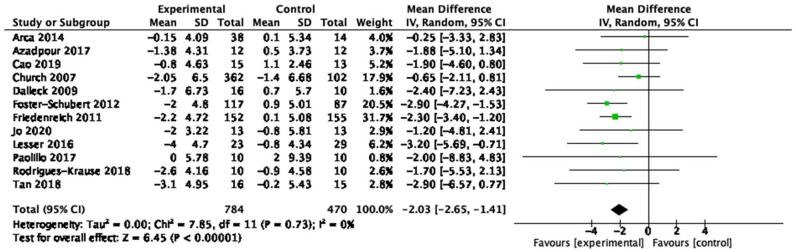
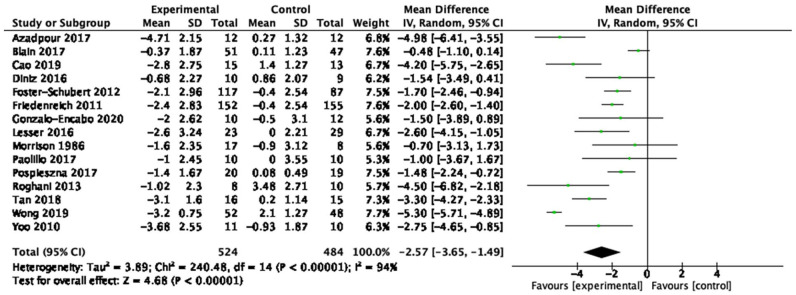
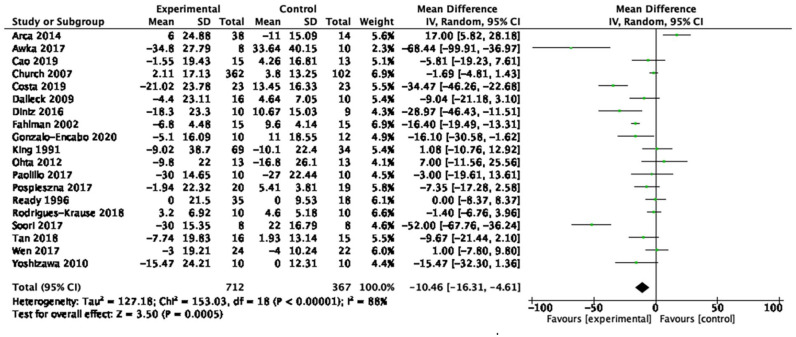
Fifty-nine studies (n = 4,225; 45–78 years old) were identified, 53 (n = 3,821) were included in the quantitative analyses. Aerobic training interventions varied in frequency (3–21×/week), intensity, type, time (8–60 min/session), and duration (3–52 weeks). Aerobic training improved systolic blood pressure (mean difference (MD) = −4.41 mmHg, 95% confidence interval (95%CI) [−7.29, −1.52], p = 0.01), resting heart rate (MD = −3.08 bpm, 95%CI [−5.11, −1.05], p < 0.01), body mass index (BMI, MD = −0.65 kg/m2, 95%CI [−0.99, −0.31], p < 0.01), waist circumference (MD = −2.03 cm, 95%CI [−2.65, −1.41], p < 0.01), body fat (MD = −2.57 kg, 95%CI [−3.65, −1.49], p < 0.01), low-density lipoprotein cholesterol (MD = −10.46 mg/dL, 95%CI [−16.31, −4.61], p < 0.01), high-density lipoprotein cholesterol (MD = 3.28 mg/dL, 95%CI [0.20, 6.36], p = 0.04) and cardiorespiratory fitness (standardized MD = 1.38, 95%CI [1.13, 1.64], p < 0.01). There was a very low certainty of evidence for all outcomes. In subgroup analyses, light- and vigorous intensities were beneficial for BMI with no effect for moderate-intensity exercise (p < 0.01). Light intensity showed a beneficial effect (p = 0.02) for glucose levels (p < 0.01) and triglycerides; there was no effect with moderate or vigorous intensities.
Conclusion:
Aerobic training may improve cardiometabolic health outcomes in postmenopausal females. There may be differential effects of exercise intensity on BMI, blood triglycerides, and blood glucose; however, this warrants further investigation.
Registration:
PROSPERO—CRD42022313350
Keywords: aerobic exercise, cardiovascular health, menopause, systematic review, meta-analysis
Introduction
Menopause is the permanent cessation of menstruation due to ovarian follicular depletion, with females considered postmenopausal after 12 consecutive months of amenorrhoea.1,2 Following menopause, there is a reduction in estrogen and its cardioprotective effects, 3 which can lead to worsening cardiovascular health, characterized by increased blood pressure, body fat, dyslipidemia, and reduced glucose tolerance. 4 Postmenopausal females are at a two-fold higher risk for cardiometabolic disease compared to premenopausal females 5 and the risk of stroke nearly doubles in the 10 years following menopause. 6
Although pharmacological treatments may be used to mitigate cardiometabolic decline, their effectiveness attenuates with duration of use; thus, alternative strategies to sustain long-term cardiometabolic health are needed. 7 Aerobic exercise training is an increasingly popular, 8 and effective9,10 strategy to promote cardiometabolic health. A systematic review of 93 trials with 5,223 males and females >18 years of age with normal blood pressure, prehypertension, and hypertension found that exercise, including aerobic exercise, decreases systolic blood pressure (SBP) and diastolic blood pressure (DBP). 11 In other reviews of individuals who are overweight or obese, 12 living with metabolic syndrome 13 and type 2 diabetes, 14 aerobic exercise training is effective for improving SBP, 14 DBP, 13 body composition,12 –14 high-density lipoprotein cholesterol (HDL-C), 13 serum triglycerides,13,14 blood glucose, 13 and cardiorespiratory fitness (CRF). 13 Importantly, the American College of Sports Medicine suggests that intensity may be the most important exercise training parameter for improving CRF,15,16 which has been corroborated by evidence for individuals undergoing cardiac rehabilitation 17 and in middle-aged sedentary adults. 18
To date, four systematic reviews have focused specifically on postmenopausal females and reported that aerobic or resistance training interventions were effective in improving SBP,19,20 DBP,19,20 CRF (n = 398),20,21 waist circumference (WC) (n = 711),19,21,22 blood triglycerides, 19 and blood glucose (n = 568). 21 Three of the reviews excluded studies involving females with conditions commonly seen postmenopause, such as metabolic syndrome, 23 hypertension, 24 diabetes mellitus,25,26 obesity,27,28 and dyslipidemia, 29 limiting the generalizability of their findings. To date, only one review 15 examined the potential influence of exercise intensity on outcomes in postmenopausal females, a potent variable in the exercise prescription for optimizing dose-related benefits. 16 We note however that this review focused specifically on metabolic syndrome risk factors (blood glucose, HDL-C, triglycerides, SBP, DBP, WC), and aggregated aerobic and resistance training together. 19 We also note that all previous reviews carefully defined menopausal status (i.e., confirmed/self-reported postmenopausal status) but may have missed the body of evidence from studies including older females of postmenopausal age where postmenopausal status may not have been confirmed. Last, these reviews did not conduct an analysis of the certainty of evidence, making it challenging to evaluate the true effectiveness of exercise on these cardiometabolic outcomes. There is an opportunity to build upon these previous reviews by synthesizing a broader body of literature on older females at postmenopausal ages, including those with common metabolic conditions, and conducting an analysis of the certainty of evidence.
Thus, the primary objective of this systematic review was to examine the effects of aerobic exercise training on traditional cardiometabolic health measures in a broad population of postmenopausal females. Based on the importance of exercise intensity, the secondary objective was to examine the association between different aerobic exercise intensities and traditional cardiometabolic health measures.
Methods
This systematic review and meta-analysis was conducted following Cochrane guidelines for Systematic Reviews of Interventions, 30 and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. 31 The protocol is registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42022313350).
Eligibility criteria
This review was restricted to peer-reviewed, published randomized controlled clinical trials in English. Non-randomized trials, conference abstracts, cross-sectional studies, study protocols, and ongoing trials were excluded.
In this review, we considered the study sample to represent postmenopausal females if they met at least one of the following three criteria: (1) explicitly confirmed participants to be postmenopausal (e.g., via self-report); (2) if postmenopausal status was not explicitly reported but age range was, a conservative lower bound of age ⩾55 years old, or (3) if age range was not reported, mean age ⩾63 years old. The latter two criteria were based on data from large population-based studies that report the average age of menopause to be 50–52 years old,32 –36 with onset of menopause varying by 1 or 2 years due to factors such as race, body composition, physical activity levels, diet, socioeconomic status, and smoking. 37 Furthermore, a mean age of 63 with an estimated standard deviation (SD) of 4 years yields a 95% confidence interval (95% CI) with a lower limit age of 55 years. Lastly, to increase the generalizability of our findings, we included studies of participants with hypertension, diabetes mellitus, obesity, and dyslipidemia, as these metabolic conditions are common postmenopause.23 –29 Participants with a history of overt cardiovascular events were excluded (e.g., stroke and myocardial infarction).
Included trials were aerobic exercise training interventions (i.e., activities that use large muscle groups that are maintained continuously and in a rhythmic nature) 38 that reported at least two of the following training parameters: frequency, intensity, type, and duration. There was no restriction to the frequency, intensity, type, and duration of aerobic interventions. To better isolate the effect of aerobic exercise training, co-interventions, and non-aerobic exercise interventions, such as resistance training, yoga, tai chi, virtual reality, pharmacological, or nutritional interventions were excluded. Control groups included non-exercise, non-diet, or non-pharmacological interventions, such as health education or stretching.
We included trials that examined the most prominent modifiable cardiometabolic health outcomes in females, (1) Resting SBP and DBP39,40; (2) resting heart rate (HR)41,42; (3) body composition and anthropometric measures43,44 (body mass index (BMI), 45 body fat, 46 waist-to-hip ratio (WHR) and WC 47 ); (4) blood lipids (low-density lipoprotein cholesterol (LDL-C), 48 HDL-C, 49 and blood triglycerides 50 ); (5) blood glucose51,52 and (6) CRF.53 –55
Studies not reporting at least one of these traditional cardiometabolic outcomes were excluded.
Information sources
The initial search of six electronic databases (OVID MEDLINE, EMBASE, CINAHL, CENTRAL, SPORTDiscus, and Web of Science) was conducted from inception to April 25, 2022 and updated on July 21, 2023. The search strategy was developed in consultation with a research librarian and included terms related to “cardiometabolic health,” “aerobic exercise,” “older adults,” and “menopause.” The final search strategies are displayed in Supplemental File 1.
Data extraction
Two sets of independent reviewers (EH and EW, EH and KSN) assessed study eligibility based on titles and abstracts followed by full-text review. All reviewers (EH, EW, KSN) piloted title and abstract screening by screening two sets of abstracts independently (n = 100). Disagreements were resolved by discussion and if necessary, resolution with a third reviewer (AT).
Data was extracted into Covidence systematic review manager (Covidence systematic review software; Veritas Health Innovation, Melbourne, Australia) by three sets of independent reviewers (EH and EW, EH and KSN, EH and HF) and included lead author name, year of publication, summary of participant eligibility criteria, population characteristics, control group characteristics, exercise intervention parameters (i.e., frequency, intensity, type, and duration), qualitative summary of effect on cardiometabolic health outcome, sample size and change in outcome (pre- to post-intervention) in intervention and control groups, respectively.
Statistical analysis
To assess the effects of aerobic exercise training on measures of cardiometabolic health (objective 1), random-effects meta-analyses and inverse variance-weighted estimation were used. Mean difference (MD) or standardized mean difference (SMD) in change scores from pre- to post-intervention with 95%CIs were used to determine the effect of aerobic exercise training on outcomes of interest. 30 Pooled SDs were calculated. Studies that contained two or more intervention groups were combined, outcome measures reported in median (interquartile range), mean (standard error) or mean (95%CI) were transformed to mean and SDs, and SDs for change scores were calculated per Cochrane guidelines. 30 Corresponding authors were contacted for studies with missing pre- and post-intervention means and SDs; otherwise, these studies were excluded. Forest plots were created to visually display the overall effects of aerobic exercise on outcomes of interest.
A priori subgroup analyses were conducted to compare the individual effects of different intensities (light, moderate, and vigorous) of aerobic exercise on cardiometabolic health (objective 2). Subgroups were categorized according to the American College of Sport Medicine criteria. 56 To preserve number of studies in each intensity category, categories of very-light and light, and vigorous and near-maximal to maximal were combined. For studies that progressed exercise intensity throughout the intervention period, studies were categorized based on the intensity performed at ⩾50% of the total intervention duration.
The robustness of the findings was tested with four a priori sensitivity analyses excluding studies of (1) older age females without confirmed postmenopausal status; (2) females with reported chronic conditions (i.e., reported hypertension, diabetes mellitus, dyslipidemia, and obesity); (3) outliers where high heterogeneity was observed; and (4) comparator groups of stretching, health education, relaxation, and placebo drug interventions.
Heterogeneity was assessed using Higgins I 2 statistic 30 and all analyses were conducted using Review Manager (RevMan version 5.3).
Quality appraisal
The methodological quality of included studies was evaluated by three sets of two independent reviewers (EH and EW, EH and KSN, EH and HF) using the Cochrane Risk of Bias 2 tool 57 (low, high, some concerns) for domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting. Certainty of evidence was evaluated using the Grading of Recommendation, Assessment, Development and Evaluations (GRADE) tool for high, moderate, low, or very low certainty, based on assessments of risk of bias, imprecision, inconsistency, indirectness, and publication bias. 58 Publication bias was assessed using visual inspection of funnel plots and the Egger’s test conducted using Stata statistical software (Version 16.1; Stata CorpLLC, College Station, TX, USA). Disagreements were resolved by discussion and if necessary, resolution with a third reviewer (AT).
Results
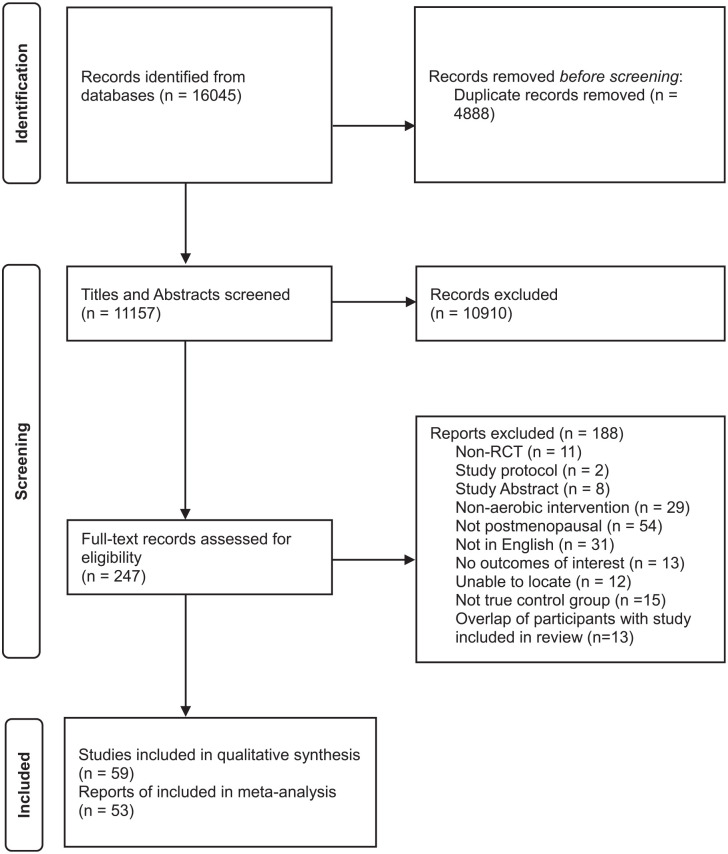
Study flow diagram is displayed in Figure 1. Fifty-nine articles59 –117 from 57 trials were included in this review. Of these 59 articles, 6 were excluded from the quantitative synthesis due to incomplete reporting of means and/or SDs.59 –63,118 Thus, 53 articles65 –117 from 51 trials were included in the meta-analysis. Thirteen articles119 –131 were also excluded from the meta-analysis as the data were already represented by the primary trial article which had the largest sample for the reported outcomes of interest.
Figure 1.
Study flow diagram.
Qualitative synthesis
Details of all included studies are summarized in Supplemental Table S1.
Participant characteristics
A total of 4,225 participants were included in this review, with study sample sizes ranging from 14 68 to 464. 105 Participants’ ages ranged from 45 years old 73 with confirmed postmenopausal status to 78 years old. 93 Forty-eight studies (81%) reported menopausal status, and 11 studies were included based on age criteria.62,66,68,71,79,93,101,104,108,116,117 Forty studies (68%) included sedentary postmenopausal females without reported chronic disease, and 19 studies included postmenopausal females living with 1 or more cardiometabolic conditions.59,60,67,71,72,76,86 –89,94,95,100,104,105,108,110,113,117
Aerobic exercise intervention
Aerobic exercise intervention durations ranged from 3 96 to 52 weeks,86,90,98 –100,106,107 with 8 to 60 min per session, except four studies87,105,111,112 that had a calorie or step goal. Interventions took place 3 to 21 times per week (3 times/day × 7 days). Aerobic interventions consisted of treadmill or cycle ergometer training, aquatic aerobic training, outdoor walking, and bench stepping. Forty-nine studies (83%) contained 1 aerobic intervention group, whereas 10 studies59,78,87,90,92,103,105,111 –113 contained multiple intervention groups that varied in intensity, duration, or type.
Comparator
Fifty-one studies compared aerobic exercise with no intervention (maintain usual diet and activity), and eight studies used stretching,77,111,112,116 health education,60,117 relaxation,104,116 or placebo drug interventions 65 as the control group.
Outcomes
SBP and DBP were investigated in 26 of 59 studies (44%), and 16 studies (27%) examined HR. Among body composition and anthropometric measures, BMI was the most frequently investigated outcome (n = 36, 61%), followed by body fat (n = 15, 25%), WC (n = 13, 22%), and WHR (n = 6, 10%). Regarding blood lipids, 19 studies (32%) investigated LDL-C and 21 studies (36%) investigated HDL-C and blood triglycerides. Blood glucose was investigated in 11 studies (19%), whereas CRF was investigated in 33 studies (56%) (Supplemental Table S1).
Quality assessment
From the quality assessment using the Cochrane Risk of Bias 2 tool, most studies were determined to be at high risk of bias due to failure to report participant attrition data (drop-out, reasons for drop-out) or had missing outcome data with failure to estimate intervention effects (intention-to-treat analyses) (Supplemental Table S2).
Adverse events
Thirty (51%) of the 59 studies did not report monitoring of adverse events.59,61 –63,65,66,68,70,73 –76,78 –80,84 –86,88,91,93 –96,101,102,110,113,117,118 Of the studies that monitored adverse events, 19 (32%)67,69,71,72,77,81 –83,89,92,97 –100,103,104,107 –109 reported no occurrence of adverse events, 4 (7%)87,105,112,116 studies reported adverse events unrelated to the exercise intervention, 3 (5%)60,114,115 studies reported adverse events but did not specify its relation to the intervention, and 3 (5%)90,106,111 studies reported adverse events due to the intervention.
Quantitative synthesis
Fifty-three studies (n = 3,821 participants) were included for meta-analysis. Nine studies reported outcome data from two or more intervention groups. All outcomes were reported as MD, except CRF where 12 studies61,68,69,88,91,99,101,108,109,115 –117 measured CRF using different submaximal tests (e.g., 1-mile walk test, Astrand test, and Blake test) and therefore was reported as SMD.
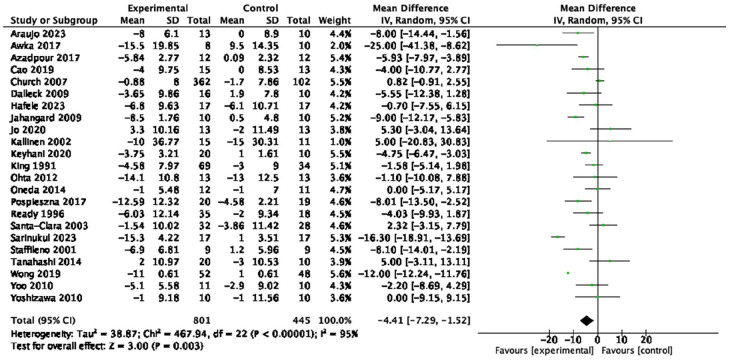
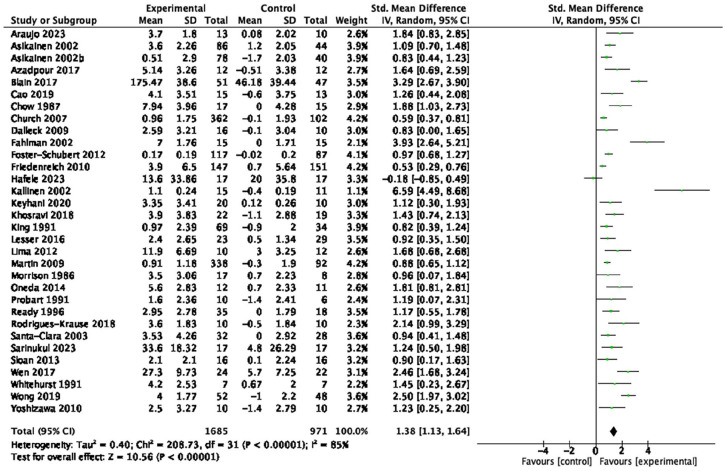
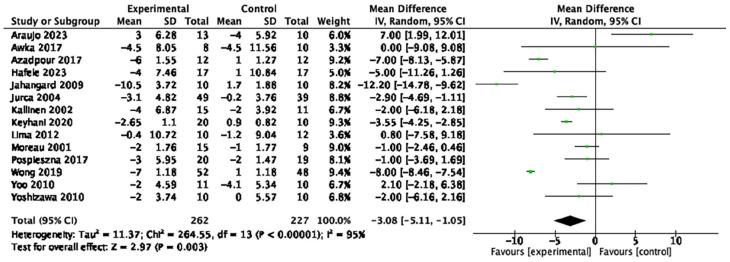
Table 1 provides a summary of findings from the meta-analyses to examine the exercise-related effects on cardiometabolic outcomes of interest with the GRADE certainty of evidence. There were beneficial effects of aerobic exercise on all measures of cardiometabolic health except DBP, WHR, blood triglycerides, or blood glucose. Forest plots for beneficial effects are displayed in Figures 2 to 9; non-significant findings are displayed in Supplemental Figures S1–S4. All outcomes were downgraded from high to very low certainty of evidence due to multiple factors, including “very serious” or “serious” risk of bias, heterogeneity, indirectness, imprecision, and the suspicion of publication bias (Table 2).
Table 1.
Effect of aerobic exercise on traditional cardiometabolic health measures in postmenopausal females.
| Cardiometabolic health measure | Mean difference | [95%CI] | p-value | Certainty of evidence |
|---|---|---|---|---|
| Systolic blood pressure (23 studies, n = 1,246) | −4.41 mmHg | [−7.29, −1.52] | <0.01 | ⊕◯◯◯ Very low |
| Diastolic blood pressure (23 studies, n = 1,246) | −2.32 mmHg | [−4.76, 0.11] | 0.06 | ⊕◯◯◯ Very low |
| Resting heart rate (14 studies, n = 489) | −3.08 bpm | [−5.11, −1.05] | <0.01 | ⊕◯◯◯ Very low |
| Body mass index (33 studies, n = 1,887) | −0.65 kg/m2 | [−0.99, −0.31] | <0.01 | ⊕◯◯◯ Very low |
| Waist circumference (12 studies, n = 1,254) | −2.03 cm | [−2.65, −1.41] | <0.01 | ⊕◯◯◯ Very low |
| Waist-to-hip ratio (6 studies, n = 196) | −0.01 | [−0.02, 0.00] | 0.06 | ⊕◯◯◯ Very low |
| Body fat (15 studies, n = 1,008) | −2.57 kg | [−3.65, −1.49] | <0.01 | ⊕◯◯◯ Very low |
| Low-density lipoprotein cholesterol (19 studies, n = 1,079) | −10.46 mg/dL | [−16.31, −4.61] | <0.01 | ⊕◯◯◯ Very low |
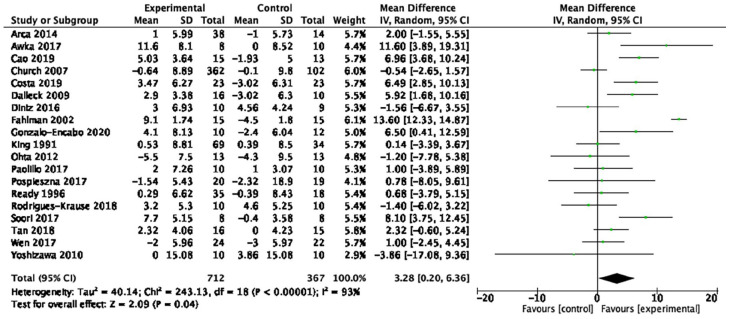
| High-density lipoprotein cholesterol (19 studies, n = 1,079) | 3.28 mg/dL | [0.20, 6.36] | 0.04 | ⊕◯◯◯ Very low |
| Blood triglycerides (19 studies, n = 1,079) | −11.59 mg/dL | [−25.82, 2.65] | 0.11 | ⊕◯◯◯ Very low |
| Blood glucose (10 studies, n = 875) | −0.92 mg/dL | [−2.79, 0.95] | 0.33 | ⊕◯◯◯ Very low |
| Cardiorespiratory fitness (32 studies, n = 2,656) | SMD: 1.38 | [1.13, 1.64] | <0.01 | ⊕◯◯◯ Very low |
95%CI: 95% confidence interval; SMD: standardized mean difference.
Bold values donote statistical significance at p < 0.05.
Figure 2.
Forest plot of the effect of aerobic exercise on systolic blood pressure.
Figure 9.
Forest plot of the effect of aerobic exercise on cardiorespiratory fitness.
Table 2.
Certainty of evidence of included studies using GRADE tool.
| Outcome | Number of studies (n participants) | Certainty assessment | Effect [95%CI] | Certainty | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ||||
| Systolic blood pressure | 23 studies (n = 1,246) | Very serious a | Serious b | Very serious c | Not serious | None | MD = −4.41 mmHg [−7.29, −1.52] | ⊕◯◯◯ Very low |
| Diastolic blood pressure | 23 studies (n = 1,246) | Very serious a | Serious b | Very serious c | Very serious d | None | MD = −2.32 mmHg [−4.76, 0.11] | ⊕◯◯◯ Very low |
| Resting heart rate | 14 studies (n = 489) | Very serious a | Serious b | Very serious c | Serious e | Highly suspected f | MD = −3.08 bpm [−5.11, −1.05] | ⊕◯◯◯ Very low |
| Body mass index | 33 studies (n = 1,887) | Very serious a | Serious b | Very serious c | Not serious | Highly suspected f | MD = −0.65 kg/m2 [−0.99, −0.31] | ⊕◯◯◯ Very low |
| Waist circumference | 12 studies (n = 1,254) | Serious g | Not serious | Very serious c | Not serious | None | MD = −2.03 cm [−2.65, −1.41] | ⊕◯◯◯ Very low |
| Waist-to-hip ratio | 6 studies (n = 196) | Serious g | Serious b | Very serious c | Very serious d | None | MD = −0.01 [−0.02, 0.00] | ⊕◯◯◯ Very low |
| Body fat | 15 studies (n = 1,008) | Very serious a | Very serious h | Very serious c | Serious e | None | MD = −2.57 kg [−3.65, −1.49] | ⊕◯◯◯ Very low |
| Low-density lipoprotein cholesterol | 19 studies (n = 1,079) | Serious g | Very serious h | Very serious c | Not serious | Highly suspected f | MD = −10.46 mg/dL [−16.31, −4.61] | ⊕◯◯◯ Very low |
| High-density lipoprotein cholesterol | 19 studies (n = 1,079) | Very serious a | Very serious h | Very serious c | Serious e | None | MD = 3.28 mg/dL [0.2, 6.36] | ⊕◯◯◯ Very low |
| Blood triglycerides | 19 studies (n = 1,079) | Very serious a | Very serious h | Very serious c | Very serious d | None | MD = −11.59 mg/dL [−25.82, 2.65] | ⊕◯◯◯ Very low |
| Blood glucose | 10 studies (n = 875) | Serious g | Very serious h | Very serious c | Serious i | None | MD = −0.92 mg/dL [−2.79, 0.95] | ⊕◯◯◯ Very low |
| CRF | 32 studies (n = 2,656) | Very serious a | Very serious h | Very serious c | Not serious | Highly suspected f | SMD = 1.38 [1.13, 1.64] | ⊕◯◯◯ Very low |
95% CI: 95% confidence interval; MD: mean difference; CRF: cardiorespiratory fitness; SMD: standardized mean difference; GRADE, Grading of Recommendation, Assessment, Development and Evaluations.
Downgraded for high risk of bias in majority of RoB-2 domains including attrition bias.
Downgraded for high statistical heterogeneity with overlapping CIs.
Downgraded due to heterogeneity in population and heterogeneity in comparison groups.
Downgraded for large 95%CI and crosses line of no effect.
Downgraded for large 95%CI, but sample >400 participants.
Downgraded for evidence of publication bias from Egger test and visual funnel plot assessment.
Downgraded for “some concerns” in majority of RoB-2 domains.
Downgraded for high statistical heterogeneity with minimal overlapping CIs.
Downgraded for crossing line of no effect but narrow 95%CI and sample >400 participants.
Figure 3.
Forest plot of the effect of aerobic exercise on resting heart rate.
Figure 4.
Forest plot of the effect of aerobic exercise on body mass index.
Figure 5.
Forest plot of the effect of aerobic exercise on waist circumference.
Figure 6.
Forest plot of the effect of aerobic exercise on body fat.
Figure 7.
Forest plot of the effect of aerobic exercise on low-density lipoprotein cholesterol.
Figure 8.
Forest plot of the effect of aerobic exercise on high-density lipoprotein cholesterol.
Positive effects in all outcomes were maintained following sensitivity analyses removing studies of (1) older age females without confirmed postmenopausal status; (2) females with reported chronic conditions; (3) outliers where high heterogeneity was observed; and (4) comparator groups of stretching, health education, relaxation, and placebo drug interventions.
Subgroup analyses
To conduct subgroup analyses based on intensity, 5 (10%)71,73,91,94,108 studies were categorized as light intensity, 25 (51%)65,67,68,72,74,76 –80,87 –89,93,96,103 –105,107,109,111 –115 were moderate intensity, and 19 (39%)66,69,75,81,82,84,86,90,92,95,97 –102,106,110,116 were vigorous intensity. Forest plots for the subgroup analyses are displayed in Supplemental Figures S5–S7. There was significant subgroup effect for BMI (χ2 = 9.79, df = 2, p < 0.01), blood triglycerides (χ2 = 7.70, df = 2, p = 0.02), and blood glucose (χ2 = 20.98, df = 2, p < 0.01). For BMI, light (n = 5 studies, MD = −1.17 kg/m2, 95%CI [−1.88, −0.47], p < 0.01) and vigorous (n = 12 studies, MD = −1.02 kg/m2, 95%CI [−1.74, −0.29], p < 0.01) intensities were beneficial, while there was no effect of moderate intensity (n = 14 studies, MD = −0.21 kg/m2, 95%CI [−0.45, −0.03], p = 0.09). Light intensity also showed a significant effect on blood triglycerides (n = 3 studies, MD = −35.39 mg/dL, 95%CI [−57.09, −13.70], p < 0.01) and blood glucose (n = 1 study, MD = −18.36 mg/dL, 95%CI [−26.04, −10.68], p < 0.01); there was no effect observed for moderate and vigorous intensities (Table 3). There were no other statistically significant subgroup effects observed.
Table 3.
Comparison between aerobic exercise intensity subgroups on cardiometabolic health measures.
| Cardiometabolic health measure | Studies | n participants | Mean difference [95%CI] | p | Pearson chi-square | ||
|---|---|---|---|---|---|---|---|
| χ2 | Degrees of freedom | p | |||||
| Body mass index | |||||||
| Light | 5 | 134 | −1.17 kg/m2 [−1.88, −0.47] | <0.01 | 9.79 | 2 | <0.01* |
| Moderate | 14 | 801 | −0.21 kg/m2 [−0.45, −0.03] | 0.09 | |||
| Vigorous | 12 | 896 | −1.02 kg/m2 [−1.74, −0.29] | <0.01 | |||
| Blood triglycerides | |||||||
| Light | 3 | 75 | −35.39 mg/dL [−57.09, −13.70] | <0.01 | 7.70 | 2 | 0.02* |
| Moderate | 9 | 738 | −3.11 mg/dL [−10.78, −4.57] | 0.43 | |||
| Vigorous | 6 | 240 | −12.75 mg/dL [−44.03, −18.53] | 0.42 | |||
| Blood glucose | |||||||
| Light | 1 | 31 | −18.36 mg/dL [−26.04, −10.68] | <0.01 | 20.98 | 2 | <0.01* |
| Moderate | 4 | 549 | 0.40 mg/dL [−2.72, 3.51] | 0.80 | |||
| Vigorous | 3 | 245 | −0.45 mg/dL [−1.52, 0.62] | 0.62 | |||
95%CI: 95% confidence interval.
Denotes p < 0.05.
No significant differences were found in the effects of exercise intensities on BMI and blood triglycerides when studies involving females with comorbidities were excluded. The results of the subgroup analyses remained after excluding studies that (1) did not explicitly report menopausal status; (2) studies with high heterogeneity; and (3) studies with comparator groups of stretching, health education, relaxation, and placebo drug interventions.
Discussion
This systematic review is the first to examine the effects of aerobic exercise in older postmenopausal females and those with common metabolic conditions. It is also the first to complete a comprehensive assessment of the certainty of evidence using the GRADE assessment, allowing clinicians and guideline developers to consider this information in their knowledge utilization and translation.
The positive effects of aerobic exercise on body fat, WC, LDL-C, HDL-C, and CRF found in this review are aligned with previous reviews,21,22 and is the first to report improvements in HR, and BMI. Moreover, these beneficial effects are consistent with reviews in adults with obesity and type 2 diabetes, which included combined interventions of aerobic training and diet, 132 and aerobic and resistance training. 133 There was little to no effect observed for DBP, WHR, blood triglycerides, and blood glucose, although the evidence is very uncertain. We note that many outcomes that demonstrated positive effects had a large proportion of studies with baseline values outside of desired levels and thus had greater potential for improvement. For example, most studies had participants who began the interventions in prehypertensive or hypertensive ranges (⩾120 mmHg) for SBP (68%), 134 or with high resting HR (50%), 135 BMI ⩾25 kg/m2 (84%), 136 WC ⩾90 cm (91%), 136 LDL-C ⩾100 mg/dL (89%) 137 and HDL-C ⩽50 mg/dL (44%). 138 This is likely a product of the broader range of studies eligible for this review, which included participants with common metabolic conditions and may explain, in part, the expanded positive effects observed in comparison to prior reviews. This approach, nonetheless, enables a more comprehensive representation of postmenopausal females, thereby increasing the generalizability of findings to underscore the benefit of aerobic exercise for individuals at higher risk for cardiovascular disease.
The null effect of aerobic exercise on DBP may be attributed to low baseline levels making it challenging to induce further reductions. The age of participants may play a factor; we note that >70% of studies in our review had a mean age of greater than 58 years old, and previous research has reported that DBP increases with age until approximately 58 years for females, after which it tends to decline, 139 possibly contributing to the normotensive DBP observed. We also did not observe an effect of aerobic exercise on WHR, although this analysis may have been underpowered due to the small number of participants included in the analysis (n = 196). Additionally, considering that adipose tissue is typically stored in the gluteofemoral regions for females, 140 it is plausible that simultaneous reductions in both waist and hip circumference may have occurred thus resulting in no change in the overall ratio. Lastly, aerobic exercise training did not have an impact on blood triglyceride levels or blood glucose levels, however, mean blood triglyceride was in normolipidemic levels (⩽150 mg/dL) 141 at baseline for 68% of studies and blood glucose in normoglycemic levels (63–100 mg/dL) for 70% of studies). 142
The physiological mechanisms that underlie the positive effects of aerobic exercise training on cardiometabolic health are well supported. Aerobic exercise training decreases sympathetic nerve activity and increases parasympathetic activity leading to long-term peripheral vasodilation. 143 Exercise may also improve SBP by attenuating age-related decreases in arterial elasticity and compliance through increased pulse pressures and mechanical distension of collagen fibers. 144 Moreover, exercise-induced enhancements in stroke volume, leading to increased cardiac output during rest, can potentially contribute to a reduction in HR. 145 Aerobic exercise can improve body composition through increased total energy expenditure, 146 leading the body to rely on adipose tissue for energy. 147 Additionally, the upregulation of Proprotein Convertase Subtilisin/Kexin Type 9 due to aerobic exercise may accelerate the absorption and excretion of LDL-C, and upregulation of the liver X receptor gene has a strong effect on the formation of HDL-C. 148 CRF may be increased through changes to cardiac output by improved stroke and blood volumes,145,149 and arteriovenous oxygen content difference. 149
The studies included in our review found either beneficial or null effects, and the 95%CIs encompassed clinically important values for most outcomes, but we acknowledge that the positive effects observed are very uncertain based on the GRADE assessment. Exercise intervention trials are often poorly reported across a range of health conditions, 150 and the overall quality is inferior to pharmacological trials. 151 Nonetheless, we propose that clinicians should consider recommending aerobic exercise to improve cardiometabolic health and mitigate menopause-related cardiovascular risk. This conservative proposition also considered in part that postmenopausal females have shown interest in physical activity including aerobic exercise to improve body composition 152 and subsequently cardiometabolic health, and that some aerobic exercise training interventions such as walking are both feasible and low in cost making them a practical option. 153
In our secondary analyses, we observed that both light and vigorous intensities were effective in reducing BMI compared to moderate intensity, and light-intensity exercise for improving blood triglycerides and blood glucose levels. The positive benefits of light-intensity exercise but not moderate-intensity exercise were unexpected but may be a product of exercise volume,154 –156 which was not examined in this review. The small number of studies in the low-intensity category may have contributed to type I error, although we note that the baseline characteristics of the study samples were elevated in terms of cardiometabolic disease risk factors and thus may have been more responsive to change. For example, the light-intensity subgroup showed the largest effect on BMI but comprised a single study of overweight or obese females, two out of the three studies on blood triglycerides focused on females with hyperlipidemia, and the only study in the light-intensity subgroup for blood glucose specifically targeted females with type 2 diabetes mellitus. In sensitivity analyses with studies of overweight and obese females and females with hyperlipidemia removed, subgroup differences in exercise intensity on BMI and blood triglycerides were no longer observed. Hence, the positive effects of light aerobic exercise should be interpreted with caution.
Limitations
We acknowledge the limitations of this review. We only included studies published in English; there may have been potentially eligible studies from the 31 papers published in other languages. Moreover, while we used conservative age criteria to ensure with a degree of confidence that the females included were postmenopausal, there is a small possibility of including premenopausal females and excluding postmenopausal females who did not meet our age criteria. Next, although we conducted a sensitivity analysis excluding studies of older females to confirm our results, we were underpowered to perform a split analysis to observe the true effects of the influence of age. Finally, recent evidence suggests that while females with lifelong exercise behaviors accrue the greatest benefit to vascular health, 157 exercises initiated early in the menopause transition may mitigate age-related declines in vascular health more so than later in life. 158 Our review however was not designed to assess the influence of lifetime exercise patterns. Future work should incorporate data such as age and duration since the final menstrual period, and consider performing a split analysis or meta-regression to better understand the influence of these factors on the effects of aerobic training.
Conclusion
Aerobic exercise training may be an important strategy to mitigate the increased cardiometabolic risk that occurs following menopause. Aerobic exercise should be considered by clinicians and clinical practice guidelines for postmenopausal females as a non-pharmacological and non-diet primary preventative approach for cardiometabolic disease.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Supplemental material, sj-docx-3-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Supplemental material, sj-docx-4-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Acknowledgments
EH was supported by a Canadian Institute for Health Research-Masters Canada Graduate Scholarship. EW and KSN are supported by the Ontario Graduate Scholarship. MB supported by a tier 2 Canada Research Chair in Mobility, Aging, and Chronic Disease.
Footnotes
ORCID iDs: Maureen J MacDonald  https://orcid.org/0000-0001-9851-3610
https://orcid.org/0000-0001-9851-3610
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Eric Huynh: Conceptualization; Investigation; Writing – original draft; Writing – review & editing; Methodology; Formal analysis; Project administration.
Elise Wiley: Writing – review & editing; Methodology; Formal analysis.
Kenneth S Noguchi: Methodology; Writing – review & editing; Formal analysis.
Hanna Fang: Methodology; Writing – review & editing; Formal analysis.
Marla K Beauchamp: Writing – review & editing.
Maureen J MacDonald: Writing – review & editing.
Ada Tang: Conceptualization; Supervision; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All data generated during and/or analyzed during this current study are available from the corresponding author on reasonable request.
References
- 1. Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999; 353: 571–580. [DOI] [PubMed] [Google Scholar]
- 2. Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 1987; 65: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 3. Iorga A, Cunningham CM, Moazeni S, et al. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 2017; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosano GM, Vitale C, Marazzi G, et al. Menopause and cardiovascular disease: the evidence. Climacteric 2007; 10(Suppl 1): 19–24. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, Hjortland MC, McNamara PM, et al. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 1976; 85: 447–452. [DOI] [PubMed] [Google Scholar]
- 6. Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol 2012; 11: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canoy D, Copland E, Nazarzadeh M, et al. Antihypertensive drug effects on long-term blood pressure: an individual-level data meta-analysis of randomised clinical trials. Heart 2022; 108: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newsome ANM, Reed R, Sansone J, et al. 2024 ACSM Worldwide Fitness Trends: future directions of the health and fitness industry. ACSMS Health Fitness J 2024; 28: 14–26. [Google Scholar]
- 9. Batrakoulis A, Jamurtas AZ, Fatouros IG. High-intensity interval training in metabolic diseases. ACSMS Health Fitness J 2021; 25: 54–59. [Google Scholar]
- 10. Batrakoulis A, Jamurtas AZ, Metsios GS, et al. Comparative efficacy of 5 exercise types on cardiometabolic health in overweight and obese adults: a systematic review and network meta-analysis of 81 randomized controlled trials. Circ Cardiovasc Qual Outcomes 2022; 15: e008243. [DOI] [PubMed] [Google Scholar]
- 11. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013; 2: e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ismail I, Keating SE, Baker MK, et al. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev 2012; 13: 68–91. [DOI] [PubMed] [Google Scholar]
- 13. Wewege MA, Thom JM, Rye KA, et al. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018; 274: 162–171. [DOI] [PubMed] [Google Scholar]
- 14. Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care 2011; 34: 1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Sports Medicine,Liguori G, Feito Y, et al. ACSM’s guidelines for exercise testing and prescription, 11th ed. Philadelphia, PA: Wolters Kluwer, 2022. [Google Scholar]
- 16. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell BL, Lock MJ, Davison K, et al. What is the effect of aerobic exercise intensity on cardiorespiratory fitness in those undergoing cardiac rehabilitation? A systematic review with meta-analysis. Br J Sports Med 2019; 53: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 18. Ross R, de Lannoy L, Stotz PJ. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin Proc 2015; 90: 1506–1514. [DOI] [PubMed] [Google Scholar]
- 19. Tan A, Thomas RL, Campbell MD, et al. Effects of exercise training on metabolic syndrome risk factors in post-menopausal women—a systematic review and meta-analysis of randomised controlled trials. Clin Nutr 2023; 42: 337–351. [DOI] [PubMed] [Google Scholar]
- 20. Li T, Zhang L. Effect of exercise on cardiovascular risk in sedentary postmenopausal women: a systematic review and meta-analysis. Ann Palliat Med 2023; 12: 150–162. [DOI] [PubMed] [Google Scholar]
- 21. Park SH, Kim CG. Effects of aerobic exercise on waist circumference, VO2 max, blood glucose, insulin and lipid index in middle-aged women: a meta-analysis of randomized controlled trials. Health Care Women Int 2022; 43(10–11): 1158–1180. [DOI] [PubMed] [Google Scholar]
- 22. Yeh ML, Liao RW, Hsu CC, et al. Exercises improve body composition, cardiovascular risk factors and bone mineral density for menopausal women: a systematic review and meta-analysis of randomized controlled trials. Appl Nurs Res 2018; 40: 90–98. [DOI] [PubMed] [Google Scholar]
- 23. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003; 88: 2404–2411. [DOI] [PubMed] [Google Scholar]
- 24. Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 2012; 14: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heianza Y, Arase Y, Kodama S, et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care 2013; 36: 4007–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang M, Gan W, Kartsonaki C, et al. Menopausal status, age at natural menopause and risk of diabetes in China: a 10-year prospective study of 300,000 women. Nutr Metab (Lond) 2022; 19: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sternfeld B, Wang H, Quesenberry CP, Jr., et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am J Epidemiol 2004; 160: 912–922. [DOI] [PubMed] [Google Scholar]
- 28. Guthrie JR, Dennerstein L, Dudley EC. Weight gain and the menopause: a 5-year prospective study. Climacteric 1999; 2: 205–211. [DOI] [PubMed] [Google Scholar]
- 29. Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009; 54: 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JPT, Thomas J, Chandler J, et al. (eds.). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022), http://www.training.cochrane.org/handbook (2022, accessed, June 18 2024).
- 31. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gottschalk MS, Eskild A, Hofvind S, et al. Temporal trends in age at menarche and age at menopause: a population study of 312 656 women in Norway. Hum Reprod 2020; 35: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwawukume EY, Ghosh TS, Wilson JB. Menopausal age of Ghanaian women. Int J Gynecol Obstet 1993; 40: 151–155. [DOI] [PubMed] [Google Scholar]
- 34. Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001; 153: 865–874. [DOI] [PubMed] [Google Scholar]
- 35. Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol 1994; 139: 64–76. [DOI] [PubMed] [Google Scholar]
- 36. Beard JH, Jacoby SF, Maher Z, et al. Trends in age at natural menopause and reproductive life span among US women, 1959–2018. JAMA 2021; 325: 1327–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011; 38: 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel H, Alkhawam H, Madanieh R, et al. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J Cardiol 2017; 9: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wenger NK, Arnold A, Bairey Merz CN, et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol 2018; 71: 1797–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lobo RA, Davis SR, De Villiers TJ, et al. Prevention of diseases after menopause. Climacteric 2014; 17: 540–556. [DOI] [PubMed] [Google Scholar]
- 41. Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007; 50: 823–830. [DOI] [PubMed] [Google Scholar]
- 42. Zhang D, Shen X, Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. CMAJ 2016; 188: E53–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res 2016; 118: 1752–1770. [DOI] [PubMed] [Google Scholar]
- 44. Zalesin KC, Franklin BA, Miller WM, et al. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am 2008; 37: 663–684, ix. [DOI] [PubMed] [Google Scholar]
- 45. Katzmarzyk PT, Reeder BA, Elliott S, et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Health 2012; 103: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev 2002; 3: 209–215. [DOI] [PubMed] [Google Scholar]
- 47. Huxley R, Mendis S, Zheleznyakov E, et al. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr 2010; 64: 16–22. [DOI] [PubMed] [Google Scholar]
- 48. Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. Lancet 2020; 396: 1644–1652. [DOI] [PubMed] [Google Scholar]
- 49. Toth PP. High-density lipoprotein and cardiovascular risk. Circulation 2004; 109: 1809–1812. [DOI] [PubMed] [Google Scholar]
- 50. Harchaoui KE, Visser ME, Kastelein JJ, et al. Triglycerides and cardiovascular risk. Curr Cardiol Rev 2009; 5: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haffner SJ, Cassells H. Hyperglycemia as a cardiovascular risk factor. Am J Med 2003; 115(Suppl 8A): 6S–11S. [DOI] [PubMed] [Google Scholar]
- 52. Marks JB, Raskin P. Cardiovascular risk in diabetes. J Diabetes Complications 2000; 14: 108–115. [DOI] [PubMed] [Google Scholar]
- 53. Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory fitness and cardiovascular disease prevention: an update. Curr Atheroscler Rep 2018; 20: 1. [DOI] [PubMed] [Google Scholar]
- 54. Al-Mallah MH, Juraschek SP, Whelton S, et al. Sex differences in cardiorespiratory fitness and all-cause mortality: the Henry Ford ExercIse Testing (FIT) Project. Mayo Clin Proc 2016; 91: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009; 301: 2024–2035. [DOI] [PubMed] [Google Scholar]
- 56. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 10th ed. Philadelphia, PA: Wolters Kluwer, 2021. [Google Scholar]
- 57. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 58. Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008; 336: 1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adams-Campbell LL, Taylor T, Hicks J, et al. The effect of a 6-month exercise intervention trial on allostatic load in Black women at increased risk for breast cancer: the FIERCE study. J Racial Ethn Health Disparities 2022; 9(5): 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coletta AM, Brewster AM, Chen M, et al. High-intensity interval training is feasible in women at high risk for breast cancer. Medicine and Science in Sports and Exercise 2019; 51: 2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cowan MM, Gregory LW. Responses of pre- and post-menopausal females to aerobic conditioning. Med Sci Sports Exerc 1985; 17: 138–143. [PubMed] [Google Scholar]
- 62. Hamdorf PA, Withers RT, Penhall RK, et al. Physical training effects on the fitness and habitual activity patterns of elderly women. Arch Phys Med Rehabil 1992; 73: 603–608. [PubMed] [Google Scholar]
- 63. Tartibian B, FitzGerald LZ, Azadpour N, et al. A randomized controlled study examining the effect of exercise on inflammatory cytokine levels in post-menopausal women. Post Reprod health 2015; 21: 9–15. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Liu G, Hong D, et al. White matter injury in ischemic stroke. Prog Neurobiol 2016; 141: 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoshizawa M, Maeda S, Miyaki A, et al. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens 2010; 23: 368–372. [DOI] [PubMed] [Google Scholar]
- 66. Yoo EJ, Jun TW, Hawkins SA. The effects of a walking exercise program on fall-related fitness, bone metabolism, and fall-related psychological factors in elderly women. Res Sports Med 2010; 18: 236–250. [DOI] [PubMed] [Google Scholar]
- 67. Wong A, Kwak Y-S, Scott SD, et al. The effects of swimming training on arterial function, muscular strength, and cardiorespiratory capacity in postmenopausal women with stage 2 hypertension. Menopause 2019; 26: 653–658. [DOI] [PubMed] [Google Scholar]
- 68. Whitehurst M. Reaction time unchanged in older women following aerobic training. Percept Mot Skills 1991; 72: 251–256. [DOI] [PubMed] [Google Scholar]
- 69. Wen HJ, Huang TH, Li TL, et al. Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos Int 2017; 28: 539–547. [DOI] [PubMed] [Google Scholar]
- 70. Tanahashi K, Akazawa N, Miyaki A, et al. Aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am J Hypertens 2014; 27: 415–421. [DOI] [PubMed] [Google Scholar]
- 71. Tan S, Du P, Zhao W, et al. Exercise training at maximal fat oxidation intensity for older women with type 2 diabetes. Int J Sports Med 2018; 39: 374–381. [DOI] [PubMed] [Google Scholar]
- 72. Staffileno BA, Braun LT, Rosenson RS. The accumulative effects of physical activity in hypertensive post-menopausal women. J Cardiovasc Risk 2001; 8: 283–290. [DOI] [PubMed] [Google Scholar]
- 73. Soori R, Rezaeian N, Khosravi N, et al. Effects of water-based endurance training, resistance training, and combined water and resistance training programs on visfatin and ICAM-1 levels in sedentary obese women. Sci Sports 2017; 32: 144–151. [Google Scholar]
- 74. Sloan CA, Engels HJ, Fahlman MM, et al. Effects of exercise on S-IGA and URS in postmenopausal women. Int J Sports Med 2013; 34: 81–86. [DOI] [PubMed] [Google Scholar]
- 75. Santa-Clara H, Szymanski L, Fernhall B. Effect of exercise training on blood pressure in postmenopausal Caucasian and African-American women. Am J Cardiol 2003; 91: 1009–1011, A8. [DOI] [PubMed] [Google Scholar]
- 76. Roghani T, Torkaman G, Movasseghe S, et al. Effects of short-term aerobic exercise with and without external loading on bone metabolism and balance in postmenopausal women with osteoporosis. Rheumatol Int 2013; 33: 291–298. [DOI] [PubMed] [Google Scholar]
- 77. Rodrigues-Krause J, Farinha JB, Ramis TR, et al. Effects of dancing compared to walking on cardiovascular risk and functional capacity of older women: a randomized controlled trial. Exp Gerontol 2018; 114: 67–77. [DOI] [PubMed] [Google Scholar]
- 78. Ready AE, Naimark B, Ducas J, et al. Influence sur la sante du kilometrage effectue en marchant par des femmes post-menopausees [Influence of walking volume on health benefits in women post-menopause]. Med Sci Sports Exerc 1996; 28: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 79. Probart CK, Notelovitz M, Martin D, et al. The effect of moderate aerobic exercise on physical fitness among women 70 years and older. Maturitas 1991; 14: 49–56. [DOI] [PubMed] [Google Scholar]
- 80. Pospieszna B, Karolkiewicz J, Tarnas J, et al. Influence of 12-week Nordic Walking training on biomarkers of endothelial function in healthy postmenopausal women. J Sports Med Phys Fitness 2017; 57: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 81. Paolillo FR, Borghi-Silva A, Arena R, et al. Effects of phototherapy plus physical training on metabolic profile and quality of life in postmenopausal women. J Cosmet Laser Ther 2017; 19: 364–372. [DOI] [PubMed] [Google Scholar]
- 82. Oneda B, Cardoso CG, Jr., Forjaz CLM, et al. Effects of estrogen therapy and aerobic training on sympathetic activity and hemodynamics in healthy postmenopausal women: a double-blind randomized trial. Menopause 2014; 21: 369–375. [DOI] [PubMed] [Google Scholar]
- 83. Ohta M, Hirao N, Mori Y, et al. Effects of bench step exercise on arterial stiffness in post-menopausal women: contribution of IGF-1 bioactivity and nitric oxide production. Growth Horm IGF Research 2012; 22: 36–41. [DOI] [PubMed] [Google Scholar]
- 84. Morrison DA, Boyden TW, Pamenter RW, et al. Effects of aerobic training on exercise tolerance and echocardiographic dimensions in untrained postmenopausal women. Am Heart J 1986; 112: 561–567. [DOI] [PubMed] [Google Scholar]
- 85. Moreau KL, Degarmo R, Langley J, et al. Increasing daily walking lowers blood pressure in postmenopausal women. Med Sci Sports Exerc 2001; 33: 1825–1831. [DOI] [PubMed] [Google Scholar]
- 86. Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med 2011; 41: 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martin CK, Church TS, Thompson AM, et al. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med 2009; 169: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lima AH, Couto HE, Cardoso GA, et al. Aerobic training does not alter blood pressure in menopausal women with metabolic syndrome. Arq Bras Cardiol 2012; 99: 979–987. [DOI] [PubMed] [Google Scholar]
- 89. Lesser IA, Singer J, Hoogbruin A, et al. Effectiveness of exercise on visceral adipose tissue in older South Asian women. Med Sci Sports Exerc 2016; 48: 1371–1378. [DOI] [PubMed] [Google Scholar]
- 90. King AC, Haskell WL, Taylor CB, et al. Group- vs home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA 1991; 266: 1535–1542. [PubMed] [Google Scholar]
- 91. Khosravi N, Eskandari Z, Farajivafa V, et al. Effect of 6 months of aerobic training on adipokines as breast cancer risk factors in postmenopausal women: a randomized controlled trial. J Cancer Res Ther 2018; 14: 1336–1340. [DOI] [PubMed] [Google Scholar]
- 92. Keyhani D, Tartibian B, Dabiri A, et al. Effect of high-intensity interval training versus moderate-intensity aerobic continuous training on galectin-3 gene expression in postmenopausal women: a randomized controlled trial. J Aging Phys Act 2020; 28(6): 987–995. [DOI] [PubMed] [Google Scholar]
- 93. Kallinen M, Sipilä S, Alen M, et al. Improving cardiovascular fitness by strength or endurance training in women aged 76–78 years. A population-based, randomized controlled trial. Age Ageing 2002; 31: 247–254. [DOI] [PubMed] [Google Scholar]
- 94. Jurca R, Church TS, Morss GM, et al. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J 2004; 147: e21. [DOI] [PubMed] [Google Scholar]
- 95. Jo E-A, Wu S-S, Han H-R, et al. Effects of exergaming in postmenopausal women with high cardiovascular risk: a randomized controlled trial. Clin Cardiol 2020; 43: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jahangard T, Torkaman G, Ghoosheh B, et al. The effect of short-term aerobic training on coagulation and fibrinolytic factors in sedentary healthy postmenopausal women. Maturitas 2009; 64: 223–227. [DOI] [PubMed] [Google Scholar]
- 97. Gonzalo-Encabo P, Valades D, Garcia-Honduvilla N, et al. Exercise type and fat mass loss regulate breast cancer-related sex hormones in obese and overweight postmenopausal women. Eur J Appl Physiol 2020; 120: 1277–1287. [DOI] [PubMed] [Google Scholar]
- 98. Friedenreich CM, Woolcott CG, McTiernan A, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond) 2011; 35: 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol 2010; 28: 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012; 20(8): 1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fahlman MM, Boardley D, Lambert CP, et al. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J Gerontol A Biol Sci Med Sci 2002; 57: B54–B60. [DOI] [PubMed] [Google Scholar]
- 102. Diniz TA, Fortaleza ACS, Rossi FE, et al. Short-term program of aerobic training prescribed using critical velocity is effective to improve metabolic profile in postmenopausal women. Sci Sports 2016; 31: 95–102. [Google Scholar]
- 103. Dalleck LC, Allen BA, Hanson BA, et al. Dose-response relationship between moderate-intensity exercise duration and coronary heart disease risk factors in postmenopausal women. J Womens Health (Larchmt) 2009; 18: 105–113. [DOI] [PubMed] [Google Scholar]
- 104. Costa RR, Buttelli ACK, Coconcelli L, et al. Water-based aerobic and resistance training as a treatment to improve the lipid profile of women with dyslipidemia: a randomized controlled trial. J Phys Act Health 2019; 16: 348–354. [DOI] [PubMed] [Google Scholar]
- 105. Church TS, Earnest CP, Skinner JS, et al. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 2007; 297: 2081–2091. [DOI] [PubMed] [Google Scholar]
- 106. Chow R, Harrison JE, Notarius C. Effect of two randomised exercise programmes on bone mass of healthy postmenopausal women. Br Med J (Clin Res Ed) 1987; 295: 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Carrasco M, Vaquero M. Water training in postmenopausal women: effect on muscular strength. Eur J Sport Sci 2012; 12: 193–200. [Google Scholar]
- 108. Cao L, Jiang Y, Li Q, et al. Exercise training at maximal fat oxidation intensity for overweight or obese older women: a randomized study. J Sports Sci Medicine 2019; 18: 413–418. [PMC free article] [PubMed] [Google Scholar]
- 109. Blain H, Jaussent A, Picot MC, et al. Effect of a 6-month brisk walking program on walking endurance in sedentary and physically deconditioned women aged 60 or older: a randomized trial. J Nutr Health Aging 2017; 21: 1183–1189. [DOI] [PubMed] [Google Scholar]
- 110. Azadpour N, Tartibian B, Kosar SN. Effects of aerobic exercise training on ACE and ADRB2 gene expression, plasma angiotensin II level, and flow-mediated dilation: a study on obese postmenopausal women with prehypertension. Menopause 2017; 24: 269–277. [DOI] [PubMed] [Google Scholar]
- 111. Asikainen TM, Miilunpalo S, Oja P, et al. Walking trials in postmenopausal women: effect of one vs two daily bouts on aerobic fitness. Scand J Med Sci Sports 2002; 12: 99–105. [DOI] [PubMed] [Google Scholar]
- 112. Asikainen TM, Miilunpalo S, Oja P, et al. Randomised, controlled walking trials in postmenopausal women: the minimum dose to improve aerobic fitness? Br J Sports Med 2002; 36: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Arca EA, Martinelli B, Martin LC, et al. Aquatic exercise is as effective as dry land training to blood pressure reduction in postmenopausal hypertensive women. Physiother Res Int 2014; 19: 93–98. [DOI] [PubMed] [Google Scholar]
- 114. Akwa LG, Moses MO, Emikpe AO, et al. Lipid profile, cardiorespiratory function and quality of life of postmenopausal women improves with aerobic exercise. J Hum Sport Exerc 2017; 12: 698–709. [Google Scholar]
- 115. Araujo RC, Rodrigues GD, Ferreira LF, et al. The time course of cardiorespiratory adaptations to rowing indoor training in post-menopausal women. Int J Environ Res Public Health 2023; 20(4): 3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Häfele MS, Alberton CL, Häfele V, et al. Water-based training programs improve functional capacity, cognitive and hemodynamic outcomes? The ACTIVE randomized clinical trial. Res Q Exerc Sport 2023; 94: 24–34. [DOI] [PubMed] [Google Scholar]
- 117. Sarinukul C, Janyacharoen T, Donpunha W, et al. The effects of stepping exercise on blood pressure, physical performance, and quality of life in female older adults with stage 1 hypertension: a randomized controlled trial. Can Geriatr J 2023; 26: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang CH, Chung MH, Chan P, et al. Effects of endurance exercise training on risk components for metabolic syndrome, interleukin-6, and the exercise capacity of postmenopausal women. Geriatr Nurs 2014; 35: 212–218. [DOI] [PubMed] [Google Scholar]
- 119. Swift DL, Johannsen NM, Lavie CJ, et al. Racial differences in the response of cardiorespiratory fitness to aerobic exercise training in Caucasian and African American postmenopausal women. J Appl Physiol 2013; 114: 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Swift DL, Johannsen NM, Earnest CP, et al. Effect of different doses of aerobic exercise training on total bilirubin levels. Med Sci Sports Exerc 2012; 44: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Swift DL, Earnest CP, Katzmarzyk PT, et al. The effect of different doses of aerobic exercise training on exercise blood pressure in overweight and obese postmenopausal women. Menopause 2012; 19: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Swift DL, Earnest CP, Blair SN, et al. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med 2012; 46: 753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stewart LK, Earnest CP, Blair SN, et al. Effects of different doses of physical activity on C-reactive protein among women. Med Sci Sports Exerc 2010; 42: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Santa-Clara H, Szymanski L, Ordille T, et al. Effects of exercise training on resting metabolic rate in postmenopausal African American and Caucasian women. Metabolism 2006; 55: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 125. Mason C, de Dieu Tapsoba J, Duggan C, et al. Eating behaviors and weight loss outcomes in a 12-month randomized trial of diet and/or exercise intervention in postmenopausal women. Int J Behav Nutr Phys Act 2019; 16: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Johannsen NM, Swift DL, Johnson WD, et al. Effect of different doses of aerobic exercise on total white blood cell (WBC) and WBC subfraction number in postmenopausal women: results from DREW. PLoS One 2012; 7: e31319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dash C, Taylor TR, Makambi KH, et al. Effect of exercise on metabolic syndrome in black women by family history and predicted risk of breast cancer: the FIERCE Study. Cancer 2018; 124: 3355–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Church TS, Martin CK, Thompson AM, et al. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One 2009; 4: e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cardoso CG, Jr., Rosas FC, Oneda B, et al. Aerobic training abolishes ambulatory blood pressure increase induced by estrogen therapy: a double blind randomized clinical trial. Maturitas 2011; 69: 189–194. [DOI] [PubMed] [Google Scholar]
- 130. Cardoso CG, Jr., Medina FL, Pinto LG, et al. Oral estrogen therapy may mitigate the effects of aerobic training on cardiorespiratory fitness in postmenopausal women: a double-blind, randomized clinical pilot study. Menopause 2014; 21: 376–382. [DOI] [PubMed] [Google Scholar]
- 131. Anaya SA, Church TS, Blair SN, et al. Exercise dose-response of the V(E)/VCO(2) slope in postmenopausal women in the DREW study. Med Sci Sports Exerc 2009; 41: 971–976. [DOI] [PubMed] [Google Scholar]
- 132. Al-Mhanna SB, Rocha-Rodriguesc S, Mohamed M, et al. Effects of combined aerobic exercise and diet on cardiometabolic health in patients with obesity and type 2 diabetes: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil 2023; 15: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Al-Mhanna SB, Batrakoulis A, Wan Ghazali WS, et al. Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis. PeerJ 2024; 12: e17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hardy ST, Sakhuja S, Jaeger BC, et al. Maintaining normal blood pressure across the life course: the JHS. Hypertension 2021; 77: 1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Avram R, Tison GH, Aschbacher K, et al. Real-world heart rate norms in the Health eHeart study. NPJ Digit Med 2019; 2: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020; 16: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rong S, Li B, Chen L, et al. Association of low-density lipoprotein cholesterol levels with more than 20-year risk of cardiovascular and all-cause mortality in the general population. J Am Heart Assoc 2022; 11: e023690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ahmed HM, Miller M, Nasir K, et al. Primary low level of high-density lipoprotein cholesterol and risks of coronary heart disease, cardiovascular disease, and death: results from the multi-ethnic study of atherosclerosis. Am J Epidemiol 2016; 183: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Webb AJS. Progression of arterial stiffness is associated with midlife diastolic blood pressure and transition to late-life hypertensive phenotypes. J Am Heart Assoc 2020; 9: e014547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lumish HS, O’Reilly M, Reilly MP. Sex Differences in genomic drivers of adipose distribution and related cardiometabolic disorders: opportunities for precision medicine. Arterioscler Thromb Vasc Biol 2020; 40: 45–60. [DOI] [PubMed] [Google Scholar]
- 141. Laufs U, Parhofer KG, Ginsberg HN, et al. Clinical review on triglycerides. Eur Heart J 2020; 41: 99–109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Guemes M, Rahman SA, Hussain K. What is a normal blood glucose? Arch Dis Child 2016; 101: 569–574. [DOI] [PubMed] [Google Scholar]
- 143. Hamer M. The anti-hypertensive effects of exercise: integrating acute and chronic mechanisms. Sports Med 2006; 36: 109–116. [DOI] [PubMed] [Google Scholar]
- 144. Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000; 102: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 145. Fernandes T, Hashimoto NY, Magalhaes FC, et al. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1–7). Hypertension 2011; 58: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hills AP, Mokhtar N, Byrne NM. Assessment of physical activity and energy expenditure: an overview of objective measures. Front Nutr 2014; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Purdom T, Kravitz L, Dokladny K, et al. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr 2018; 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis 2017; 16: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Schmidt W, Prommer N. Impact of alterations in total hemoglobin mass on VO2max. Exerc Sport Sci Rev 2010; 38: 68–75. [DOI] [PubMed] [Google Scholar]
- 150. Hansford HJ, Wewege MA, Cashin AG, et al. If exercise is medicine, why don’t we know the dose? An overview of systematic reviews assessing reporting quality of exercise interventions in health and disease. Br J Sports Med 2022; 56: 692–700. [DOI] [PubMed] [Google Scholar]
- 151. Adams SC, McMillan J, Salline K, et al. Comparing the reporting and conduct quality of exercise and pharmacological randomised controlled trials: a systematic review. BMJ Open 2021; 11: e048218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Marlatt KL, Beyl RA, Redman LM. A qualitative assessment of health behaviors and experiences during menopause: a cross-sectional, observational study. Maturitas 2018; 116: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Med 2004; 34: 753–778. [DOI] [PubMed] [Google Scholar]
- 154. Ishikawa-Takata K, Ohta T, Tanaka H. How much exercise is required to reduce blood pressure in essential hypertensives: a dose-response study. Am J Hypertens 2003; 16: 629–633. [DOI] [PubMed] [Google Scholar]
- 155. Ohkawara K, Tanaka S, Miyachi M, et al. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 2007; 31: 1786–1797. [DOI] [PubMed] [Google Scholar]
- 156. Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 2001; 33: S459–S471; discussion S493–S454. [DOI] [PubMed] [Google Scholar]
- 157. Carrick-Ranson G, Sloane NM, Howden EJ, et al. The effect of lifelong endurance exercise on cardiovascular structure and exercise function in women. J Physiol 2020; 598: 2589–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tamariz-Ellemann A, Wickham KA, Norregaard LB, et al. The time is now: regular exercise maintains vascular health in ageing women. J Physiol 2023; 601: 2085–2098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Supplemental material, sj-docx-3-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health
Supplemental material, sj-docx-4-whe-10.1177_17455057241290889 for The effects of aerobic exercise on cardiometabolic health in postmenopausal females: A systematic review and meta-analysis of randomized controlled trials by Eric Huynh, Elise Wiley, Kenneth S Noguchi, Hanna Fang, Marla K Beauchamp, Maureen J MacDonald and Ada Tang in Women’s Health