Abstract
To investigate the importance of different processes to heat stress tolerance, 45 Arabidopsis (Arabidopsis thaliana) mutants and one transgenic line were tested for basal and acquired thermotolerance at different stages of growth. Plants tested were defective in signaling pathways (abscisic acid, salicylic acid, ethylene, and oxidative burst signaling) and in reactive oxygen metabolism (ascorbic acid or glutathione production, catalase) or had previously been found to have temperature-related phenotypes (e.g. fatty acid desaturase mutants, uvh6). Mutants were assessed for thermotolerance defects in seed germination, hypocotyl elongation, root growth, and seedling survival. To assess oxidative damage and alterations in the heat shock response, thiobarbituric acid reactive substances, heat shock protein 101, and small heat shock protein levels were determined. Fifteen mutants showed significant phenotypes. Abscisic acid (ABA) signaling mutants (abi1 and abi2) and the UV-sensitive mutant, uvh6, showed the strongest defects in acquired thermotolerance of root growth and seedling survival. Mutations in nicotinamide adenine dinucleotide phosphate oxidase homolog genes (atrbohB and D), ABA biosynthesis mutants (aba1, aba2, and aba3), and NahG transgenic lines (salicylic acid deficient) showed weaker defects. Ethylene signaling mutants (ein2 and etr1) and reactive oxygen metabolism mutants (vtc1, vtc2, npq1, and cad2) were more defective in basal than acquired thermotolerance, especially under high light. All mutants accumulated wild-type levels of heat shock protein 101 and small heat shock proteins. These data indicate that, separate from heat shock protein induction, ABA, active oxygen species, and salicylic acid pathways are involved in acquired thermotolerance and that UVH6 plays a significant role in temperature responses in addition to its role in UV stress.
Plants and other organisms have both an inherent ability to survive exposure to temperatures above the optimal for growth (basal thermotolerance) and an ability to acquire tolerance to otherwise lethal heat stress (acquired thermotolerance). Acquired thermotolerance is induced by a short acclimation period at moderately high (but survivable) temperatures or by treatment with other nonlethal stress prior to heat stress (Kapoor et al., 1990; Vierling, 1991; Flahaut et al., 1996; Burke et al., 2000; Hong and Vierling, 2000; Massie et al., 2003; Larkindale et al., 2005). The ability to withstand and to acclimate to supra-optimal temperatures results from both prevention of heat damage and repair of heat-sensitive components. Organisms must also maintain metabolic homeostasis during stress or be able to reestablish homeostasis subsequent to the stress period. Although plants are frequently subjected to dramatic heating to above the optimal growth temperature, relatively little is known about the critical genes controlling either basal or acquired thermotolerance in plants.
Heat stress has a complex impact on cell function, suggesting that many processes are involved in thermotolerance. Some processes may be specific to basal thermotolerance, others may be induced during acquired thermotolerance, and many may be involved in both. High temperatures are known to affect membrane-linked processes due to alterations in membrane fluidity and permeability (Alfonso et al., 2001; Sangwan et al., 2002). Enzyme function is also sensitive to changes in temperature. Heat-induced alterations in enzyme activity can lead to imbalance in metabolic pathways, or heat can cause complete enzyme inactivation due to protein denaturation (Vierling, 1991; Kampinga et al., 1995). Membrane and protein damage lead to the production of active oxygen species that cause heat-induced oxidative stress (Dat et al., 1998a, 1998b; Gong et al., 1998; Larkindale and Knight, 2002). Heat can also promote programmed cell death (Swidzinski et al., 2002; Vacca et al., 2004). In plants, these different types of damage translate into reduced photosynthesis, impaired translocation of assimilates, and reduced carbon gain, leading to altered growth and reproduction (Hall, 2001).
The best-characterized aspect of acquired thermotolerance is the production of heat shock proteins (HSPs; Vierling, 1991). During acclimation, plants, like other organisms, induce massive transcription and translation of HSPs. These proteins are proposed to act as molecular chaperones to protect cellular proteins against irreversible heat-induced denaturation and to facilitate refolding of heat-damaged proteins (Boston et al., 1996). Genetic evidence has established that the Hsp100 family proteins are essential for the acquisition of thermotolerance in plants. Loss-of-function mutants of Hsp101 in Arabidopsis (Arabidopsis thaliana; hot1; Hong and Vierling, 2000, 2001) and maize (Zea mays; Nieto-Sotelo et al., 1999) are unable to acquire thermotolerance at several different growth stages.
Several lines of evidence indicate, however, that HSP synthesis is only one aspect of protection against heat-induced damage. Using an assay for acquired thermotolerance of hypocotyl elongation of 2.5-d dark-grown seedlings, we identified seven loci, designated hot1 through 7, with reduced acquired thermotolerance (Hong and Vierling, 2000; Hong et al., 2003; S.-W. Hong, U. Lee, and E. Vierling, unpublished data). The hot1 mutation in the HSP101 gene is the only one of these mutations that mapped to a locus encoding an HSP or heat shock transcription factor gene, and all of these mutants except hot3 accumulate HSPs normally. hot1 also shows a very severe heat-sensitive phenotype at all growth stages. In addition, although efforts have also been made to link HSP levels to cultivar differences in heat tolerance in a number of plant species, most of these studies have been inconclusive (for review, see Klueva et al., 2001). Thus, non-HSP genes are clearly essential for thermotolerance.
Genetic data also indicate that different genes contribute to heat tolerance at different stages of the plant life cycle and that different genes may be essential for basal and acquired thermotolerance. Several of the hot mutants are defective in acquired thermotolerance only when assayed as 2.5-d seedlings but not at later growth stages (Hong et al., 2003; S.-W. Hong, U. Lee, and E. Vierling, unpublished data). In contrast, NahG transgenic plants and npr1 mutants both acquire thermotolerance normally as 2.5-d seedlings but show decreased basal thermotolerance as 10-d seedlings (Clarke et al., 2004).
As described above, in our previous efforts to identify genes involved in acquired thermotolerance, we screened for mutants defective in acquired thermotolerance of hypocotyl elongation of 2.5-d dark-grown seedlings. Although the screen was not carried out to saturation (second alleles were found for three of seven loci), the small number of loci identified was surprising, given the diverse processes perturbed by heat. Therefore, to determine the contribution of additional genes to acquired and basal thermotolerance, in this study we examined the heat stress phenotypes of 45 existing Arabidopsis mutants and one transgenic line (NahG). As described in Table I, the mutants selected were mutants that disrupt processes that could be predicted, based on previous data, to play a role in responses to heat. These included mutants defective in signaling pathways (abscisic acid [ABA], salicylic acid, ethylene, and oxidative burst signaling) and in reactive oxygen metabolism (ascorbic acid or glutathione production, catalase) or mutants previously found to have temperature-related phenotypes (e.g. fatty acid desaturase mutants, uvh6). Although some of these mutants have been shown to be defective in heat tolerance, their responses to heat at different growth stages had not been systematically tested nor had their basal and acquired thermotolerance responses been compared. We tested each mutant for basal and acquired thermotolerance at five growth stages and assessed levels of thiobarbituric acid reactive substances and HSPs. The data indicate that, separate from HSP induction, ABA, active oxygen species, and salicylic acid pathways are involved in acquired thermotolerance and that the uvh6 gene plays a significant role in temperature responses in addition to its previously described role in UV light stress. The results further support the conclusions that thermotolerance is a complex multigenic process, with different gene sets involved in acquired and basal thermotolerance and in thermotolerance at different plant growth stages.
Table I.
Rationale for selection of mutants analyzed
| Cellular Process | Evidence for Involvement in Heat Responses | Previous Mutants Tested/Condition | Mutants Tested in This Study | References |
|---|---|---|---|---|
| ABA signaling/biosynthesis | Pretreatment with ABA increases basal thermotolerance | abi1, abi2, defective in basal thermotolerance, 10 d | aba1-3, aba2-1, aba3-1, abi1-1, abi2-1, abi3-1, and ade1-1 | Robertson et al. (1994); Gong et al. (1998); Larkindale and Knight (2002); Larkindale and Huang (2004) |
| SA signaling | Pretreatment with SA increases basal thermotolerance | NahG transgenic line (unable to accumulate SA) and npr1 defective in basal thermotolerance, 10 d | NahG and npr1-1 | Dat et al. (1998a, 1998b); Larkindale and Knight (2002); Clarke et al. (2004); Larkindale and Huang (2004) |
| Ethylene signaling | Pretreatment with ACCa (ethylene precursor) increases basal thermotolerance | etr1 defective in basal thermotolerance, 10 d | etr1-1 and ein2-1 | Larkindale and Huang (2004); Larkindale and Knight (2002) |
| AOS signaling | Pretreatment with H2O2 increases basal thermotolerance | None | atrbohA, atrbohB, atrbohC, atrbohD, atrbohE, atrbohF, atrbohG, and atrbohH | Dat et al. (1998b); Larkindale and Huang (2004) |
| Membrane fatty acid composition | Reducing trienoic fatty acid levels by chemical treatment or mutation results in increased stability of photosynthesis and increased tolerance to chronic heat stress | fad7/fad8 double mutant showed increased tolerance to chronic heat stress | fad2-1, fad2-2, fad3-1, fad4-1, fad5-1, fad6-1, fad7-1, fad 7-2, fad7-1/8-1, and fab2-1 | Horváth et al. (1998); Murakami et al. (2000); Alfonso et al. (2001); Sangwan et al. (2002) |
| Antioxidant pathway | Heat stress causes photooxidative damage and results in increased antioxidant production | None | npq1-2, cad2-1, vtc1-1, vtc2-1, and cat1/3 | Dat et al. (1998a, 1998b); Gong et al. (1998); Larkindale and Knight (2002) |
| Ubiquitin pathway | Ubiquitin-mediated cytoplasmic proteolysis can compensate for the absence of HSPs in yeast | ubp1, ubp2, and ubp1/2 were defective in prevention of damage due to protein aggregation | ubp1-1, ubp2-1, and ubp1-1/2-1 | Yan et al. (2000); Friant et al. (2003); Riezman (2004) |
| Programmed cell death | High temperature treatments induce programmed cell death in plants | None | acd2-2 | Swidzinski et al. (2002); Vacca et al. (2004) |
| UV-sensitive mutants | UV stress and heat stress might be expected to cause similar kinds of damage | uvh6 has previously been shown to be defective in chronic heat tolerance | uvh1-1, uvh3-1, and uvh6-1 | Jenkins et al. (1997) |
| Other | This or similar mutants have previously been shown to be sensitive to high temperatures | tsl4 and the auxin resistant mutant TU8 were shown to be sensitive to chronic heat stress | rns1, tsl4, and axr1-3 | Bariola et al. (1999); Ludwig-Muller et al. (2000); Berardini et al. (2001); C. Somerville, unpublished data |
ACC, 1-Aminocyclopropane-1-carboxylic acid.
RESULTS
Identification of Mutants with Altered Heat Tolerance as 7-d Seedlings
The rationale for choosing each of the 45 mutants used in this study is summarized in Table I. Further information about each mutant, including the nature of the gene product and the specific gene locus (when available), is provided in Table II (see also Supplemental Table I). In most cases, only a single allele at each locus was examined, although multiple mutants in the same process (e.g. ABA signaling) were studied. The number of the allele used is provided in Table I, but will otherwise not be used in the text. Most of the mutations are point mutations generated by ethyl methanesulfonate mutagenesis (Supplemental Table I), and therefore it should be kept in mind that they may not represent complete loss-of-function alleles.
Table II.
Mutant lines analyzed for heat stress phenotypes
Further information is provided in Supplemental Table I. ACC, 1-Aminocyclopropane-1-carboxylic acid; N/A, not applicable.
| Mutant Allele | Gene | Atg No. | Functional Category |
|---|---|---|---|
| aba1-3 | Zeaxanthin epoxidase | At5g67030 | ABA biosynthesis |
| aba2-1 | Short-chain dehydrogenase/reductase (SDR protein) involved in the conversion of xanthoxin to ABA-aldehyde | At1g52340 | ABA biosynthesis |
| aba3-1 | Molybdenum cofactor sulfurase | At1g16540 | ABA biosynthesis |
| ade1-1 | Unknown | Unknown | Enhanced ABA production |
| abi1-1 | Protein phosphatase 2C | At4g26080 | ABA insensitive |
| abi2-1 | Protein phosphatase 2C | At5g57050 | ABA insensitive |
| abi3-1 | ABA-dependent transcription factor | At3g24650 | ABA insensitive |
| NahG | Transgenic plant expressing the salicylate hydroxylase gene from Pseudomonas syringae | N/A | Cannot accumulate salicylic acid |
| npr1-1 | Similar to the transcription factor inhibitor IκB | At1g64280 | Does not acquire systemic resistance to pathogens |
| etr1-1 | Ethylene receptor | At1g66340 | Insensitive to ethylene |
| ein2-1 | NRAMP metal transporter family | At5g03280 | Insensitive to ethylene |
| eto1-1 | Negative regulator of ACC synthase 5 | At3g51770 | Higher ethylene levels |
| eto2-1 | ACC synthase 5 | At5g65800 | Higher ethylene levels |
| eto3-1 | ACC synthase 9 | At3g49700 | Higher ethylene levels |
| atrbohA | Respiratory burst oxidase homolog A | At5g07390 | Unknown |
| atrbohB | Respiratory burst oxidase homolog B | At1g09090 | Unknown |
| atrbohC | Respiratory burst oxidase homolog C | At5g51060 | Unknown |
| atrbohD | Respiratory burst oxidase homolog D | At5g47910 | Unknown |
| atrbohE | Respiratory burst oxidase homolog E | At1g19230 | Unknown |
| atrbohF | Respiratory burst oxidase homolog F | At1g64060 | Unknown |
| atrbohG | Respiratory burst oxidase homolog G | At4g25090 | Unknown |
| atrbohH | Respiratory burst oxidase homolog H | At5g60010 | Unknown |
| fad2-1 | Endoplasmic reticulum Δ12 desaturase | At3g12120 | Fatty acid synthesis |
| fad2-2 | Endoplasmic reticulum Δ12 desaturase | At3g12120 | Fatty acid synthesis |
| fad3-1 | Endoplasmic reticulum omega-3 fatty acid desaturase | At2g29980 | Fatty acid synthesis |
| fad4-1 | Predicted to encode the chloroplastic phosphatidylglycerol desaturase | Unknown | Fatty acid synthesis |
| fad5-1 | Chloroplastic fatty acid desaturase family, responsible for synthesis of 16:1 fatty acids | At3g15850 | Fatty acid synthesis |
| fad6-1 | Chloroplastic omega-6 fatty acid desaturase responsible for the synthesis of 16:2 and 18:2 fatty acids | At4g30950 | Fatty acid synthesis |
| fad7-1 | Chloroplastic omega-3 fatty acid desaturase | At3g11170 | Fatty acid synthesis |
| fad7-2 | Chloroplastic omega-3 fatty acid desaturase | At3g11170 | Fatty acid synthesis |
| fad7-1/fad8-1 | Double mutant in two chloroplastic omega-3 fatty acid desaturases | At3g11170 | Fatty acid synthesis |
| fab2-1 | Mutant in acyl-carrier-protein desaturase/stearoyl-ACP desaturase (SSI2) | At2g43710 | Fatty acid synthesis |
| vtc1-1 | GDP-Man pyrophosphorylase | At2g39770 | Vitamin C deficient |
| vtc2-1 | Expressed protein | At4g26850 | Vitamin C deficient |
| npq1-2 | Violaxanthin deepoxidase | At1g08550 | Altered nonphotochemical quenching |
| cad2-1 | γ-Glutamylcysteine synthetase | At4g23100 | Cadmium sensitive |
| cat1/3 | Catalase (both peroxisomal) | At1g20630/At1g20620 | Catalase deficient |
| acd2-2 | Expressed protein | At4g37000 | Spontaneous lesions in the absence of infection |
| ubp1-1 | Ubiquitin-specific protease 1 | At2g32780 | Ubiquitin specific protease 1 |
| ubp2-1 | Ubiquitin-specific protease 2 | At1g04860 | Ubiquitin specific protease 2 |
| axr1-3 | Similar to a ubiquitin-like activating enzyme | At1g05180 | Auxin resistant |
| uvh6-1 | DNA repair helicase, presumed transcription factor | At1g03190 | UV light sensitive |
| uvh3-1 | Excision repair nuclease | At3g28030 | UV light sensitive |
| uvh1-1 | Excision repair nuclease | At5g41150 | UV light sensitive |
| tsl4 | Unknown gene | Unknown | Grows at 17°C but not at 37°C |
| rns1 | S-like ribonuclease | At2g02990 | Induced by inorganic phosphate (Pi) starvation |
| hot1-1 | HSP101 | At1g74310 | Hsp100/ClpB family HSP |
All of the mutants were initially tested for their responses to a heat stress applied to 7-d seedlings grown on agar plates in the light. We chose this growth stage with an interest in evaluating heat tolerance of photosynthetically active, autotrophic seedlings. Furthermore, we had already performed a forward genetic screen for thermotolerance in 2.5-d-old dark-grown seedlings as described above and found that only a subset of the mutants identified showed a phenotype after growth in the light (Hong et al., 2003). Plants were tested either for basal thermotolerance by heating directly to 45°C for 60 min or for acquired thermotolerance by acclimation at 38°C for 90 min plus 2 h at room temperature, followed by the challenge heat treatment of 45°C for 180 min. As controls, each assay plate also included wild-type seedlings of the corresponding Arabidopsis accession (Supplemental Table I) and hot1 (Hsp101) mutant seedlings. Both heat treatments represent the limits of survival for wild-type plants and are lethal to the hot1 mutant. The hot1 mutant was chosen as the heat-sensitive control because it is the most heat sensitive of all the thermotolerance (hot) mutants that we have characterized at all growth stages (Hong et al., 2003). None of the mutants suffer any damage from the 38°C heat acclimation treatment alone (data not shown). Five days after heat treatment, the percentage of seedlings that survived (i.e. remained green and growing) was determined and compared to the number of surviving wild-type plants on the same plate.
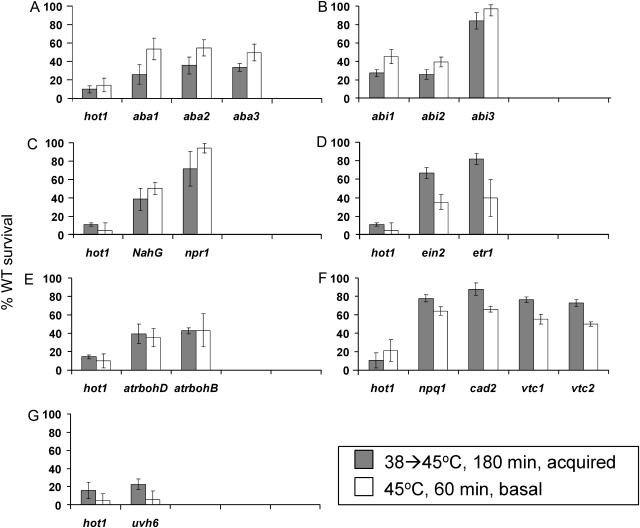
As shown in Figure 1, 15 of the 46 mutants/transgenic lines tested showed significant phenotypes in these thermotolerance assays. Mutants with some of the stronger phenotypes included plants with mutations in genes required for ABA biosynthesis (aba1, aba2, and aba3; Fig. 1A) and plants with mutations in genes involved in ABA signaling (abi1 and abi2; Fig. 1B). These mutants all showed reduced basal thermotolerance (approximately 40%–55% wild-type) and a more severe reduction in acquired thermotolerance (approximately 30%–40% wild-type). Of the ABA mutants we tested, only abi3 (Fig. 1B) and ade1 (data not shown) showed no significant reduction in thermotolerance as compared to wild-type plants. These results implicate ABA in thermotolerance responses.
Figure 1.
Thermotolerance phenotype of mutants showing heat sensitivity as 7-d-old seedlings. Seedlings grown on agar plates in the light for 7 d were heated to 38°C for 90 min, cooled to room temperature for 120 min, then heated to 45°C for 180 min (acquired thermotolerance, gray bars) or heated to 45°C for 60 min (basal thermotolerance, white bars). Percentage of survival of plants relative to the wild-type control on the same plate was determined 5 d after heat stress. A, ABA biosynthesis mutants; B, ABA-insensitive mutants; C, SA signaling transgenic line and mutant; D, ethylene signaling mutants; E, NADPH oxidase mutants; F, antioxidant mutants; G, uvh6. Each experiment was performed on a minimum of five separate plates, each with at least 20 plants of each line, including the wild-type and hot1 controls. Further controls were performed using unheated plants and plants given only the 38°C pretreatment, and all of these plants survived (data not shown). Data for the hot1 controls in each section of the figure were derived from the same plates as the mutants in that section; the exact percentage of survival varied from plate to plate due to minor differences in temperature within the incubator. B does not show the hot1 control, as the abi1 and abi2 mutants are in the Ler background, while hot1 is in Col. Mutants in C and D were tested on the same plate, so the hot1 controls are identical. Error bars represent the sd from the average value over all experiments. WT, Wild type.
Three other mutants link additional signaling pathways to thermotolerance. NahG transgenic plants, unable to accumulate salicylic acid (SA), and two respiratory burst oxidase mutants (atrbohB and atrbohD), proposed to be involved in active oxygen species signaling (Torres et al., 1998, 2002), showed similar reductions in acquired thermotolerance (approximately 40% wild-type) as well as statistically similar reductions in basal thermotolerance (Fig. 1, C and E). In contrast, npr1, which is defective further downstream in SA-induced defense responses, showed only limited heat sensitivity in either assay. Mutations in six other Atrboh genes (see Tables I and II) did not result in any significant thermotolerance defects (data not shown).
The final group of signaling mutants, those defective in ethylene signaling (etr1 and ein2), also showed reduced basal thermotolerance (40% wild-type) but only a very minor defect in acquired thermotolerance (approximately 70%–85% wild-type; Fig. 1D). The ethylene overaccumulating mutants eto1, 2, and 3 did not show any significant defects (data not shown).
Four mutants with defects in genes relating to antioxidant metabolism (vtc1, vtc2, npq1, and cad2) showed only moderate thermotolerance defects (Fig. 1F). These mutants showed a small (approximately 20%) decrease in acquired thermotolerance and a somewhat stronger decrease in basal thermotolerance, with vtc2 showing the strongest phenotype in both assays. The catalase defective mutant tested (cat1/3) showed no phenotype in either assay (data not shown).
Finally, the strongest defect detected in our screen was exhibited by the UV-sensitive mutant uvh6, which carries a defect in a DNA repair helicase and presumed transcription factor gene (Liu et al., 2003). As seen in Figure 1G, this mutant showed substantially reduced survival (<20% wild-type) in both basal and acquired thermotolerance assays, resembling the phenotype of the hot1 control. The other UV-sensitive mutants tested (uvh1 and uvh3) showed a wild-type phenotype under these assay conditions, although in more extreme heat treatments (pretreatment and 45°C, 220 min), uvh3 actually survived better than the wild type (data not shown).
All the other mutants tested, as listed in Tables I and II, showed no decreased thermotolerance. That is, their survival was >80% of the wild type under the assay conditions.
Mutants Defective in Thermotolerance also Show Oxidative Damage
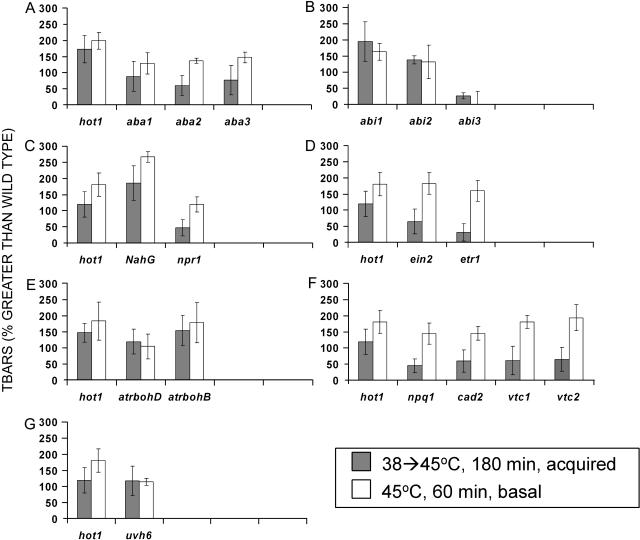
As an additional test of heat sensitivity, as well as a measure of oxidative damage, mutants found to have a phenotype as shown in Figure 1 were assayed for accumulation of thiobarbituric acid reactive substances (TBARS). High levels of TBARS correlate with high levels of oxidative damage to lipid membranes (Heath and Packer, 1968). The heat treatments for basal and acquired thermotolerance were performed on 7-d seedlings as shown in Figure 1, and tissue for the TBARs assay was harvested 2 d after the stress.
As shown in Figure 2, all of the mutants with significant phenotypes in the heat stress assays also had higher levels of TBARS than wild-type plants (50%–250% higher), indicative of increased oxidative damage. However, while in the survival assays it was clear that hot1 and uvh6 showed a stronger phenotype than the other mutants, in the TBARS assay, the NahG transgenic line and atrbohB and atrbohD also showed a similar degree of oxidative damage to hot1, under both basal and acquired thermotolerance treatments. It is possible that this represents the maximum level of TBARS that can be produced prior to death of the seedling. The abi1 and 2 mutants also had very high TBARS but cannot be directly compared to hot1 because of their different genetic backgrounds (Columbia versus Landsberg erecta; Supplemental Table I).
Figure 2.
Heat-induced oxidative damage in mutants with decreased thermotolerance. Plants were heat treated as described in Figure 1, and after 2 d of recovery, seedlings were harvested and stored in liquid nitrogen until the assay was performed. The TBARS level determined from the mutants relative to the wild-type control on each plate was determined. Values are graphed as percentage of greater than the wild type (WT; i.e. a mutant with 2 times the TBARS level seen in the wild type is recorded as a value of 100% greater than the wild type). A, ABA biosynthesis mutants; B, ABA-insensitive mutants; C, SA signaling mutant/transgenic line; D, ethylene signaling mutants; E, NADPH oxidase mutants; F, antioxidant mutants; G, uvh6. Experimental replication and controls were as described in Figure 1. Error bars represent the sd over all experiments.
We also observed that the TBARS levels for the ethylene (Fig. 2D) and oxidative stress mutants (Fig. 2F) were higher after basal thermotolerance tests than after acquired thermotolerance tests, as was expected from the survival levels seen in these mutants (survival was lower after basal treatments, Fig. 1, D and F). In contrast, this relationship does not hold for the aba1, 2, and 3 mutants (Fig. 2A). These mutants show no significant difference in their ability to survive acquired versus basal thermotolerance treatments but show slightly higher levels of oxidative damage in the basal thermotolerance tests. These data confirm that oxidative stress is a significant component of heat-induced damage for all of these mutants but that other processes also contribute to determine overall survival of heat stress.
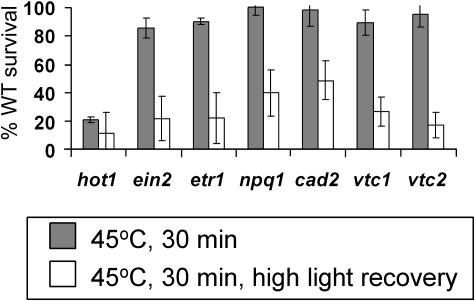
Enhancement of Heat Sensitivity by High Light Conditions
Oxidative stress is increased by high light (Niyogi, 1999), and the extent of heat damage has been observed to vary with light conditions (Larkindale and Knight, 2002). Therefore, we hypothesized that survival after heat stress might be further impaired by increasing the light intensity during recovery from heat stress, providing additional information both about differences between the responses of the mutants and about the relative contribution of oxidative damage to lethality. Increasing the light intensity during a 5-d recovery period did increase lethality of heated plants, even for wild-type plants, when testing for either acquired or basal thermotolerance (data not shown). Accordingly, to compare the mutants to the wild type, the 45°C heating period for the basal thermotolerance test was reduced to 30 min, conditions under which wild-type plants survived even when allowed to recover under high light conditions (data not shown). Like hot1, the mutants identified in Figures 1 and 2 as heat sensitive (aba1, aba2, aba3, abi1, abi1, NahG, atrbohB, atrbohD, and uvh6) all showed reduced survival compared to the wild type after this heat treatment when allowed to recover under moderate light, but essentially none of the these mutants survived when recovery was under high light (survival of mutants was 0%–20% wild-type survival, numerical data shown in Table III). Furthermore, the ethylene mutants (ein2 and etr1) and the antioxidant mutants (npq1, cad2, vtc1, and vtc2), which had only a moderate phenotype after recovery under normal light conditions (Fig. 1, D and F), showed a dramatic decrease in viability under high light as illustrated in Figure 3. Other mutants (eto mutants and acd2) that showed a small but not statistically significant defect in the initial survival assays did not show any increased defect under high light as compared to the wild type (data not shown). Thus, high light affects the ability of specific mutants to recover from heat stress, presumably because of increased oxidative stress.
Table III.
Summary of thermotolerance phenotype of mutants at different developmental stages
Wild-type and mutant plants were assayed for thermotolerance at five different developmental stages. Data shown here are all from acquired thermotolerance assays, except in the germination and high light assays, where plants were heated directly to 45°C as indicated. Plant age corresponds to the number of days of growth prior to heat treatment. The more severe mutant phenotypes are shown in boldface, and moderate phenotypes are in italics. Each experiment was repeated at least three times (at least 30 plants assayed in total), except the 25-d assay, where only five plants were assayed in each experiment. Error represents sds. Following are mutants with wild-type phenotype in hypocotyl assays: ade1, abi3, eto1, eto2, eto3, atrbohA, atrbohC, atrbohE, atrbohF, atrbohG, atrbohH, fad2, fad3, fad4, fad5, fad6, fad7, fad7/8, fab3, acd2, ubp1, ubp2, ubp1/ubp2, cat1/3, rns1, tsl4, axr1, and uvh1. WT, Wild-type.
| Mutant | Germination 45°C, 220 min (% Germinated) | 2.5-d Hypocotyl Elongation 38°C, 90 min, 45°C, 180 min (% Unheated Growth) | 4-d Root Growth 38°C, 90 min, 45°C, 180 min (% Unheated Growth) | 7-d Survival 38°C, 90 min, 45°C, 180 min (% WT Survival) | 7-d Survival, High Light 45°C, 30 min (% WT Survival) | TBARS 7 d 38°C 90 min, 45°C 180 min (% WT TBARS) | 25-d Survival 38°C, 90 min, 45°C, 180 min (Description) |
|---|---|---|---|---|---|---|---|
| Col | 93.4 ± 7.6 | 22.7 ± 2.5 | 42.7 ± 7 | 100 | 100 | 100 | Green |
| hot1 | 9.1 ± 7.5 | 0 ± 0 | 14.3 ± 1 | 13.3 ± 5 | 8.3 ± 10 | 270 ± 38 | Bleached |
| aba1 | 87.4 ± 9.1 | 24 ± 3.8 | 17.5 ± 3 | 25.4 ± 4 | 16.7 ± 12 | 207 ± 26 | Bleached |
| aba2 | 92.4 ± 8.3 | 25 ± 4.8 | 18.3 ± 5 | 32.1 ± 3 | 16.1 ± 12 | 218 ± 59 | Bleached |
| aba3 | 82.7 ± 7.3 | 26 ± 4.3 | 16.0 ± 4 | 31.7 ± 2 | 17.9 ± 11 | 216 ± 72 | Bleached |
| abi1 | 79.7 ± 16.3 | 20.3 ± 3.3 | 11.0 ± 4 | 23.1 ± 2 | 15.4 ± 11 | 294 ± 60 | Bleached |
| abi2 | 80.0 ± 16.1 | 23.8 ± 3.6 | 14.9 ± 5 | 22.3 ± 12 | 14.2 ± 10 | 238 ± 13 | Bleached |
| NahG | 85.7 ± 21.4 | 17.3 ± 3.7 | 23.4 ± 4 | 40.0 ± 14 | 16.5 ± 19 | 285 ± 54 | Bleached |
| npr1 | 87.3 ± 21.4 | 20 ± 4.4 | 39.6 ± 2 | 79.4 ± 12 | 78.9 ± 8 | 147 ± 25 | Bleached patches |
| ein2 | 71.0 ± 7.9 | 19.3 ± 2.9 | 28.9 ± 4 | 69.2 ± 15 | 21.8 ± 16 | 165 ± 37 | Bleached patches |
| etr1 | 72.3 ± 16.4 | 22.9 ± 2.4 | 29.3 ± 3 | 74.3 ± 9 | 22.1 ± 17 | 141 ± 26 | Bleached patches |
| atrbohB | 93.4 ± 7.8 | 18.0 ± 2.9 | 22.1 ± 2 | 49.7 ± 8 | 12.1 ± 12 | 254 ± 46 | Bleached |
| atrbohD | 76.8 ± 7.5 | 19.0 ± 2.4 | 23.3 ± 3 | 48.5 ± 10 | 9.7 ± 13 | 219 ± 39 | Bleached |
| npq1 | 89.1 ± 7.4 | 24.5 ± 2.8 | 29.9 ± 3 | 72.9 ± 4 | 34.6 ± 17 | 145 ± 37 | Bleached |
| cad2 | 86.8 ± 12.5 | 25.5 ± 2.4 | 35.4 ± 4 | 77.9 ± 7 | 53.5 ± 17 | 159 ± 21 | Bleached patches |
| vtc1 | 99.8 ± 16.5 | 20.0 ± 2.8 | 30.7 ± 1 | 76.7 ± 3 | 16 ± 14 | 159 ± 19 | Bleached |
| vtc2 | 95.4 ± 8.9 | 21.9 ± 2.5 | 26.9 ± 2 | 72.8 ± 4 | 12.1 ± 10 | 164 ± 43 | Bleached |
| uvh6 | 11.4 ± 11.7 | 0 ± 0 | 18.6 ± 2 | 17.4 ± 9 | 13.3 ± 11 | 216 ± 14 | Bleached |
| uvh3 | 5.2 ± 8.6 | 5.4 ± 2.8 | 47.3 ± 5.3 | 110 ± 10 | 112 ± 15 | 98.5 ± 35 | Green |
Figure 3.
High light intensity during recovery increases heat sensitivity. Seven-day-old plants were heated on plates to 45°C for 30 min without pretreatment and allowed to recover under normal or high light conditions (100 or 250 μmol m−2 s−1) for 5 d. Averages of the survival of plants relative to the wild type (WT) control on each plate are shown. Repetition and controls were as described in Figure 1, with unheated samples being allowed to recover under either high or low light conditions. Survival of unheated control plants under both high and low light conditions was 100% (data not shown). Error bars represent the sd over all experiments.
Parallel assays using high light during recovery were performed after the acquired thermotolerance heat treatment (38°C for 90 min, 2 h room temperature, 45°C for 180 min). In these assays, survival of the mutants shown to have a phenotype in Figure 1 (aba1, aba2, aba3, abi1, abi2, NahG, atrbohB, atrbohD, and uvh6) was <20%, while survival of wild-type plants was 75% ± 20%. Increased light levels do not, therefore, affect the defect in acquired thermotolerance in these plants.
The experiments above were all done with both the 38°C pretreatment and the 45°C heat treatment occurring in the dark. The same assays were done with the aba1, aba2, aba3, abi1, abi2, NahG, atrbohB, atrbohD, uvh6, and hot1 mutants using heat treatments performed under normal growth light conditions. No significant differences in viability compared to results with treatments in the dark were found (data not shown). We did not test the effects of high light during heat treatment.
Thermotolerance at Different Growth Stages
While the mutants depicted in Figures 1 and 2 were defective in thermotolerance when assayed as 7-d plants, previous work has shown that thermotolerance phenotypes can vary at different stages of growth (Hong and Vierling, 2000; Hong et al., 2003; Clarke et al., 2004). Therefore, we next tested all of the mutants listed in Tables I and II for acquired thermotolerance of hypocotyl elongation after 2.5 d of growth in the dark, the conditions used to identify the hot1 mutant. Only two of the mutants, uvh6 and uvh3, were found to be defective in acquired thermotolerance at this growth stage (Table III), revealing a significant difference in response of young dark-grown and light-grown seedlings.
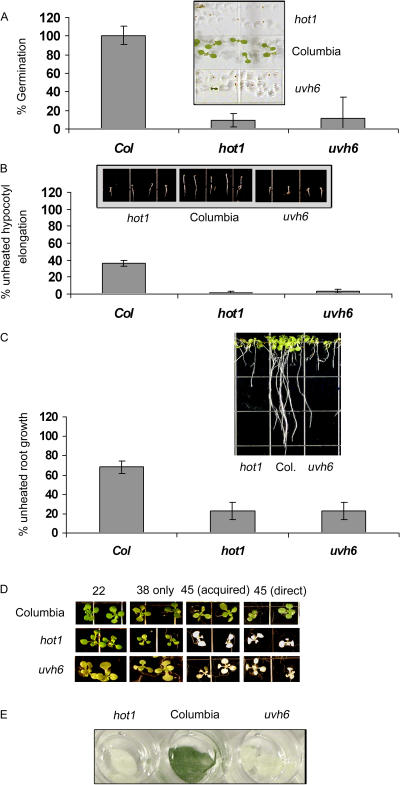
We then extended our assays to consider other growth stages, testing only the mutants found to have a phenotype in the 7-d-old seedling assay, along with uvh3 because of its heat-sensitive hypocotyl elongation phenotype. The assays used tested basal thermotolerance during germination, acquired thermotolerance of root growth of 4-d-old plants, or chlorophyll retention (extent of bleaching) in leaves from 25-d plants. Averages for the results from these assays, along with the hypocotyl and 7-d seedlings assays already discussed, are presented for all of these mutants in Table III. Because of its strong phenotypes, pictures from representative experiments along with the quantitative data for uvh6 compared to hot1 are also presented in Figure 4. It is important to note that a line of the uvh6 mutant complemented by transformation with the wild-type Uvh6 gene (Liu et al., 2003) showed wild-type responses to heat (data not shown).
Figure 4.
Heat stress phenotypes of uvh6 plants at different life stages. A, Germination of seeds after heating to 45°C for 220 min and scoring 1 week after heat treatment. B, Hypocotyl elongation of 2.5-d-old seedlings and 2.5 d recovery following a 38°C pretreatment, 120 min at room temperature, and 180 min heat stress at 45°C. C, Root elongation 5 d after heat stress for plants heated to 45°C for 180 min after pretreatment; plants were heated 4 d after germination. D, Photograph of 7-d plants heated and allowed to recover for 5 d. Plants were unheated (22), heated to 38°C for 90 min only (38), heated to 45°C for 180 min after pretreatment at 38°C (38–45), or heated directly to 45°C for 60 min (45). E, Photograph of leaves removed from 25-d plants and heated to 45°C for 180 min in a water bath and left to recover for 2 d. Error bars in A, B, and C represent the sd over five replicate experiments, each containing at least 10 plants of each line.
Although only hot1, uvh3, and uvh6 showed a phenotype in the germination or hypocotyl elongation assays, after 4 d of growth and exposure to light, all of the mutants except uvh3 showed defects in heat tolerance. The relative degree of heat sensitivity for each mutant appeared to be consistent across the assays performed in older plants, with npr1 showing the least severe phenotype and hot1, uvh6, and the abi mutants showing the strongest phenotypes (Table III; Fig. 4).
HSP Accumulation Is Unaltered in Mutants Defective in Thermotolerance
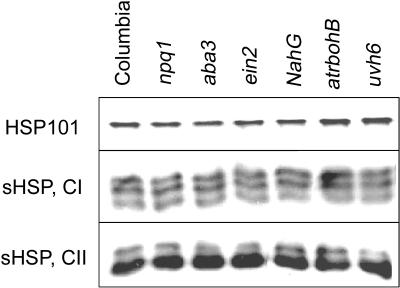
Since the expression of HSPs is known to contribute to thermotolerance, it was of interest to determine if any of the mutants with thermotolerance phenotypes as 7-d seedlings were defective in HSP accumulation. To assess HSP levels, protein was extracted from 7-d seedlings that had been heat treated at 38°C for 90 min followed by 30 min at 22°C, conditions similar to the acclimation treatment used to induce acquired thermotolerance. Levels of Hsp101 and class I and class II small heat shock proteins (sHsps) were then examined by western blotting. Results for a subset of the mutants (one from each functional group: npq1, aba3, ein2, NahG, atrbohB, and uvh6) are shown in Figure 5. Equivalent results were obtained from the other mutants tested (aba1, aba2, abi1, abi2, npr1, etr1, atrbohD, cad2, vtc1, and vtc2; data not shown). All of the mutants showed normal levels of these HSPs compared to wild-type plants at this time point. HSPs also accumulated to wild-type levels in 2.5-d dark-grown uvh6 seedlings subjected to the same heat stress (data not shown). Therefore, the observed heat sensitivities of these mutants do not correlate with defects in HSP accumulation during the acquisition of thermotolerance, consistent with the conclusion that these mutants are defective in other processes involved in the acquisition of thermotolerance.
Figure 5.
HSP accumulation in mutant plants. Seedlings were treated at 38°C for 90 min and cooled to room temperature for 30 min prior to harvesting tissue for protein extraction. HSP levels were determined by SDS-PAGE followed by western blotting with antibodies against Hsp101 and class I and class II sHsps.
Basal versus Acquired Thermotolerance
Evidence from Figures 1 and 2 suggested that the ethylene signaling mutants and the antioxidant mutants were more impaired in basal thermotolerance than in acquired thermotolerance. This observation indicates that different pathways could be involved in basal and acquired thermotolerance. It might therefore be possible to elucidate the relative importance of different signaling pathways in basal versus acquired thermotolerance by looking at the relative severity of the mutant phenotypes under different heat stress conditions. That is, a mutant that showed a phenotype greater than the average phenotype in the acquired assay but merely average in the basal assay might be expected to play some role in events specific to acquired thermotolerance. A mutant that showed a less defective phenotype in the acquired than in the basal thermotolerance assays would be anticipated to be involved in processes made redundant during acquired thermotolerance. Such processes, although critical to basal thermotolerance, are not necessary for survival after acclimation.
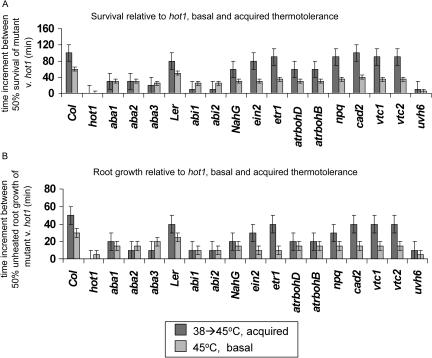
The mutants were therefore tested in the 4-d root growth and 7-d survival assays for both basal and acquired thermotolerance, with 45°C heating periods from 30 to 120 min without pretreatment (basal) or 90 to 250 min with pretreatment (acquired). Since longer heating periods impose greater stress on the plant, the most heat-sensitive mutants would be expected to show a phenotype after the shortest period of heating. Figure 6 compares the length of heat treatment required to reduce survival or root growth to 50% of unheated samples in comparison to the hot1 mutant. Results for both basal and acquired thermotolerance of seedling survival (Fig. 6A) or of root growth (Fig. 6B) are graphed for each mutant. Note that abi1 and abi2 are in the Landsberg erecta background (Ler), while the other mutants are in a Columbia background (Col), so these cannot be directly compared.
Figure 6.
Comparison of basal and acquired thermotolerance in selected mutants. The survival of 7-d plants (A) or root growth of 4-d plants (B) of each mutant was determined after progressively longer heat treatments. For basal thermotolerance, heat treatments were from 30 to 120 min at 45°C at 5-min intervals. For acquired thermotolerance, heat treatments were from 90 to 250 min at 10-min intervals following pretreatment. In each case, the time of heat treatment required to reduce survival or root growth to 50% of the unheated controls was determined. The data are presented as the additional time at 45°C required for 50% survival of that mutant as compared to hot1 (the most severe mutant). Error was ±10 min for acquired thermotolerance and ±5 min for basal thermotolerance.
As expected, in both the root growth and survival assays, hot1 and uvh6 showed the strongest phenotypes of all of the mutants. Interestingly, all the other mutants performed similarly in the basal thermotolerance tests, requiring approximately the same additional time of heating compared to hot1 to show 50% reduction in survival (approximately 30 min) or root growth (approximately 15 min). By contrast, in the acquired thermotolerance tests, aba1, aba2, and aba3 performed essentially as poorly as hot1 and uvh6, as did abi1 and abi2, although it should be noted that the Ler control was also more heat sensitive than Col. The phenotype of the NahG transgenic line and the atrbohD and atrbohB mutants were intermediate, and ein2, etr1, cad2, vtc, vtc2, and npq1 were only slightly worse than the wild type in acquiring thermotolerance. These data, which are consistent with the results in Figures 1 and 2, suggest that the ABA signaling mutants are most likely to be involved in processes specifically required for acquired thermotolerance and that the NahG transgenic line and the atrbohD and atrbohB mutants play some role in acquired thermotolerance but that the ethylene and antioxidant mutants are more likely to be critical in basal heat tolerance, with less of a role in processes required for acquired thermotolerance.
DISCUSSION
We have evaluated the importance of different processes and pathways to heat stress tolerance by testing 45 Arabidopsis mutants and one transgenic line for sensitivity to heat (basal thermotolerance) and ability to acquire thermotolerance at different stages of growth. Mutants were chosen on the basis of previous data implicating the mutant gene in responses to high temperature (Table I) and included mutants in signaling pathways, mutants with defects in the production of antioxidants, and other mutants with defects in genes previously associated with heat stress or heat-induced pathways (Larkindale et al., 2005). Of the 46 plants tested, the most significant phenotypes in both basal and acquired thermotolerance were seen in several ABA biosynthesis or signaling mutants, in plants unable to accumulate SA, in two mutants of respiratory burst oxidase homologs, and in the uvh6 mutant, which is defective in a DNA helicase. All of these mutants accumulated HSP101 and class I and II sHsps normally, suggesting that the processes affected by the mutant genes are independent of the heat shock response. This indicates that there are multiple processes involved in the acquisition of thermotolerance, of which HSP synthesis is only one critical aspect.
Although some of these mutant plants are small (e.g. vtc1) or show other unrelated phenotypes (uvh6 is yellow-green), we argue that the heat phenotypes seen in this study are not due to inherent weaknesses in the mutants. We base this assumption on the fact that some of the mutants tested that showed no phenotype in any assay were also small (e.g. axr1) and that the mutants that did show a phenotype showed it only under specific conditions, i.e. at specific stages of development, more so during basal than acquired thermotolerance, or only after specific heat treatments. Therefore, we conclude that the selected mutants show genuine heat-defective phenotypes.
Results of this study demonstrate that there are many protective pathways that contribute to survival of plants at high temperatures and suggest that the relative importance of the different pathways changes throughout plant development. This conclusion is supported by the observation that mutants showed differential heat sensitivity depending on the stage of growth at which they were assayed. Strikingly, of the mutants tested, only uvh6 and uvh3 (and hot1 as described previously) showed heat sensitivity during seed germination or when tested as 2.5-d dark-grown seedlings. The other mutants were sensitive only after 4 or more d of growth in the light, growth stages at which uvh6 and hot1 (but not uvh3) were also sensitive. Our results also agree with those of Clarke et al. (2004), in which they demonstrated that NahG and npr1 were defective in basal thermotolerance in 10-d-old plants, but neither exhibited a defect in acquired thermotolerance of 2.5-d seedlings. In contrast to these results, four of seven different hot mutants, which were isolated as mutants defective in acquired thermotolerance as 2.5-d seedlings, acquire thermotolerance normally as older plants (Hong et al., 2003; S.-W. Hong, U. Lee, and E. Vierling, unpublished data). The absence of a 2.5-d phenotype in most of the mutants tested here explains why these mutations were not isolated in this hot mutant screen (Hong and Vierling, 2000) and that none of the unpublished hot mutants have been mapped to loci close to these genes (Hong et al., 2003; S.-W. Hong, U. Lee, and E. Vierling, unpublished data). In total, the data support the idea that plants suffer different types of damage at different growth stages (e.g. prevention/repair of oxidative damage appears more critical in light-grown plants compared to young dark-grown plants), which then require a different balance of protective mechanisms, and/or that different protective mechanisms vary in their redundancy during growth.
ABA signaling appears to be more critical for acquired thermotolerance than for basal thermotolerance (while playing a part in both), while the converse is true for ethylene signaling and antioxidant protection. The ABA mutants aba1, aba2, aba3, abi1, and abi2 were unique in showing significantly stronger phenotypes in the acquired thermotolerance assays than in the basal thermotolerance assays. In contrast, the ethylene and antioxidant pathway mutants all showed significantly stronger basal than acquired thermotolerance phenotypes, especially under high light conditions, suggesting that control of oxidative damage is more critical in nonacclimated plants. Together, these data support the hypothesis that heat tolerance involves multiple processes, some of which are primarily of importance during basal thermotolerance (ethylene and antioxidant pathways), some of which are more critical during the acquisition of thermotolerance (ABA pathways), and a number of which are required for survival of any heat treatment (involving an oxidative burst, SA, UVH6, and HSPs). These components may function in multiple pathways acting together to allow plant survival at high temperatures, and the exact balance of components needed for survival depends both on the plant growth stage and on the duration and severity of the heat stress.
The abi3 mutant was unique among the ABA signaling mutants in that it did not appear to be involved in the acquisition of thermotolerance at any growth stage. This transcription factor has previously been associated with developmental control of HSP accumulation in seeds (Rojas et al., 1999; Wehmeyer and Vierling, 2000). The lack of a phenotype in the abi3 plants further supports the model that ABI3 is involved in seed-specific expression but not in heat-dependent expression of HSPs.
Given that both ABA biosynthesis mutants (aba mutants) and ABA-insensitive mutants (abi1 and 2) were found to be sensitive to heat stress, it can be hypothesized that ABA is likely to accumulate during heat stress, triggering downstream responses. In Arabidopsis, pretreatment of plants with exogenous ABA has been shown to improve basal thermotolerance (Larkindale and Knight, 2002), and Ristic and Cass (1992) have shown that a high ABA-accumulating line of maize has increased tolerance to chronic heat and drought. Therefore, it was somewhat surprising that ade1, a mutant known to overaccumulate ABA, had the same heat resistance as the wild type and furthermore did not survive any better than heat-stressed wild-type plants (data not shown).
ABA appears to play a part in some pathway distinct to the induction of HSPs that is nonetheless essential to the acquisition of thermotolerance in light-grown plants. At present, little is known about what a heat-stress ABA pathway might involve, although it is reasonable to hypothesize that similar genes might be induced by ABA during heat stress as during drought stress. Large numbers of stress- and ABA-regulated genes have been implicated in drought tolerance (Zhu, 2002), and specific transcripts have been shown to be induced by both heat and drought stress (Rizhsky et al., 2004). In our study, plants were heated on sealed agar plates, so the plants did not suffer from dehydration in conjunction with heat stress. In confirmation of this assumption, we have found that the genes identified as drought- but not heat-inducible by Rizhsky et al. (2004) are not up-regulated during our heating regimes (data not shown). The combination of heat and drought stress, however, is common in nature, so coordination of heat and drought responsive pathways may be beneficial to the plant.
The NahG transgenic line and atrbohB and atrbohD mutants all showed a significant phenotype both after a direct heat treatment and after acquisition of thermotolerance. It is difficult to determine to what extent these plants are defective in processes specifically involved in acquired thermotolerance, since their defect in basal thermotolerance may also affect plants after acquisition of thermotolerance. Heat pretreatment does not compensate for the effects of the mutations, however, as the plants do not survive as well as wild-type plants after acclimation. These results suggest that signaling pathways involving SA and active oxygen species (AOS) are critical for events during both basal and acquired thermotolerance. The involvement of SA and AOS signals in the acquisition of thermotolerance requires further investigation, as end points for these signaling pathways during heat stress have not been determined. SA and AOS signaling pathways during biotic stresses have been well established (Sandermann, 2000; Van Breusegem et al., 2001; Thatcher et al., 2005), but no correlation between these pathways and heat stress has been established.
In contrast to the other mutants, the ethylene and the antioxidant pathway mutants showed almost wild-type acquired thermotolerance but a severe defect in basal thermotolerance (Figs. 1, 2, and 6). Thus, these mutants are defective in some pathway that is essential to basal thermotolerance but that is made redundant by processes induced during the acquisition of thermotolerance. The affect of heat on the ein2 and etr1 mutants and on the mutants compromised in antioxidant defenses was dramatically enhanced by increasing the light intensity during recovery from heat stress (Fig. 3), and these mutants show no phenotype in assays performed during early seedling growth in the dark. Photooxidative damage has been shown to be a critical aspect of 10-d-old seedling survival during basal thermotolerance treatments (Larkindale and Knight, 2002), so the increased lethality seen in these mutants may be due to increased levels of heat-induced oxidative stress. This hypothesis is supported by the uniformly high TBARS measurements in all plants sensitive to heat stress and the relatively high TBARS levels for the ethylene and antioxidant mutants even under conditions where only a small decrease in survival was observed (Fig. 2). As ethylene has been associated with oxidative stress responses (Kato et al., 2000; Argandona et al., 2001; Bortier et al., 2001; Moeder et al., 2002; Nie et al., 2002; Manning et al., 2003), ethylene might act as a signal to activate oxidative defenses during heat stress. Taken together, these observations indicate that oxidative damage is a major component in heat damage under basal thermotolerance conditions. In contrast, such damage is less critical during acquired thermotolerance either because there are fewer free radicals produced or because free radicals are dealt with by other systems.
Of the mutants in this study, only uvh6 showed a strong defect in both basal thermotolerance and acquired thermotolerance at all stages of its life cycle from germination through to 25 d, similar to the Hsp101 mutant hot1. Therefore, this DNA helicase appears to control some function essential to both basal and acquired heat tolerance that is neither age nor light dependent. The ability of uvh6 to accumulate Hsp101 and sHsps indicates that its essential function is distinct from the induction of HSPs or the function of Hsp101. This DNA helicase is believed to have two cellular functions based on the known functions of human and yeast (Saccharomyces cerevisiae) homologs. These homologs act both as a DNA helicase involved in DNA damage repair by the nuclear excision pathway and as a core component of the TFIIH transcription factor complex (Svejstrup et al., 1996). We hypothesize that the latter function is more essential for thermotolerance in plants based on two lines of reasoning. First, the uvh6-1 allele used in this study shows only a moderate defect in DNA repair (Liu et al., 2003), and yet its affect on thermotolerance is severe. Second, recent data have shown that cells carrying certain mutant alleles of the human homolog of Uvh6, XPD, have defective transcriptional responses to specific hormone treatments involving only a subset of transcriptional activators (Dubaele et al., 2003; Drané et al., 2004). The current model explaining this observation is that XPD regulates the phosphorylation activity of the TFIIH-associated cyclin-dependent kinase-activating kinase complex, directing it to phosphorylate and activate specific transcription factors, thus inducing transcription of specific target genes. Hence, we suggest that the uvh6 phenotype arises from failure to induce genes distinct from HSPs that are required for heat stress tolerance.
We also tested 30 mutants that showed no heat sensitivity under any of the conditions used, including mutants that had previously been documented as sensitive to high temperatures, e.g. tsl4 and some of the fad mutants (Hugly et al., 1991; James and Dooner, 1991; C. Somerville, unpublished data). The contrast between our results and those previously published is most likely due to differences in the assays used. Heat sensitivity in these mutants had been identified through chronic, moderate heat stresses as opposed to their response to severe heat stress or their ability to acclimate to high temperatures. Different processes again might be expected to be involved in long-term heat acclimation than are involved in either basal or acquired thermotolerance.
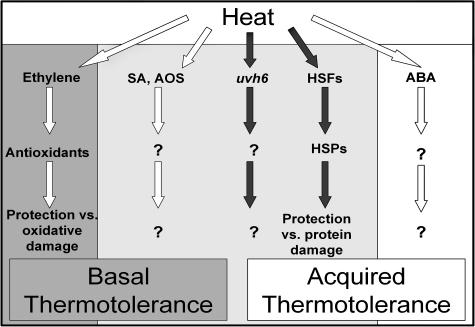
Based on our data, we can now diagram a framework for understanding how different signaling pathways contribute to both basal and acquired thermotolerance as shown in Figure 7. Depending on the stress applied and the age of the plant, it appears that different aspects of heat-induced damage impact plant survival, and different types of heat-induced damage are prevented or repaired through different cellular systems. The signaling molecules AOS, SA, and ABA and the transcription component uvh6 potentially activate or modulate other pathways involved in the plant response to heat that are different from the HSP pathway. Because they affect both basal and acquired thermotolerance, they must have effects distinct from the antioxidant pathway. What kind of damage is being prevented or repaired by such pathways has yet to be determined. The SA transgenic line and the AOS mutants showed similar phenotypes and affected basal and acquired thermotolerance equally; therefore, in Figure 7, they are depicted as potentially part of the same pathway. ABA is depicted as part of a separate signaling pathway to the other components, although some aspects of ABA signaling may be common to a pathway involving SA and AOS. The effect of uvh6 is different from the effects of the other mutants in that the gene is essential for thermotolerance in plants of all ages. Although UVH6 function may impact other signaling pathways, it is clear that at least some aspect of UVH6 function is unique and essential for survival in plants prior to exposure to light.
Figure 7.
Framework for heat stress responses and thermotolerance in Arabidopsis. The different signaling components and protective factors postulated to be involved in thermotolerance are shown grouped into pathways where there is evidence that there are interactions between a specific signaling component and a particular end response. Question marks indicate some of the places in the schema where components remain unidentified. The areas highlighted in dark gray represent pathways shown to be critical for basal thermotolerance, those in white are critical for acquired thermotolerance, and areas shaded in light gray are critical for both. The pathways indicated by black arrows are critical for basal and acquired thermotolerance at all stages in development; white arrows indicate pathways critical only in 4-d plants and older after exposure to light. HSFs, heat shock transcription factors.
In Figure 7, five separate pathways are therefore illustrated, which, acting together, produce the thermotolerant phenotypes in light-grown plants. Only some of these pathways are critical in 2.5-d dark-grown plants. Heat is a complex stress causing damage to a range of cellular components, so it should not be surprising that a large number of different protective pathways are required in order to survive. Induction of any one of these pathways allows the plant to acquire some measure of thermotolerance, and the loss of any specific pathway merely limits the extent of that tolerance.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants of the different mutant lines and their associated wild-type accessions were used as shown in Table I, and the seed source/donor is shown in Supplemental Table I. Mutants were obtained in the homozygous state from the stock center or individual donor and were propagated directly to obtain sufficient material for experimentation (in some cases through three generations). The homozygosity of all mutants with easily scorable phenotypes, e.g. ABA insensitivity or presence of a transgene, was also directly verified. Seeds were surface sterilized, plated on nutrient medium (Haughn and Somerville, 1986) containing 0.5% (w/v) Suc, and kept at 4°C for a minimum of 3 d. Plants were then grown in lighted growth chambers (approximately 100 μmol m−2 s−1) on a 22°C/18°C, 16-h day/night cycle. For high light recovery treatments, plants were placed in the same cabinets at a light intensity of 250 μmol m−2 s−1 maintained at the same temperature. For the 25-d assay, plants were grown on plates for approximately 10 d, then transferred to soil, and grown for a further 15 d under the same light conditions in the growth chamber.
Heat Stress Treatments
Thermotolerance assays of seeds, 2.5-d dark-grown, 4-d light-grown, and 7-d light-grown seedlings were performed according to Hong and Vierling (2000). Because these assays were carried out on sealed minimal nutrient plates containing an equal volume of agar medium poured on a leveling table, it is assumed that plant temperatures closely match the incubator temperatures. Basal thermotolerance treatments consisted of heating plants to 45°C, while acquired thermotolerance tests were done by heating the plants initially to 38°C for 90 min, then leaving the plants at room temperature for 120 min, before finally heating to 45°C for 2 to 3 h. All heat treatments were performed in the dark. Recovery was in a growth chamber at 22°C for 5 d in the light. For the seed thermotolerance test, seeds were heat treated at 45°C for 3 to 4 h immediately after removal from the cold and then allowed to grow an additional 3 d before measurement. For hypocotyl elongation, seedlings were grown for 2.5 d in the dark and heat stressed, and then growth was measured after an additional 2.5 d in the dark. Growth after the heat treatment was measured and compared with seedlings of the same mutant line receiving no heat treatment. The 4-d root growth assay was done by growing the plants vertically on plates for 4 d in the light, then heat treating the plants and returning them to the growth incubator to continue to grow vertically for 5 d in the light. Only growth after heat treatment was measured and compared to growth of the same mutant plant given no heat treatment. The assays with 7-d seedlings were done on large plates (150 mm) that were heated directly in the incubator, and seedlings were allowed to recover for 5 d with a 16-h light/dark cycle prior to calculating the percentage survival. Plants that were still green and producing new leaves were scored as surviving. The 25-d assay was done by placing leaves from the newest whorl of leaves from a 25-d-old plant into a 12-well plate, with 2 mL of water in each well. The plate was then floated on the surface of water baths at either 38°C or 45°C as appropriate. In all cases, the results for each plant line were compared to those of the appropriate wild-type ecotype on the same plate, and the heat-sensitive positive control hot1 was included on each plate.
TBARS Assay
Analysis of TBARS was performed according to Heath and Packer (1968). Plants grown on plates were given the described heat treatments and left to recover under normal light conditions for 2 d. The tissue (0.25 g per sample) was then harvested and ground in a total of 0.5 mL of buffer (0.25 mL 0.5% [w/v] thiobarbituric acid in 20% [v/v] trichloroacetic acid and 0.25 mL 175 mm NaCl in 50 mm Tris, pH 8) and heated, and absorbance readings were taken at 532 and 600 nm. The levels of TBARS were determined by comparison to a malonaldehyde standard curve and each sample compared to that of the wild-type control from the same plate. Results were averaged over at least five separate experiments.
Western Analysis
Seedlings were treated at 38°C for 90 min and cooled to room temperature for 30 min prior to harvesting the tissue for protein extraction. Total protein from seedlings was extracted in SDS sample buffer (60 mm Tris-HCl [pH 8.0], 60 mm dithiothreitol, 2.0% [w/v] SDS, 15% [w/v] Suc, 5 mm ɛ-amino-N-caproic acid, and 1.0 mm benzamidine). Protein concentration was determined using a Coomassie Brilliant Blue dye-binding assay (Ghosh et al., 1988) with bovine serum albumin as a standard. Standard methods were used for SDS-PAGE separation of protein samples on 7.5% or 15% (w/v) polyacrylamide gels. For western analysis, proteins were blotted to nitrocellulose and processed for detection using chemiluminescence (Amersham, Piscataway, NJ) as described previously (Wehmeyer et al., 1996). Anti-Hsp101 antiserum was used at a dilution of 1:1,000 (v/v). Antiserum against class I sHsps (Wehmeyer et al., 1996) was used at a dilution of 1:1,000 (v/v). Antiserum against class II sHsps was produced against Arabidopsis Hsp17.6II (X63443; N. Buan and E. Vierling, unpublished data) and used at a dilution of 1:1,000.
Supplementary Material
Acknowledgments
We thank the many individuals who provided seeds of mutants not available in the stock center, Dr. Suk-Whan Hong (Department of Applied Plant Sciences, Chonnam National University, Korea) for initially identifying the thermotolerance phenotype of uvh6, and Dr. Nicola Evans (Department of Plant Sciences, University of Oxford) for genotyping the Atrboh mutants.
This work was supported by the National Science Foundation (grant no. IBN–0213128) and by the U.S. Department of Agriculture (grant no. NRICGP 99–351007618).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062257.
References
- Alfonso M, Yruela I, Almarcegui S, Torrado E, Perez MA, Picorel R (2001) Unusual tolerance to high temperatures in a new herbicide-resistant D1 mutant from Glycine max (L.) Merr. cell cultures deficient in fatty acid desaturation. Planta 212: 573–582 [DOI] [PubMed] [Google Scholar]
- Argandona VH, Chaman M, Cardemil L, Munoz O, Zuniga GE, Corcuera LJ (2001) Ethylene production and peroxidase activity in aphid-infested barley. J Chem Ecol 27: 53–68 [DOI] [PubMed] [Google Scholar]
- Bariola PA, MacIntosh GC, Green PJ (1999) Regulation of S-like ribonuclease levels in Arabidopsis. Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol 119: 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardini TZ, Bollman K, Sun H, Poethig RS (2001) Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291: 2405–2407 [DOI] [PubMed] [Google Scholar]
- Bortier K, Dekelver G, De Temmerman L, Ceulemans R (2001) Stem injection of Populus nigra with EDU to study ozone effects under field conditions. Environ Pollut 111: 199–208 [DOI] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E (1996) Molecular chaperones and protein folding in plants. Plant Mol Biol 32: 191–222 [DOI] [PubMed] [Google Scholar]
- Burke JJ, O'Mahoney PJ, Oliver MJ (2000) Isolation of Arabidopsis mutants lacking components of acquired thermotolerance. Plant Physiol 123: 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Mur LA, Wood JE, Scott IM (2004) Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J 38: 432–447 [DOI] [PubMed] [Google Scholar]
- Dat JF, Foyer CH, Scott IM (1998. a) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118: 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998. b) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116: 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drané P, Compe E, Catez P, Chymkowitch P, Egly J-M (2004) Selective regulation of vitamin D receptor-responsive genes by TFIIH. Mol Cell 16: 187–197 [DOI] [PubMed] [Google Scholar]
- Dubaele S, Proietti De Santis L, Bienstock RJ, Keriel A, Stefanini M, Van Houten B, Egly JM (2003) Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol Cell 11: 1635–1646 [DOI] [PubMed] [Google Scholar]
- Flahaut S, Benachour A, Giard JC, Boutibonnes P, Auffray Y (1996) Defense against lethal treatments and de novo protein synthesis induced by NaCl in Enterococcus faecalis ATCC 19433. Arch Microbiol 165: 317–324 [DOI] [PubMed] [Google Scholar]
- Friant S, Meier KD, Riezman H (2003) Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction. EMBO J 22: 3783–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Gepstein SS, Heikkila JJ, Dumbroff EB (1988) Use of a scanning densitometer of an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem 169: 227–233 [DOI] [PubMed] [Google Scholar]
- Gong M, Li YJ, Chen SZ (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153: 488–496 [Google Scholar]
- Hall AE (2001) Crop Responses to the Environment. CRC Press, Boca Raton, FL
- Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125: 189–198 [DOI] [PubMed] [Google Scholar]
- Hong SW, Lee U, Vierling E (2003) Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol 132: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2001) Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J 27: 25–35 [DOI] [PubMed] [Google Scholar]
- Horváth I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L (1998) Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA 95: 3513–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S, Kunst L, Somerville C (1991) Linkage relationships of mutations that affect fatty-acid composition in Arabidopsis. J Hered 82: 484–488 [Google Scholar]
- James DW, Dooner HK (1991) Novel seed lipid phenotypes in combinations of mutants altered in fatty-acid biosynthesis in Arabidopsis. Theor Appl Genet 82: 409–412 [DOI] [PubMed] [Google Scholar]
- Jenkins ME, Suzuki TC, Mount DW (1997) Evidence that heat and ultraviolet radiation activate a common stress-response program in plants that is altered in the uvh6 mutant of Arabidopsis thaliana. Plant Physiol 115: 1351–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Brunsting JF, Stege GJ, Burgman PW, Konings AW (1995) Thermal protein denaturation and protein aggregation in cells made thermotolerant by various chemicals: role of heat shock proteins. Exp Cell Res 219: 536–546 [DOI] [PubMed] [Google Scholar]
- Kato M, Hayakawa Y, Hyodo H, Ikoma Y, Yano M (2000) Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol 41: 440–447 [DOI] [PubMed] [Google Scholar]
- Kapoor M, Sreenivasan GM, Goel N, Lewis J (1990) Development of thermotolerance in Neurospora crassa by heat shock and other stresses eliciting peroxidase induction. J Bacteriol 172: 2798–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueva NY, Maestri E, Marmiroli N, Nguyen HT (2001) Mechanisms of thermotolerance in crops. In AS Basra ed, Crop Responses and Adaptations to Temperature Stress. Food Products Press, Binghamton, NY, pp 177–217
- Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161: 405–413 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Mishkind M, Vierling E (2005) Plant responses to high temperature. In MA Jenks, PM Hasegawa, eds, Plant Abiotic Stress. Blackwell Scientific Publications, Oxford (in press)
- Liu Z, Hong S-W, Escobar M, Vierling E, Mitchell DL, Mount DW, Hall JD (2003) Arabidopsis UVH6, a homolog of human XPD and yeast RAD3 DNA repair genes functions in DNA repair and is essential for plant growth. Plant Physiol 132: 1405–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Muller J, Krishna P, Forreiter C (2000) A glucosinolate mutant of Arabidopsis is thermosensitive and defective in cytosolic Hsp90 expression after heat stress. Plant Physiol 123: 949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning WJ, Flagler RB, Frenkel MA (2003) Assessing plant response to ambient ozone: growth of ozone-sensitive loblolly pine seedlings treated with ethylenediurea or sodium erythorbate. Environ Pollut 126: 73–81 [DOI] [PubMed] [Google Scholar]
- Massie MR, Lapoczka EM, Boggs KD, Stine KE, White GE (2003) Exposure to the metabolic inhibitor sodium azide induces stress protein expression and thermotolerance in the nematode Caenorhabditis elegans. Cell Stress Chaperones 8: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeder W, Barry CS, Tauriainen AA, Betz C, Tuomainen J, Utriainen M, Grierson D, Sandermann H, Langebartels C, Kangasjarvi J (2002) Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol 130: 1918–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K (2000) Trienoic fatty acids and plant tolerance of high temperature. Science 287: 476–479 [DOI] [PubMed] [Google Scholar]
- Nie X, Singh RP, Tai GC (2002) Molecular characterization and expression analysis of 1-aminocyclopropane-1-carboxylate oxidase homologs from potato under abiotic and biotic stresses. Genome 45: 905–913 [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J, Kannan KB, Martinez LM, Segal C (1999) Characterization of a maize heat-shock protein 101 gene, HSP101, encoding a ClpB/Hsp100 protein homologue. Gene 230: 187–195 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Riezman H (2004) Why do cells require heat shock proteins to survive heat stress? Cell Cycle 3: 61–63 [PubMed] [Google Scholar]
- Ristic Z, Cass DD (1992) Chloroplast structure after water and high temperature stress in 2 lines of maize that differ in endogenous levels of abscisic acid. Int J Plant Sci 153: 186–196 [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AJ, Ishikawa M, Gusta LV, MacKenzie SL (1994) Abscisic acid-induced heat tolerance in Bromus inermis Leyss cell-suspension cultures. Heat-stable, abscisic acid-responsive polypeptides in combination with sucrose confer enhanced thermostability. Plant Physiol 105: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Almoguera C, Jordano J (1999) Transcriptional activation of a heat shock gene promoter in sunflower embryos: synergism between ABI3 and heat shock factors. Plant J 20: 601–610 [DOI] [PubMed] [Google Scholar]
- Sandermann H (2000) Active oxygen species as mediators of plant immunity: three case studies. Biol Chem 381: 649–653 [DOI] [PubMed] [Google Scholar]
- Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31: 629–638 [DOI] [PubMed] [Google Scholar]
- Svejstrup JQ, Vichi P, Egly JM (1996) The multiple roles of transcription/repair factor TFIIH. Trends Biochem Sci 9: 346–350 [PubMed] [Google Scholar]
- Swidzinski JA, Sweetlove LJ, Leaver CJ (2002) A custom microarray analysis of gene expression during programmed cell death in Arabidopsis thaliana. Plant J 30: 431–446 [DOI] [PubMed] [Google Scholar]
- Thatcher LF, Anderson JP, Singh KB (2005) Plant defense responses: What have we learnt from Arabidopsis? Funct Plant Biol 32: 1–19 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14: 365–370 [DOI] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L (2004) Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco bright-yellow 2 cells. Plant Physiol 134: 1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Vranova E, Dat JF, Inzé D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161: 405–414 [Google Scholar]
- Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42: 579–620 [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E (1996) Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol 112: 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E (2000) The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol 122: 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Doelling JH, Falbel TG, Durski AM, Vierstra RD (2000) The ubiquitin-specific protease family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol 124: 1828–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.