Abstract
Within the central nervous system, synaptic plasticity, fundamental to processes like learning and memory, is largely driven by activity-dependent changes in synaptic strength. This plasticity often manifests as long-term potentiation (LTP) and long-term depression (LTD), which are bidirectional modulations of synaptic efficacy. Strong epidemiological and experimental evidence show that the heart–brain axis could be severely compromised by both neurological and cardiovascular disorders. Particularly, cardiovascular disorders, such as heart failure, hypertension, obesity, diabetes and insulin resistance, and arrhythmias, may lead to cognitive impairment, a condition known as cardiogenic dementia. Herein, we review the available knowledge on the synaptic and molecular mechanisms by which cardiogenic dementia may arise and describe how LTP and/or LTD induction and maintenance may be compromised in the CA1 region of the hippocampus by heart failure, metabolic syndrome, and arrhythmias. We also discuss the emerging evidence that endothelial dysfunction may contribute to directly altering hippocampal LTP by impairing the synaptically induced activation of the endothelial nitric oxide synthase. A better understanding of how CV disorders impact on the proper function of central synapses will shed novel light on the molecular underpinnings of cardiogenic dementia, thereby providing a new perspective for more specific pharmacological treatments.
Keywords: heart–brain axis, cardiovascular disorders, synaptic plasticity, cardiogenic dementia, cognitive impairment, heart failure, metabolic syndrome, arrhythmias, NMDA receptors, long-term potentiation
1. Introduction
The heart–brain axis (HBA) is based upon the bidirectional flow of information between the heart and the brain, which becomes evident when the dysfunction in one system leads to a significant impairment in the function (and even in the structure) of the other [1,2,3]. The functional interplay between the cardiovascular (CV) and nervous systems has shaped the concept of neurocardiology, a branch of medicine that aims at investigating the pathological and therapeutic implications of the HBA [4]. The HBA is perhaps more known to cardiologists rather than neurologists as the potential ability of the central nervous system (CNS) to cause CV disorders through the cardiomotor sympathetic and parasympathetic outflow of the autonomous nervous system has long been recognized [1,4]. An imbalance between the sympathetic (cardioexcitatory) and parasympathetic (cardioinhibitory) tone could lead to emotional stress-induced cardiomyopathy syndromes, including Takotsubo syndrome and neurogenic stunned myocardium [1,4,5]. In addition, the heart can promote catecholamine secretion via the hypothalamic–pituitary–adrenal (HPA) axis, which also promote cortisol release from the adrenal glands. The neuroendocrine storm triggered by the HPA further contributes to myocardial injury by predisposing the heart to insults, such as ischemia, inflammation, and ionic disturbances [6,7]. The autonomic imbalance induced by emotional and/or physical stress could also result in hypertension, increased afterload, and, ultimately, congestive heart failure (HF) by impairing endothelial signaling, mean arterial pressure, cardiac phenotype and function (e.g., left ventricular hypertrophy and arrhythmia) and the renin–angiotensin–aldosterone system [5,8]. The harmful consequences of the increased sympathetic tone on the CV system have gained further momentum during the recent COronaVIrus Disease 19 (COVID-19) pandemic, in which the HBA dysfunction has contributed to worsening the outcome of COVID-19 patients [9].
However, CV diseases, pivotal among which are HF, metabolic syndrome, and arrhythmias, may also result in severe neurological disorders as a consequence of cerebral hypoperfusion and cardioembolic stroke or, in the presence of myocardial injury, because of systemic inflammation and neurohumoral activation [2,10,11,12,13]. The ability of the CV system to regulate neuronal signaling and synaptic plasticity may lead patients with cardiac-related risk factor to present severe cognitive deficits [2,12], a condition for which the term “cardiogenic dementia” has been coined (Figure 1) [2,14]. The mechanisms by which CV dysfunction may lead to cognitive defects and induce cardiogenic dementia have been widely investigated and discussed in an excellent recent review article [2]. Nevertheless, the pathophysiological link between CV disorders and the impairment of neuronal signaling and synaptic plasticity, which underlies cardiogenic dementia at molecular/cellular levels, remains still elusive. Herein, we first describe the ionic mechanisms of learning and memory by focusing our attention on the excitatory glutamatergic synapses, which can experience an increase or a decrease in the strength of synaptic transmission that underlie cognitive and emotional processes. Then, we illustrate how HF, metabolic syndrome, including hypertension, obesity, diabetes and insulin resistance, and endothelial dysfunction, and arrhythmias may impair the ionic and synaptic mechanisms responsible for synaptic plasticity in the CNS. A better understanding of how CV disorders impact on the proper function (and structure) of central synapses is not only expected to shed novel light on the molecular underpinnings of cardiogenic dementia but also to provide a new prospect for more effective pharmacological treatments.
Figure 1.
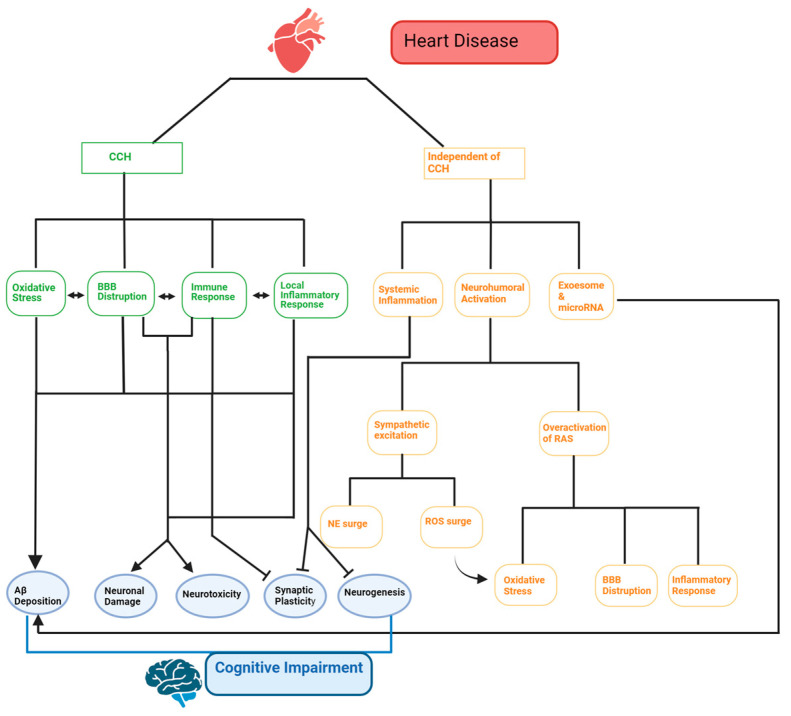
Cardiogenic dementia: a multifaceted pathway. Cardiogenic dementia can result from cognitive impairment following heart disease. This impairment can occur due to chronic cerebral hypoperfusion (CCH) or independently of it. CCH arises when long-term heart damage reduces cardiac output. This triggers a chain of events involving oxidative stress, local inflammation, immune responses, and blood–brain barrier (BBB) disruption. Cardiogenic dementia can also occur without changes in cerebral blood flow (CBF). This involves systemic inflammation, neurohumoral activation, and the release of exosomes. Increased norepinephrine (NE) and reactive oxygen species (ROS) can result from sympathetic excitation. Additionally, the overactivation of the renin–angiotensin system (RAS) leads to oxidative stress, BBB disruption, and inflammation. In conclusion, heart disease can contribute to amyloid-beta protein (Aβ) deposition, neuronal damage, and neurotoxicity. It can also hinder synaptic plasticity and neurogenesis through both CCH-dependent and -independent mechanisms. These factors collectively worsen cognitive function. Made with BioRender.
2. The Ionic Mechanisms of Learning and Memory: The Unexpected Targets of Cardiovascular Disorders
Synaptic plasticity, the ability of synapses to dynamically strengthen or weaken in response to neural activity, is a fundamental mechanism underpinning learning and memory. Therefore, cardiogenic dementia primarily affects the signaling pathways that underpin memory formation consolidation and shape emotional behavior at excitatory synapses in the hippocampus and other brain regions, such as the amygdala and prefrontal cortex (PFC). Excitatory neurotransmission in the brain is mediated by glutamate, which is released from pre-synaptic terminals and targets both ionotropic and metabotropic receptors on the postsynaptic neurons. CV diseases severely interfere with glutamatergic signaling and with the downstream pathways that lead to memory and learning and shape emotional and behavioral skills.
Long-term potentiation (LTP) and long-term depression (LTD) are two well-characterized forms of synaptic plasticity. LTP, characterized by a persistent increase in synaptic strength, is often associated with the acquisition of new information. Conversely, LTD, a long-lasting decrease in synaptic strength, is thought to be involved in processes such as forgetting and the refinement of neural circuits. While the hippocampus has been the primary model system for studying synaptic plasticity, recent evidence suggests that these mechanisms are widespread throughout the brain [15,16,17,18,19]. The molecular mechanisms underlying LTP and LTD are complex and involve a variety of signaling pathways, including those mediated by N-methyl-D-aspartate (NMDA) receptors (NMDARs), calcium ions (Ca2+), and second messenger systems (Figure 2) [17,20,21,22,23,24,25]. At excitatory synapses, synaptic plasticity is primarily mediated by alterations in the function and number of postsynaptic ionotropic glutamate receptors, particularly α-amino-3-hydroxy-5-methyl-4-isoxasolepropionic acid (AMPA) receptors (AMPARs), kainate receptors, and NMDARs (Figure 2) [26,27]. Given AMPARs’ dominant role in basal synaptic transmission, much research on LTP and LTD mechanisms has focused on understanding how AMPAR-mediated synaptic responses are modulated [17,26,27]. A prevailing theory posits that NMDARs play a crucial role in initiating various forms of activity-dependent LTP and LTD by acting as a coincidence detector for pre- and postsynaptic firing patterns [15]. This property depends on the Mg2+-dependent inhibition of the NMDAR channel at resting membrane potential and their high permeability to Ca2+. An NMDAR-mediated rise in postsynaptic Ca2+ activates kinases, notably Ca2+/Calmodulin-dependent protein kinase II (CaMKII), protein kinase A (PKA), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK), and protein phosphatases, such as calcineurin, ultimately results in an increase (Figure 2) or decrease in AMPAR density and/or conductance [17,28,29].
Figure 2.
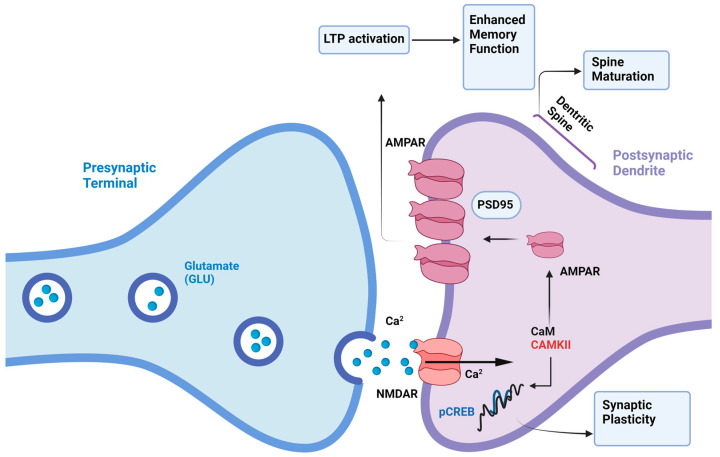
Molecular mechanisms of LTP in the hippocampus. The molecular mechanisms underlying LTP, involving signaling pathways mediated by N-methyl-D-aspartate (NMDA) receptors (NMDARs) and calcium ions (Ca2+). At excitatory synapses, synaptic plasticity is primarily mediated by alterations in postsynaptic ionotropic glutamate receptors, particularly α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs). NMDARs play a crucial role in initiating LTP by acting as coincidence detectors for pre- and postsynaptic firing patterns. An NMDAR-mediated rise in postsynaptic Ca2+ activates the Ca2+/Calmodulin (CaM)-dependent protein-kinase II (CaMKII). CaMKII-dependent phosphorylation, in turn, drives AMPAR incorporation to postsynaptic density in a post-synaptic density protein 95 (PSD-95)-dependent manner. Furthermore, CaMKII may phosphorylate cAMP response element-binding protein (CREB), the transcription factor regulating the expression of postsynaptic proteins and driving the physical expansion of dendritic spines. Made with BioRender.
While synaptic AMPARs have long been recognized as undergoing long-lasting modulation by synaptic activity, the question of whether synaptic NMDARs exhibit similar plasticity has been a subject of intense interest. Once thought to be relatively stable at synapses, synaptic NMDARs are now emerging as dynamic and capable of activity-dependent modulation, akin to AMPARs. Our understanding of the mechanisms of LTP of NMDAR-mediated excitatory postsynaptic potentials (NMDAR-EPSCs) has advanced significantly [30,31,32,33,34,35]. These studies converged on the notion that NMDAR-EPSCs may also undergo synaptic potentiation upon an increase in dendritic Ca2+ levels that is shaped by both NMDARs and group 1 metabotropic glutamate receptors (mGluR1 and mGluR5) [31,34,35,36]. In contrast to LTP, LTD of NMDAR-mediated synaptic responses has been consistently observed in response to induction protocols that elicit NMDAR-dependent LTD of AMPAR responses [37,38,39,40]. Therefore, just like synaptic AMPARs, synaptic NMDARs can also be bidirectionally modified by different patterns of synaptic activity. There is a wealth of evidence, not addressed here, that NMDARs are tightly regulated by experience during development. Our understanding of the mechanisms of activity-dependent synaptic plasticity of NMDA receptors is only beginning to emerge.
A more nuanced understanding of the temporal dynamics of synaptic plasticity has been provided by the discovery of spike timing-dependent plasticity (STDP). STDP reveals that the precise timing of pre- and postsynaptic spikes determines whether a synapse will strengthen or weaken. This bidirectional nature of STDP suggests a Hebbian-like learning rule, where synapses are strengthened when they are active during the generation of a postsynaptic spike and weakened when they are active but do not contribute to the generation of a postsynaptic spike. Recent studies have highlighted the role of various molecular mechanisms, including NMDARs, Ca2+ signaling, and protein synthesis, in regulating both LTP and LTD in canonical STDP [18,41,42].
Consistent with their critical role in learning and memory, as well as in socio-emotional and behavioral skills, LTP and LTD induction and maintenance are severely compromised in neurodegenerative and neuropsychiatric disorders, such as Alzheimer’s disease, Parkinson’s disease, dementia, schizophrenia, depression, and autism spectrum disorders [43,44,45,46,47]. Therefore, it is not surprising that cardiogenic dementia may also target the molecular mechanisms that strengthen or weaken synaptic transmission at central glutamatergic synapses, thereby leading the patients to cognitive impairment.
3. Heart Failure and Cognitive Impairment
HF is a complex clinical syndrome characterized by the heart’s inability to pump and/or refill with blood, thereby resulting in a reduced cardiac output (HF with reduced ejection fraction or HFrEF) or in an adequate cardiac output secondary to an increase in the left ventricular filling pressure due to robust neurohormonal activation (HF with preserved ejection fraction, HFpEF) [48]. HF has been estimated to affect 30–50 million patients worldwide, but its incidence is expected to rise as a consequence of the longer global average life expectancy and the availability of novel medications that substantially improve survival after HF diagnosis [49]. While the CV implications of HF are well documented, its effects on cognitive function and synaptic integrity are increasingly recognized as critical components of the disease [12,50]. Intriguingly, HF increases the risk of developing cognitive impairment, including deficits in learning and working memory, more than four-fold as compared to healthy control subjects [51]. In addition, HF patients are comorbid with anxiety and depression, which contribute to further worsening their quality of life and self-care [52]. This relationship underscores the necessity of understanding the molecular mechanisms that link HF to synaptic dysfunction. HF may affect cognitive function by reducing cerebral blood flow (CBF) [53,54], thereby resulting in hypoperfusion and/or hypoxia, and by triggering a strong neuroinflammatory axis [55,56]. These, in turn, lead to significant loss of cerebral grey matter loss and damage in brain regions that are involved in memory and emotions, such as the hippocampus, amygdala, and PFC [57,58]. However, the neural substrates and molecular mechanisms that contribute to HF-induced cognitive and emotional decline are still unclear. The brain receives ≈20% of the cardiac output [59], thereby consuming ≈60% of the energy-produced oxygen to maintain the neuronal resting potential, support neuronal firing, and enable synaptic transmission [60]. In accordance with this notion, it has long been known that persistent synaptic failure may result from mild or moderate cerebral hypoxia due to ATP depletion and down-regulation of AMPARs and NMDARs as well as of many proteins involved in synaptic vesicle trafficking, including Syntaxin-1A, Synaptogyrin-1, and SV-2 [61,62,63,64]. However, the incidence of cognitive decline does not differ significantly between HF patients with preserved versus reduced ejection fraction. The prevalence of stroke is also similar in HFrEF and HFpEV patients [55]. Therefore, additional mechanisms must contribute to impair synaptic function and favor cognitive and emotional decline in HF.
A recent investigation unveiled that neuroinflammation secondary to HF may impair synaptic function and plasticity, particularly in the dorsal hippocampus (DH) [65], which plays a pivotal role in memory and learning [16,25]. By using an ischemic HF rat model, Althammer and colleagues first confirmed that microglia, the resident immune cells of the CNS, undergo a transition towards a pro-inflammatory phenotype in DH, which is progressive in time and depends on the severity of HF [65]. Then, they found an increase in the inflammatory signal, including IL (interleukin) 1β (IL-1β), tumor necrosis factor-α (TNF-α), and C1q, which was restricted to DH and was associated with an astrocytic shift from a neuroprotective to a neurotoxic condition. In accordance with these findings, the CA1 region of HF rats showed an increase apoptotic rate and a decreased excitability of pyramidal neurons, as shown by their depolarized resting potential and reduced input/output relationship [65]. Therefore, HF reduces the ability of CA1 pyramidal neurons to properly process the incoming information by firing a train of action potential at the proper rate. Furthermore, this investigation provided additional evidence in support of the pathogenic role of the renin–angiotensin system in HF-induced neuroinflammation [56]. Angiotensin II (Ang II) is a pro-inflammatory peptide that is released in circulation after AMI [56] and may target AT1a receptors (AT1aRs) in multiple brain cells, including neurons, astrocytes, microglia, and oligodendrocytes [66]. The blood–brain barrier (BBB) was found to be leaky in the DH of HF rats, thereby potentially enabling circulating Ang II to cross the BBB and trigger the local cascade of neuroinflammation [65]. In accordance with this hypothesis, the expression of AT1aRs was primarily enhanced in the CA1 microglia of HF rats, which strongly suggests that Ang II could be critical in promoting their pro-inflammatory transition [65]. Moreover, a local increase in Ang II levels has long been known to suppress hippocampal LTP [67,68,69]. Notably, the infusion of losartan, a specific AT1aR antagonist, ameliorated hippocampal inflammation and strongly reduced hippocampal apoptosis [65], thereby rescuing the ability of CA1 pyramidal neurons to properly process the incoming information.
While the secretion of inflammatory cytokines is significantly increased, a recent investigation carried out on a rat model of chronic HF showed that the hippocampal levels of brain-derived neurotrophic factor (BDNF) are decreased [70]. BDNF is indispensable to neurotransmitter release and synaptic plasticity in central synapses [71]. BDNF binds to the tyrosine kinase tropomyosin-related kinase B receptor (TrkB) to potentiate neurotransmitter (glutamate and γ-aminobutyric or GABA) release, to induce and maintain LTP, and to participate in memory consolidation and cognitive function [71,72,73]. Consistent with these notions, BDNF in the cerebrospinal fluid was associated with a severe synaptic loss and impaired synaptic ultrastructure in the hippocampal CA1 region. In addition, chronic HF rats showed a reduction in spatial memory and a down-regulation of the cAMP/PKA/cAMP Response Element-Binding Protein (CREB) pathway, which is crucial for LTP consolidation and memory formation (Figure 2) [70]. Consistent with these findings, Parent and colleagues confirmed that the working spatial memory and the emotional long-term memory are disrupted in the hippocampus and PFC of a rat model of HFrEF due to the down-regulation of 84 genes critical for synaptic plasticity, including those encoding for NMDARs and BDNF [74]. These findings suggest that BDNF deficiency secondary to HF contributes to cognitive decline by impairing glutamatergic synapses and interfering with NMDAR signaling. Intriguingly, the pharmacological blockade of phosphodiesterase-4 (PDE4), which catalyzes cAMP hydrolysis, with Rolipram, BPN14770, or MK0952 has been proposed as a therapeutic approach to rescue cognitive functions in HF [70]. A parallel investigation further showed that the down-regulation of the BDNF/TrkB signaling pathway caused a reduction in glutamate and GABA levels in the brains of HF rats [75]. Intriguingly, a shift in the excitatory/inhibitory (E/I) balance may occur in hypothalamic magnocellular neurosecretory cells (MNCs) of HF rats due to a shift in glutamate–GABA ratio toward a relatively stronger glutamate weight [76], thereby supporting the increased neurohumoral drive in HF [77]. The imbalance of the E/I ratio is involved in the cognitive and emotional deficits described in the majority of CNS pathologies, including neurodegenerative diseases and autism spectrum disorders [22,46,47,78,79,80]. Therefore, future work might assess whether the E/I ratio is affected by HF in brain regions that are involved in emotion and cognition, such as the hippocampus, PFC, amygdala, and cerebellum. Overall, the available evidence suggests that HF may promote cognitive decline by stimulating neuroinflammation, by causing an increase in Ang II levels, and by down-regulating BDNF expression.
4. Metabolic Syndrome and Cognitive Impairment
Metabolic syndrome consists of a set of at least five cardio-metabolic disorders that include hypertension, central obesity, hyperglycemia, insulin resistance (IR), and atherogenic dyslipidemia, which may in turn increase the risk of developing type 2 diabetes mellitus (T2DM) and atherosclerosis [81,82,83]. It has been estimated that about one quarter of the world population, i.e., over a billion individuals, is now affected by metabolic syndrome [84], with an increasing prevalence among young individuals due to the larger spread of the Western diet and lifestyle [85]. Individuals with metabolic syndrome are also at strong risk of developing severe neurological deficits, such as cognitive impairment and memory loss, and neuropsychiatric disorders, such as anxiety and depression [23,81,82,86]. The emerging correlation between metabolic syndrome and cognitive impairment could be explained by the subtle reduction in microvascular perfusion that is caused by cerebrovascular atherosclerosis, which results in white matter damage and significantly reduces blood supply to firing neurons [3,87]. This hypothesis is supported by the evidence that subjects suffering from atherosclerosis or T2DM may also be affected by vascular dementia or Alzheimer’s disease [23,86,87]. However, growing evidence suggests that cardio-metabolic alterations may also lead to changes in the neural circuits and molecular mechanisms that underlie memory formation and emotion processing.
4.1. Hypertension
Hypertension is regarded as an independent risk factor for Alzheimer’s diseases and vascular cognitive impairment (VCI), i.e., the prodromal stage of cognitive decline that precedes vascular dementia (VD) [88,89,90]. An intensive lowering regimen of mean blood pressure may delay the onset of cognitive deterioration or even preserve cognition in hypertensive subjects [88]. One of the primary mechanisms by which hypertension leads to cognitive impairment is through endothelial dysfunction and microvascular rarefaction [88,89,91], which impair neurovascular coupling (NVC), i.e., the mechanism by which the increased metabolic demand of active neurons is met by an increase in local CBF [59,92]. In addition, hypertension may affect the structural and functional integrity of the BBB, promote microglia activation, and induce an inflammatory response in the brain parenchyma [88,89,91]. Hypertension-induced damage of cerebral microcirculation may obviously exert adverse effects on neuronal activity and synaptic plasticity, but emerging evidence suggests that the onset of cognitive decline in hypertensive individuals is also driven by more subtle molecular alterations.
An early investigation demonstrated an inverse relationship between mean blood pressure and glutamate concentration in the hippocampus of the hypertensive human subjects [93]. Moreover, both the early and late phase of LTP were impaired in the dentate gyrus of a genetically hypertensive rat that was deficient of the nerve growth factor (NGF). Hippocampal expression of TrkB protein was also down-regulated in hypertensive rats [94,95]. Intriguingly, the intracerebroventricular injection of NGF rescued LTP induction and maintenance [94], thereby suggesting that hypertension-induced synaptic impairment may also be due to the down-regulation of neuroprotective growth factors. A follow-up study confirmed that LTP impairment in the dentate gyrus was associated with the disruption of long-term recognition memory and a decrease in BDNF expression [96], as reported above for HF (seeSection 3). The impairment of hippocampal LTP has also been documented in a mouse model of Ang II-induced hypertension [69,97]. In addition, Tucsek and colleagues reported that hypertension was associated with a reduced synaptic density in the mouse hippocampus and with the down-regulation of several genes that are neuroprotective, such as BDNF and Igf1, or regulate postsynaptic signal transduction events, such as Homer1 [69]. For instance, insulin-like growth factor-1 (IGF-1) is critical for learning and memory via controlling the induction of NMDARs-dependent Hebbian LTP [98,99], whereas Homer1 mediates metabotropic glutamate receptors-dependent gene expression, which is indispensable for LTP consolidation [100,101]. Consistent with this finding, the administration of IGF-1 has long been known to attenuate the age-dependent decrease in learning and synaptic plasticity [102,103]. In addition, Dai and colleagues demonstrated that the p38 MAPK, which is strongly activated by inflammatory cytokines and thereby supports neuroinflammation [104], is up-regulated in the mouse hippocampus of Ang II-dependent hypertensive mice [97]. The pharmacological blockade of p38 MAPK with the selective inhibitor SKF86002 proved to be effective at rescuing hippocampal LTP induction and, therefore, might provide a suitable strategy for attenuating cognitive decline in hypertension [97]. LTP induction and retention of spatial memory are also impaired in the hippocampus of spontaneously hypertensive rats (SHRs) [105,106,107], which were originally bred from the progenitor Wistar Kyoto Rats. Interestingly, SHRs represent the most widespread animal model of Attention-Deficit/Hyperactivity Disorder (ADHD), which is characterized by a severe deficit in neuropsychological and psychosocial functions, including hyperactivity, inattention, and impulsivity [108,109]. The impairment of synaptic plasticity in SHRs may be due to the increase in oxidative stress [107], the down-regulation of NMDARs [105], CaMKII [110], dopamine D5 receptors [106], and BDNF [111], and the up-regulation of the endosomal Na+/H+ exchanger member 9 (NHE9) [112,113]. The latter can impair the postsynaptic trafficking of AMPARs [114] and deregulate dendritic Ca2+ signaling [113,115,116] during LTP induction. Pre-clinical studies showed that the most effective strategies to rescue synaptic plasticity and spatial learning in SHRs are physical exercise [107,117] and chronic swimming [118]. It should be noted that, unlike the SHR model [105], the hippocampal expression of NMDARs is not affected by Ang II-induced hypertension [69]. As hypertension is a multifactorial disease [119], the molecular mechanisms that favor the cognitive decline are likely to subtly change depending on the underlying etiology. Our current knowledge suggests that hypertension may favor synaptic impairment by reducing glutamate concentration, down-regulating the expression of neuroprotective genes, such as BDNF and Igf-1, up-regulating Ang II levels and oxidative stress, and interfering with several signaling pathways that support synaptic plasticity, such as NMDARs, AMPARs, and CaMKII.
4.2. Obesity
The global obesity epidemic has emerged over the last half century, thereby leading to the widespread diffusion of many chronic diseases that affect the population in the 21st century, including T2DM, insulin resistance, and obesity-related malignancies [120,121]. Many aspects of our contemporary environment may contribute to weight gain, including, but not limited to, sleep deprivation and stress, technology, and the high-caloric Western diet, which is based upon the massive consumption of refined sugars and saturated fat [121,122]. Recent studies revealed a bidirectional relationship between overweight and cognitive functions [123]. While it has long been known that obesity may alter synaptic processes [124,125], detrimental food intake habits may be a consequence of the dysregulation of specific neuronal circuits that are involved in appetite regulation [123,126]. On the other hand, obesity may cause a poorer cognitive performance by impairing learning and memory functions, including episodic memory and working memory [120,127]. The hippocampus has been shown to be rather vulnerable to the metabolic dysfunctions associated with obesity [124,125]. Accordingly, hippocampal-dependent spatial learning and memory are severely affected by a high-caloric diet [128,129,130]. Therefore, obesity is regarded as a novel predisposing risk factor for cognitive disorders, including Alzheimer’s disease and dementia [131,132]. The primary mechanisms by which obesity may lead to cognitive decline include down-regulation of BDNF/TrkA signaling, increased neuro-inflammation and oxidative stress, neurovascular uncoupling, and weakened BBB integrity [127,132,133,134]. However, obesity may also alter the neural circuits and molecular mechanisms that shape spatial learning and memory.
Early work showed that a high-fat diet impaired both LTP and LTD at the Schaffer collateral-CA1 synapse in mice by impairing glutamate metabolism and down-regulating NMDAR expression [135,136]. A more recent investigation revealed that NF-E2-related factor 2 (Nrf2) deficiency contributes to LTP dysfunction at the CA1 region of mice exposed to a high-caloric diet [137]. In accordance with this, Nrf2 is a transcription factor that is critical to mounting an antioxidant response [138,139] and to preventing cognitive decline with aging [140]. Intriguingly, obesity further exacerbated aging-induced cognitive decline and LTP impairment at the Schaffer collateral-CA1 synapse by promoting the degradation of vasorelaxing and pro-LTP epoxy-eicosatrienoic acids (EETs), such as 8,9-EET and 11,12-EET, and by decreasing the expression of several genes involved in memory formation and storage [141]. Similarly, LTP was decreased at the dentate gyrus of high-fat-fed rats due to the inhibition of group II metabotropic glutamate receptors (mGluR2/3) [142], although neurogenesis was not affected [130]. Conversely, LTP was only reduced in the CA1 region, but not in the dentate gyrus, of Obese Zucker Rats due to calcineurin down-regulation in the latter region that maintains adequate levels of phospho-CaMKII [143]. On the other hand, monosodium glutamate-induced obese mice, which are featured by glucose intolerance, showed enhanced LTP and LTD, as well as impaired excitatory neurotransmission, in the CA1 region due to the up-regulation of the vesicular glutamate transporter 1 [144,145]. Nevertheless, the increased hippocampal excitability led to significant recognition memory deficits, as highlighted by the novel object recognition test [144]. Obesity is also a multifactorial disease [121] and, therefore, it may differentially affect cognitive functions depending on the underlying etiology and the brain region. In this view, it is noteworthy that a high-fat diet was found to abolish LTP in the CA1 region and enhance LTP in the basolateral amygdala [146], which is consistent with the impairment of amygdala-dependent emotional memory reported in both humans and high-fat-fed rats [86,147,148]. Our current knowledge suggests that obesity may promote cognitive decline by interfering with glutamate-mediated neurotransmission, which could be either decreased or enhanced, and impairing the Nrf-2 dependent anti-oxidant response.
A variety of pharmacological strategies have been described aiming to rescue hippocampal LTP induction and restore cognitive functions in obesity, including the following: catecholaminergic stimulation [149]; administration of Glucagon-like peptide-1 (GLP-1) agonists, such as exendin-4 [150], or the insulin sensitizer, metformin [151]; blocking IL-1 signaling with the IL-1 receptor antagonist (IL-1RA) [152]; and rescuing the E/I ratio with a mixture of memantine and allopregnanolone, which, respectively, block NMDARs and GABAA receptors. These studies strongly suggest that the molecular mechanisms responsible for obesity-induced cognitive decline include the alteration of these signaling pathways. However, the multifactorial nature of obesity is unlikely to benefit of a generalized approach, e.g., supplementation of diets enriched with curcumin and omega-3 [122] or Mediterranean diet [82], but rather requires an individualized approach to effectively manage obesity according to its causation [121].
4.3. Hyperglycemia and Insulin Resistance
Long-term chronic hyperglycemia can be due to a deficiency in insulin production or the development of insulin resistance, which can result in type 1 diabetes mellitus (T1DM) or T2DM [153]. Both types of diabetes may cause mild to moderate cognitive impairment, a significant pathological condition known as diabetic encephalopathy [154]. Furthermore, diabetes mellitus is regarded as a risk factor for Alzheimer’s disease [155,156]. Endothelial dysfunction is critical in mediating the impairment of cognitive decline by hyperglycemia/diabetes due to the disruption of the BBB [157] and neurovascular uncoupling [158]. Studies carried out in rodent models of hyperglycemia, T1DM, and T2DM also suggested that oxidative stress and neuroinflammation may cause cognitive impairment by damaging myelinated tracts, hippocampal neuronal circuits, and synaptic contacts [159,160,161,162]. Furthermore, electrophysiological abnormalities, including alterations in glutamatergic transmission throughout the CNS and in hippocampal-dependent learning, memory, and cognitive tasks, have been reported [163,164,165].
By using the streptozotocin-induced rat model of T1DM, it has been shown that high-frequency stimulation (HFS) of the Schaffer collateral only results in weak LTP induction, if any, while low-frequency stimulation (LFS) induces a larger LTD as compared to healthy animals [166,167,168,169]. In accord, spatial learning and recognition memory were impaired only in severely hyperglycemic rats [166,170,171]. However, LFS results in a weaker LTD in juvenile streptozotocin-induced rats due to the reduction of cholinergic stimulation of the hippocampus [172,173,174]. These findings suggesting the age of diabetes onset could determine whether it also results in cognitive defects or not. Several mechanisms accounted for the impairment of LTP in the hippocampal CA1 region, including a defect in presynaptic glutamate release [155], the down-regulation of NMDAR expression [175] and CaMKII-dependent phosphorylation [176,177], a reduction in the Ca2+-dependent recruitment of postsynaptic AMPARs [167] and AMPAR-mediated EPSCs [178], a rightward shift in the threshold of LTP induction, and a leftward shift in the threshold of LTD induction [179]. More recent investigations further showed that T1DM may interfere with synaptic potentiation by decreasing the activity of the Na+/K+ ATPase [171,180], thereby preventing the restoration of the ionic milieu during sustained neuronal activity [181]. Preliminary evidence indicates that the impairment of LTP induction at the Schaffer collateral-CA1 synapse was associated with a reduction in AMPA/NMDA ratio in young adult, but not juvenile, streptozotocin-injected rats [172]. This finding strongly suggests that the age of onset of T1DM might be considered for selecting the most appropriate therapy to prevent cognitive decline in patients. It should, however, be noted that insulin treatment rescues synaptic potentiation in the hippocampal CA1 region [182,183]. Our current knowledge suggests that T1DM may impair LTP induction by interfering with glutamate release and glutamate-dependent postsynaptic signaling, including the down-regulation of NMDARs, AMPARs, and CaMKII activation, and by preventing the restoration of the ionic gradients across the neuronal membrane after intense synaptic activity upon Na+/K+ ATPase inhibition.
Therapeutic strategies that improved hippocampal-dependent learning and memory by boosting NMDAR signaling and ameliorating LTP impairment in T1DM include the nerve-protective drug extracted from the seed of Chinese celery, l-3-n-Butylphthalide (NBP) [184], the isoquinoline alkaloid berberine [185], the phenolic compound vanillic acid [186], probiotics treatment [187], inhibition of the receptor for advanced glycation end products (RAGE) with the specific blocker FPS-ZM1 [188], physical exercise [189], and stem cell transplantation [190].
Similar results have been obtained in multiple rodent models of T2DM. Impairment of LTP induction in the hippocampal CA1 region of Obese Zuker rats is caused by the alteration of pre-synaptic glutamate release [143,191]. Reduced LTP and impaired spatial learning ability have also been documented in high-glucose-fed rats due to a significant reduction in postsynaptic spine density and BDNF levels [192,193]. Finally, the magnitude of hippocampal LTP was dramatically attenuated in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, which spontaneously develop T2DM [194]. HFS-induced LTP at the Schaffer collateral-CA1 region was also impaired in mouse models of T2DM, including a transgenic murine model of adipocyte insulin resistance (AtENPP1-Tg) [195], diabetic db/db mice [196,197], spontaneous obese KK-Ay type 2 diabetic mice [198], and transgenic mice deficient of insulin receptor β subunit [199] or GLP-1 [200]. The molecular mechanisms involved in T2DM-dependent impairment of LTP and hippocampal-related learning and memory functions include the alteration in the molecular assortment of AMPAR subunit [198], down-regulation of NMDAR expression and phosphorylation [198], reduced glutamate release [200], CaMKII activation [196,201], CREB expression [201] during HFS, and deregulation of GABAergic signaling [197]. The deficiency in hippocampal LTP, as well as the impairment of memory recognition and spatial learning, could be ameliorated by a variety of treatments, including diet intervention [198], NBP [196], RAGE inhibition with FPS-ZM1 [202], stem cell transplantation [195], metformin, and environmental enrichment [193]. Sodium-glucose cotransporter-2 (SGLT2) inhibitors, which have been developed as anti-diabetic drugs, also proved to be more effective at tempering cognitive dysfunction as compared to other anti-diabetic strategies [203,204,205,206]. Preliminary evidence showed that SGLT2 inhibitors could rescue synaptic plasticity by increasing the hippocampal levels of BDNF and NGF [204] and that dapagliflozin was able to improve LTP at the Schaffer collateral-CA1 synapse [207]. Due to the emergence of SGLT2 inhibitors as pleiotropic drugs that exert a broad range of beneficious systemic effects, future investigations should assess whether and how they directly affect pre- or postsynaptic mechanisms of excitatory and inhibitory neurotransmission.
4.4. Dysregulated Endothelial Ion Signaling
As anticipated above, endothelial dysfunction is involved in metabolic syndrome-induced cognitive decline by mining the BBB integrity, by supporting arterial stiffening and microvascular rarefaction, and by favoring neurovascular uncoupling. Recent investigations showed that cerebrovascular endothelial cells may sense synaptic activity and thereby release nitric oxide (NO) to increase blood supply to firing neurons [59,92,208]. Synaptically released neurotransmitters and neuromodulators, such as glutamate, may induce endothelial Ca2+ oscillations at the postarteriolar transition zone by activating Gq-protein-coupled receptors that lead to Ca2+ release from the endoplasmic reticulum through inositol-1,4,5-trisphosphate receptors [116,209,210]. This oscillatory increase in endothelial Ca2+ concentration can be enhanced by endothelial hyperpolarization through inward rectifier K+ channels (KIR2.1) [209,211] and stimulates the endothelial NO synthase (eNOS), thereby leading to NO-dependent vasorelaxation and NVC [116,209,212]. In addition, cerebrovascular endothelial cells express NMDARs that can also be activated by synaptic activity and are physically coupled to eNOS and NO production [25,213,214,215]. The metabolic syndrome may compromise the endothelial ion signaling machinery [92,134,216,217,218,219,220,221], and a growing body of evidence shows that endothelial KIR2.1 channels [222,223,224] and NMDARs [213] may be impaired in Alzheimer’s disease [225]. This would, in turn, lead to reduced eNOS activation and impaired NO signaling at the neurovascular unit. Intriguingly, eNOS-derived NO may also regulate LTP induction and maintenance in the hippocampal CA1 region [226,227,228,229], and the LTP/LTD balance is shifted towards LTD upon the genetic deletion of eNOS [230]. Endothelial ion signaling can be targeted by pharmacological manipulation and dietary interventions [92,134,229]. Therefore, future work should assess whether the metabolic syndrome also affects endothelial ion signaling at the neurovascular unit and whether this contributes to the defects observed in hippocampal-dependent learning and memory cognitive tasks.
5. Arrhythmias
Cardiac arrhythmias, including atrial fibrillation, ventricular tachycardia, and ventricular fibrillation, may cause cognitive decline and dementia through a variety of mechanisms, such as cerebral hypoperfusion, thromboembolism, inflammation, and stroke [231,232,233]. Stroke is primarily caused by atrial fibrillation and is known to cause a maximum reduction in cognitive function [234], whereas anoxic brain injury is more commonly associated with ventricular arrhythmias [235]. Untangling the molecular mechanisms linking cardiac arrhythmias to the impairment of the synaptic mechanisms underlying memory formation and storage requires the use of appropriate transgenic animal models. But fatal arrhythmic events could interfere with the successful exploitation of electrophysiological experiments or voltage-sensitive dye imaging in ex vivo slices and in vivo brain preparations. For instance, several mouse models of spontaneous atrial fibrillation have been developed, but a straightforward analysis of LTP induction and maintenance, e.g., in the hippocampal CA1 region, is still missing [236]. Notably, myocardial fibrosis, which is the primary adaptative response to myocardial infarction, may also predispose HF patients to ventricular tachycardia and ventricular fibrillation [237]. Therefore, we cannot rule out that, in these patients, cognitive decline is driven by HF rather than by cardiac arrhythmias. An intriguing possibility is that inheritable arrhythmias underlain by mutations in ion channels that are expressed in both the heart and the brain are also associated with cognitive impairment. Mutations in type 2 ryanodine receptors (RyR2) may lead to cardiac arrhythmias, such as catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic cardiomyopathy [238,239,240], and to cognitive dysfunctions, such as Alzheimer’s disease [241]. However, mutations in the RYR2 gene cause either cardiac or neurological disorders, not both. Conversely, a genetic mouse model of the Timothy syndrome, a multi-organ form of long QT syndrome that is caused by mutations of the CACNA1C gene [238,242], has recently been exploited to investigate the molecular mechanisms of the neurological deficits, such as autism spectrum disorders, associated with this rare cardiac disease. In accord, the CACNA1C gene encodes for the voltage-dependent L-type Ca2+ channel, CaV1.2, which regulates the excitation–contraction coupling mechanism in the heart [243] and Ca2+-dependent gene expression and synaptic plasticity in the brain [244]. A sporadic single nucleotide change, which produces a missense mutation (G406R) in the pore-forming subunit of CaV1.2, is the most common cause of the Timothy syndrome [242]. This mutation exerts a gain-of-function effect that shifts the threshold of activation towards more negative potentials and removes the voltage-dependent inactivation of L-type Ca2+ currents, thereby leading to intracellular Ca2+ overload [242]. A preliminary investigation confirmed that the intracellular Ca2+ signals produced by CaV1.2 G406R channels showed larger spatial spread and amplitude as compared to wild-type channels. However, the duration of the increase in intracellular Ca2+ concentration was significantly shorter, thus suggesting the involvement of negative-feedback mechanisms that limit Ca2+ entry through CaV1.2 G406R channels in neurons [245]. It has been suggested that the cellular outcome of the G406R mutation in the CNS could be slightly different than in the heart due to splice variants and the interaction with other ancillary subunits and regulatory proteins [246]. A subsequent report showed that the mutant CaV1.2 G406R channels drive CREB-dependent gene expression in a depolarization-independent manner due to the channel activation at sub-threshold potentials [247]. Intriguingly, the occupancy of the Ca2+-binding site within the selectivity filter, but not Ca2+ entry into the cytoplasm, is required for the basal transcriptional activity of CaV1.2 G406R channels [247]. This finding is consistent with the emerging view that both ionotropic receptors and voltage-gated channels, including CaV1.2, may signal in a flux-independent manner [25,248,249]. A recent investigation explored the impact of the G406R mutation in hippocampal synaptic plasticity by exploiting a novel transgenic mouse model, in which the expression of the CaV1.2 G406R mutant protein from exon 8 was blunted via transcriptional interference to prevent fatal cardiac arrhythmias [246]. Ca2+ entry through L-type CaV1.2 channels during plasticity induction may support the NMDARs-mediated recruitment of Ca2+-dependent signaling pathways that stimulate AMPARs and GABAA receptor trafficking and postsynaptic spine remodeling and expansion [18,22,250,251,252]. This novel transgenic mouse model showed that the E/I balance was shifted towards excitation in the CA1 region due to an increase in AMPARs-mediated excitatory transmission and a decrease in GABAA receptors-mediated inhibitory transmission [246]. The loss of GABAergic inhibition could also be due to a reduction in interneuron migration, which is also driven by Ca2+ influx through CaV1.2 channels [253]. That the CACNA1 gene could provide a molecular link between inherited arrhythmias and cognitive dysfunction is also suggested by the deletion of exon 33, which causes a gain-of-function mutation in the CaV1.2 protein. The absence or decrease in exon 33-containing CaV1.2 channels results in ventricular tachycardia and lengthened QT interval [254], as well as in severe neurological deficits [255]. In accord, the deletion of exon 33 caused an increase in late LTP and favored the transition of early LTP to long-lasting LTP at the Schaffer collateral-CA1 synapse. Furthermore, LFS did not induce LTD but rather synaptic potentiation [255]. The LTP/LTD imbalance did not improve hippocampal-dependent functions, such as associative memory, while disrupting social behaviors as they became less aggressive [255]. In accordance with this evidence, the CACNA1 gene is also widely expressed in the amygdala [244], but the electrophysiological evidence that synaptic mechanisms are altered by CaV1.2 mutation in this brain region is missing. Future work is required to assess whether the deletion of exon 33 from the CACNA1 gene has any pathological relevance for human health.
6. Conclusions
Cardiogenic dementia is a detrimental consequence of HBA that can exacerbate the progression of CV disorders and worsen the prognosis and management of the patients. Cardiogenic dementia can increase mortality, reduce the quality of life, and enhance the economic burden imposed on the families of CV patients. The framework of the pathophysiological mechanisms by which disruption of the HBA leads to cognitive impairment, i.e., cerebral hypoperfusion, neuroinflammation, BBB breakdown, microvascular rarefaction, and neurovascular uncoupling, has roughly been delineated. But obtaining novel insights into the subtle cellular and molecular mechanisms that underpin memory storage and formation, as well social behavior and emotional responses, requires a strong interaction between cardiovascular physiologists and molecular neurobiologists. Most of the attention has hitherto been paid to the CA1 region, which is critical to the formation, consolidation, and retrieval of hippocampal-dependent memories. The available evidence suggests that CV disorders tend to converge on the dysregulation of the same synaptic mechanisms, e.g., glutamate release, AMPARs, NMDARs, and CaMKII activation, to reduce the antioxidant defenses and to down-regulate neuroprotective genes, such as BDNF and Igf-1. In our opinion, future studies will have to do the following: (1) consolidate these preliminary findings, not only in the CA1 region but also in the other hippocampal areas; (2) gain further insights on the alterations in the E/I balance that could favor LTD over LTP and thereby boost the cognitive decline; (3) confirm that endothelial signaling plays a critical role in cognitive tasks, as also recently shown in [256], and assess the contribution of endothelial dysfunction to cognitive impairment not only in terms of cerebral hypoperfusion but also of direct regulation of neuronal activity and synaptic transmission [25,229]; (4) investigate whether the age of onset of the CV disorder, e.g., obesity and diabetes, may affect the extent and the mode of cognitive dysfunction; and (5) exploit induced pluripotent stem cell-derived neuronal cultures generated from CV patients to boost the therapeutic translation of animal studies. As recently outlined by an American Heart Association scientific statement [257], there is still a gap between our understanding of how CV diseases impact on brain function at macroscopic (e.g., cerebral hypoperfusion and cerebral microembolism) and microscopic (e.g., synaptic transmission and LTP induction) levels.
We expect that future work will have to focus on other brain regions, such as the PFC, amygdala, and cerebellum, that also play a crucial role in various cognitive and behavioral functions. For instance, PFC degeneration has been shown to correlate with cognitive decline in older adults [258]. The PFC function is seemingly insensitive to changes in the heart rate [259]. However, recent studies provided evidence that children and adolescents with congenital heart disease showed lower cognitive performance, mainly in episodic memory, executive function, and language, which are associated with cerebello–PFC connectivity [260,261]. Furthermore, the structure and/or the activity of PFC, amygdala, and cerebellum may also be affected by HF [262,263,264], insulin resistance [265], overweight and obesity [266,267,268], and atrial fibrillation [269,270,271]. Functional magnetic resonance imaging has clearly demonstrated that the different brain areas that underlie cognitive, executive, and emotional functions are intimately interconnected and effectively interact to shape human behavior. Therefore, clinical studies are expected to unveil how CV disorders affect the resting-state functional connectivity and the coordination of these interactions, whereas basic research will have to exploit animal models of such diseases to dissect the underlying alterations at cellular and molecular levels.
The advent of novel, high-resolution recording tools, such as high-density multielectrode arrays and multiphoton imaging combined with rapid voltage-sensitive dye imaging, is predicted to offer novel insights on the alterations of neural circuits and synaptic mechanisms, both ex vivo and in vivo. The design of more appropriate therapeutic strategies to delay or prevent cardiogenic dementia will also benefit from optogenetics, which is yet to be applied to investigate the synaptic mechanisms of cognitive decline in mouse models of CV diseases.
Author Contributions
Conceptualization, T.S. and F.M.; validation, T.S., T.P., G.D.S. and F.M.; writing—original draft preparation, T.S. and F.M.; writing—review and editing, T.S., T.P., G.D.S. and F.M.; visualization, T.S., T.P., G.D.S. and F.M.; supervision, T.S.; funding acquisition, G.D.S. and F.M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data was created for this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research has been supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022) (G.D.S. and F.M.) and European Commission’s FESR FSE 2014–2020 and POR CALABRIA FESR AZIONE 1.5.1 “Support for research infrastructures considered critical/crucial for regional systems” Nuova Piattaforma di Farmacologia Integrata e Tecnologie Avanzate (G.D.S.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tahsili-Fahadan P., Geocadin R.G. Heart-Brain Axis: Effects of Neurologic Injury on Cardiovascular Function. Circ. Res. 2017;120:559–572. doi: 10.1161/CIRCRESAHA.116.308446. [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Xiao G., Liang Y., He S., Lyu M., Zhu Y. Heart-brain interaction in cardiogenic dementia: Pathophysiology and therapeutic potential. Front. Cardiovasc. Med. 2024;11:1304864. doi: 10.3389/fcvm.2024.1304864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeed A., Lopez O., Cohen A., Reis S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart-Brain Axis. J. Am. Heart. Assoc. 2023;12:e030780. doi: 10.1161/JAHA.123.030780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osteraas N.D., Lee V.H. Neurocardiology. Handb. Clin. Neurol. 2017;140:49–65. doi: 10.1016/B978-0-444-63600-3.00004-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Venkat P., Seyfried D., Chopp M., Yan T., Chen J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ. Res. 2017;121:451–468. doi: 10.1161/CIRCRESAHA.117.311170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang S., Zhang W. Heart-Brain Axis: A Narrative Review of the Interaction between Depression and Arrhythmia. Biomedicines. 2024;12:1719. doi: 10.3390/biomedicines12081719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X., Gu J., Zhang X. Brain-Heart Axis and the Inflammatory Response: Connecting Stroke and Cardiac Dysfunction. Cardiology. 2024;149:369–382. doi: 10.1159/000538409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armando I., Seltzer A., Bregonzio C., Saavedra J.M. Stress and angiotensin II: Novel therapeutic opportunities. Curr. Drug Targets CNS Neurol. Disord. 2003;2:413–419. doi: 10.2174/1568007033482661. [DOI] [PubMed] [Google Scholar]
- 9.Lionetti V., Bollini S., Coppini R., Gerbino A., Ghigo A., Iaccarino G., Madonna R., Mangiacapra F., Miragoli M., Moccia F., et al. Understanding the heart-brain axis response in COVID-19 patients: A suggestive perspective for therapeutic development. Pharmacol. Res. 2021;168:105581. doi: 10.1016/j.phrs.2021.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley R.E., Kelley B.P. Heart-Brain Relationship in Stroke. Biomedicines. 2021;9:1835. doi: 10.3390/biomedicines9121835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M., Sun D., Wang Y., Yan M., Zheng J., Ren J. Cognitive Impairment in Heart Failure: Landscape, Challenges, and Future Directions. Front. Cardiovasc. Med. 2021;8:831734. doi: 10.3389/fcvm.2021.831734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doehner W., Celutkiene J., Yilmaz M.B., Coats A.J.S. Heart failure and the heart-brain axis. QJM. 2023;116:897–902. doi: 10.1093/qjmed/hcad179. [DOI] [PubMed] [Google Scholar]
- 13.Polidori M.C., Marvardi M., Cherubini A., Senin U., Mecocci P. Heart disease and vascular risk factors in the cognitively impaired elderly: Implications for Alzheimer’s dementia. Aging. 2001;13:231–239. doi: 10.1007/BF03351481. [DOI] [PubMed] [Google Scholar]
- 14.Cardiogenic Dementia. Lancet. 1977;1:27–28. [PubMed] [Google Scholar]
- 15.Malenka R.C., Bear M.F. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Hagena H., Manahan-Vaughan D. Interplay of hippocampal long-term potentiation and long-term depression in enabling memory representations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024;379:20230229. doi: 10.1098/rstb.2023.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luscher C., Malenka R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb. Perspect. Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sgritta M., Locatelli F., Soda T., Prestori F., D’Angelo E.U. Hebbian Spike-Timing Dependent Plasticity at the Cerebellar Input Stage. J. Neurosci. 2017;37:2809–2823. doi: 10.1523/JNEUROSCI.2079-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Angelo E. The organization of plasticity in the cerebellar cortex: From synapses to control. Prog. Brain Res. 2014;210:31–58. doi: 10.1016/B978-0-444-63356-9.00002-9. [DOI] [PubMed] [Google Scholar]
- 20.Bliss T.V., Collingridge G.L. Expression of NMDA receptor-dependent LTP in the hippocampus: Bridging the divide. Mol. Brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezra-Nevo G., Prestori F., Locatelli F., Soda T., Ten Brinke M.M., Engel M., Boele H.J., Botta L., Leshkowitz D., Ramot A., et al. Cerebellar Learning Properties Are Modulated by the CRF Receptor. J. Neurosci. 2018;38:6751–6765. doi: 10.1523/JNEUROSCI.3106-15.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locatelli F., Soda T., Montagna I., Tritto S., Botta L., Prestori F., D’Angelo E. Calcium Channel-Dependent Induction of Long-Term Synaptic Plasticity at Excitatory Golgi Cell Synapses of Cerebellum. J. Neurosci. 2021;41:3307–3319. doi: 10.1523/JNEUROSCI.3013-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yong J., Song J. CaMKII activity and metabolic imbalance-related neurological diseases: Focus on vascular dysfunction, synaptic plasticity, amyloid beta accumulation, and lipid metabolism. Biomed. Pharmacother. 2024;175:116688. doi: 10.1016/j.biopha.2024.116688. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda R., Hayashi Y., Hell J.W. CaMKII: A central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci. 2022;23:666–682. doi: 10.1038/s41583-022-00624-2. [DOI] [PubMed] [Google Scholar]
- 25.Brunetti V., Soda T., Berra-Romani R., De Sarro G., Guerra G., Scarpellino G., Moccia F. Two Signaling Modes Are Better than One: Flux-Independent Signaling by Ionotropic Glutamate Receptors Is Coming of Age. Biomedicines. 2024;12:880. doi: 10.3390/biomedicines12040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen K.B., Yi F., Perszyk R.E., Furukawa H., Wollmuth L.P., Gibb A.J., Traynelis S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018;150:1081–1105. doi: 10.1085/jgp.201812032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerchner G.A., Nicoll R.A. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newpher T.M., Ehlers M.D. Spine microdomains for postsynaptic signaling and plasticity. Trends. Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Grosshans D.R., Clayton D.A., Coultrap S.J., Browning M.D. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat. Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- 31.Harney S.C., Rowan M., Anwyl R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J. Neurosci. 2006;26:1128–1132. doi: 10.1523/JNEUROSCI.2753-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harney S.C., Jane D.E., Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J. Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harnett M.T., Bernier B.E., Ahn K.C., Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron. 2009;62:826–838. doi: 10.1016/j.neuron.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebola N., Lujan R., Cunha R.A., Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Kwon H.B., Castillo P.E. Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron. 2008;60:1082–1094. doi: 10.1016/j.neuron.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor J.J., Rowan M.J., Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: Investigations of the involvement of mGlu receptors. J. Neurosci. 1995;15:2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery J.M., Madison D.V. State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron. 2002;33:765–777. doi: 10.1016/S0896-6273(02)00606-2. [DOI] [PubMed] [Google Scholar]
- 38.Selig D.K., Hjelmstad G.O., Herron C., Nicoll R.A., Malenka R.C. Independent mechanisms for long-term depression of AMPA and NMDA responses. Neuron. 1995;15:417–426. doi: 10.1016/0896-6273(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 39.Gean P.W., Lin J.H. D-2-amino-5-phosphonovaleate blocks induction of long-term depression of the NMDA receptor-mediated synaptic component in rat hippocampus. Neurosci. Lett. 1993;158:170–172. doi: 10.1016/0304-3940(93)90256-K. [DOI] [PubMed] [Google Scholar]
- 40.Xiao M.Y., Wigstrom H., Gustafsson B. Long-term depression in the hippocampal CA1 region is associated with equal changes in AMPA and NMDA receptor-mediated synaptic potentials. Eur. J. Neurosci. 1994;6:1055–1057. doi: 10.1111/j.1460-9568.1994.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 41.Feldman D.E. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrade-Talavera Y., Fisahn A., Rodriguez-Moreno A. Timing to be precise? An overview of spike timing-dependent plasticity, brain rhythmicity, and glial cells interplay within neuronal circuits. Mol. Psychiatry. 2023;28:2177–2188. doi: 10.1038/s41380-023-02027-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appelbaum L.G., Shenasa M.A., Stolz L., Daskalakis Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology. 2023;48:113–120. doi: 10.1038/s41386-022-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H., Bramham C.R. Bidirectional Dysregulation of AMPA Receptor-Mediated Synaptic Transmission and Plasticity in Brain Disorders. Front. Synaptic. Neurosci. 2020;12:26. doi: 10.3389/fnsyn.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuestas Torres D.M., Cardenas F.P. Synaptic plasticity in Alzheimer’s disease and healthy aging. Rev. Neurosci. 2020;31:245–268. doi: 10.1515/revneuro-2019-0058. [DOI] [PubMed] [Google Scholar]
- 46.Soda T., Mapelli L., Locatelli F., Botta L., Goldfarb M., Prestori F., D’Angelo E. Hyperexcitability and Hyperplasticity Disrupt Cerebellar Signal Transfer in the IB2 KO Mouse Model of Autism. J. Neurosci. 2019;39:2383–2397. doi: 10.1523/JNEUROSCI.1985-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mapelli L., Soda T., D’Angelo E., Prestori F. The Cerebellar Involvement in Autism Spectrum Disorders: From the Social Brain to Mouse Models. Int. J. Mol. Sci. 2022;23:3894. doi: 10.3390/ijms23073894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozkurt B., Coats A.J.S., Tsutsui H., Abdelhamid C.M., Adamopoulos S., Albert N., Anker S.D., Atherton J., Bohm M., Butler J., et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 49.Savarese G., Becher P.M., Lund L.H., Seferovic P., Rosano G.M.C., Coats A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023;118:3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 50.Mene-Afejuku T.O., Pernia M., Ibebuogu U.N., Chaudhari S., Mushiyev S., Visco F., Pekler G. Heart Failure and Cognitive Impairment: Clinical Relevance and Therapeutic Considerations. Curr. Cardiol. Rev. 2019;15:291–303. doi: 10.2174/1573403X15666190313112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sauve M.J., Lewis W.R., Blankenbiller M., Rickabaugh B., Pressler S.J. Cognitive impairments in chronic heart failure: A case controlled study. J. Card. Fail. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Lossnitzer N., Herzog W., Stork S., Wild B., Muller-Tasch T., Lehmkuhl E., Zugck C., Regitz-Zagrosek V., Pankuweit S., Maisch B., et al. Incidence rates and predictors of major and minor depression in patients with heart failure. Int. J. Cardiol. 2013;167:502–507. doi: 10.1016/j.ijcard.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 53.David H., Ughetto A., Gaudard P., Plawecki M., Paiyabhroma N., Zub E., Colson P., Richard S., Marchi N., Sicard P. Experimental Myocardial Infarction Elicits Time-Dependent Patterns of Vascular Hypoxia in Peripheral Organs and in the Brain. Front. Cardiovasc. Med. 2020;7:615507. doi: 10.3389/fcvm.2020.615507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vallabhajosyula S., Dunlay S.M., Prasad A., Kashani K., Sakhuja A., Gersh B.J., Jaffe A.S., Holmes D.R., Jr., Barsness G.W. Acute Noncardiac Organ Failure in Acute Myocardial Infarction with Cardiogenic Shock. J. Am. Coll. Cardiol. 2019;73:1781–1791. doi: 10.1016/j.jacc.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 55.Toledo C., Andrade D.C., Diaz H.S., Inestrosa N.C., Del Rio R. Neurocognitive Disorders in Heart Failure: Novel Pathophysiological Mechanisms Underpinning Memory Loss and Learning Impairment. Mol. Neurobiol. 2019;56:8035–8051. doi: 10.1007/s12035-019-01655-0. [DOI] [PubMed] [Google Scholar]
- 56.Diaz H.S., Toledo C., Andrade D.C., Marcus N.J., Del Rio R. Neuroinflammation in heart failure: New insights for an old disease. J. Physiol. 2020;598:33–59. doi: 10.1113/JP278864. [DOI] [PubMed] [Google Scholar]
- 57.Woo M.A., Macey P.M., Fonarow G.C., Hamilton M.A., Harper R.M. Regional brain gray matter loss in heart failure. J. Appl. Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 58.Lu Z., Teng Y., Wang L., Jiang Y., Li T., Chen S., Wang B., Li Y., Yang J., Wu X., et al. Abnormalities of hippocampus and frontal lobes in heart failure patients and animal models with cognitive impairment or depression: A systematic review. PLoS ONE. 2022;17:e0278398. doi: 10.1371/journal.pone.0278398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Negri S., Faris P., Soda T., Moccia F. Endothelial signaling at the core of neurovascular coupling: The emerging role of endothelial inward-rectifier K+ (Kir2.1) channels and N-methyl-d-aspartate receptors in the regulation of cerebral blood flow. Int. J. Biochem. Cell Biol. 2021;135:105983. doi: 10.1016/j.biocel.2021.105983. [DOI] [PubMed] [Google Scholar]
- 60.Engl E., Attwell D. Non-signalling energy use in the brain. J. Physiol. 2015;593:3417–3429. doi: 10.1113/jphysiol.2014.282517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hofmeijer J., van Putten M.J. Ischemic cerebral damage: An appraisal of synaptic failure. Stroke. 2012;43:607–615. doi: 10.1161/STROKEAHA.111.632943. [DOI] [PubMed] [Google Scholar]
- 62.Cui C., Jiang X., Wang Y., Li C., Lin Z., Wei Y., Ni Q. Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review. Cell. Mol. Neurobiol. 2024;44:58. doi: 10.1007/s10571-024-01491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofmeijer J., Mulder A.T., Farinha A.C., van Putten M.J., le Feber J. Mild hypoxia affects synaptic connectivity in cultured neuronal networks. Brain Res. 2014;1557:180–189. doi: 10.1016/j.brainres.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 64.le Feber J., Erkamp N., van Putten M., Hofmeijer J. Loss and recovery of functional connectivity in cultured cortical networks exposed to hypoxia. J. Neurophysiol. 2017;118:394–403. doi: 10.1152/jn.00098.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Althammer F., Roy R.K., Kirchner M.K., Campos-Lira E., Whitley K.E., Davis S., Montanez J., Ferreira-Neto H.C., Danh J., Feresin R., et al. Angiotensin II-Mediated Neuroinflammation in the Hippocampus Contributes to Neuronal Deficits and Cognitive Impairment in Heart Failure Rats. Hypertension. 2023;80:1258–1273. doi: 10.1161/HYPERTENSIONAHA.123.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Z., Orchard S.G., Nelson M.R., Fravel M.A., Ernst M.E. Angiotensin Receptor Blockers and Cognition: A Scoping Review. Curr. Hypertens. Rep. 2024;26:1–19. doi: 10.1007/s11906-023-01266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wayner M.J., Armstrong D.L., Polan-Curtain J.L., Denny J.B. Role of angiotensin II and AT1 receptors in hippocampal LTP. Pharmacol. Biochem. Behav. 1993;45:455–464. doi: 10.1016/0091-3057(93)90265-U. [DOI] [PubMed] [Google Scholar]
- 68.Scheinman S.B., Tseng K.Y., Alford S., Tai L.M. Higher Neuronal Facilitation and Potentiation with APOE4 Suppressed by Angiotensin II. Mol. Neurobiol. 2024;61:120–131. doi: 10.1007/s12035-023-03556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tucsek Z., Noa Valcarcel-Ares M., Tarantini S., Yabluchanskiy A., Fulop G., Gautam T., Orock A., Csiszar A., Deak F., Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: Implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J., Wei X., Wu X., Zhang Q., Xia G., Xia H., Shang H., Lin S. Disorder of neuroplasticity aggravates cognitive impairment via neuroinflammation associated with intestinal flora dysbiosis in chronic heart failure. Aging. 2024;16:10882–10904. doi: 10.18632/aging.205960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song J. BDNF Signaling in Vascular Dementia and Its Effects on Cerebrovascular Dysfunction, Synaptic Plasticity, and Cholinergic System Abnormality. J. Lipid Atheroscler. 2024;13:122–138. doi: 10.12997/jla.2024.13.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo W., Nagappan G., Lu B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev. Neurobiol. 2018;78:647–659. doi: 10.1002/dneu.22592. [DOI] [PubMed] [Google Scholar]
- 73.Johnstone A., Mobley W. Local TrkB signaling: Themes in development and neural plasticity. Cell Tissue Res. 2020;382:101–111. doi: 10.1007/s00441-020-03278-7. [DOI] [PubMed] [Google Scholar]
- 74.Parent M.B., Ferreira-Neto H.C., Kruemmel A.R., Althammer F., Patel A.A., Keo S., Whitley K.E., Cox D.N., Stern J.E. Heart failure impairs mood and memory in male rats and down-regulates the expression of numerous genes important for synaptic plasticity in related brain regions. Behav. Brain Res. 2021;414:113452. doi: 10.1016/j.bbr.2021.113452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L., Lu Z., Teng Y., Pan W., Li Y., Su S., Chang J., Zhao M. Cognitive impairment is associated with BDNF-TrkB signaling mediating synaptic damage and reduction of amino acid neurotransmitters in heart failure. FASEB J. 2024;38:e23351. doi: 10.1096/fj.202301699RR. [DOI] [PubMed] [Google Scholar]
- 76.Potapenko E.S., Biancardi V.C., Florschutz R.M., Ryu P.D., Stern J.E. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J. Neurophysiol. 2011;106:1545–1557. doi: 10.1152/jn.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manolis A.A., Manolis T.A., Manolis A.S. Neurohumoral Activation in Heart Failure. Int. J. Mol. Sci. 2023;24:15472. doi: 10.3390/ijms242015472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W., Xiong B.R., Zhang L.Q., Huang X., Yuan X., Tian Y.K., Tian X.B. The Role of the GABAergic System in Diseases of the Central Nervous System. Neuroscience. 2021;470:88–99. doi: 10.1016/j.neuroscience.2021.06.037. [DOI] [PubMed] [Google Scholar]
- 79.Vico Varela E., Etter G., Williams S. Excitatory-inhibitory imbalance in Alzheimer’s disease and therapeutic significance. Neurobiol. Dis. 2019;127:605–615. doi: 10.1016/j.nbd.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 80.Allen P., Sommer I.E., Jardri R., Eysenck M.W., Hugdahl K. Extrinsic and default mode networks in psychiatric conditions: Relationship to excitatory-inhibitory transmitter balance and early trauma. Neurosci. Biobehav. Rev. 2019;99:90–100. doi: 10.1016/j.neubiorev.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Rojas M., Chavez-Castillo M., Pirela D., Parra H., Nava M., Chacin M., Angarita L., Anez R., Salazar J., Ortiz R., et al. Metabolic Syndrome: Is It Time to Add the Central Nervous System? Nutrients. 2021;13:2254. doi: 10.3390/nu13072254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kouvari M., D’Cunha N.M., Travica N., Sergi D., Zec M., Marx W., Naumovski N. Metabolic Syndrome, Cognitive Impairment and the Role of Diet: A Narrative Review. Nutrients. 2022;14:333. doi: 10.3390/nu14020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 84.Saklayen M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 86.Song J. Amygdala activity and amygdala-hippocampus connectivity: Metabolic diseases, dementia, and neuropsychiatric issues. Biomed. Pharmacother. 2023;162:114647. doi: 10.1016/j.biopha.2023.114647. [DOI] [PubMed] [Google Scholar]
- 87.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pacholko A., Iadecola C. Hypertension, Neurodegeneration, and Cognitive Decline. Hypertension. 2024;81:991–1007. doi: 10.1161/HYPERTENSIONAHA.123.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baggeroer C.E., Cambronero F.E., Savan N.A., Jefferson A.L., Santisteban M.M. Basic Mechanisms of Brain Injury and Cognitive Decline in Hypertension. Hypertension. 2024;81:34–44. doi: 10.1161/HYPERTENSIONAHA.123.19939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mone P., Pansini A., Calabro F., De Gennaro S., Esposito M., Rinaldi P., Colin A., Minicucci F., Coppola A., Frullone S., et al. Global cognitive function correlates with P-wave dispersion in frail hypertensive older adults. J. Clin. Hypertens. 2022;24:638–643. doi: 10.1111/jch.14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ungvari Z., Toth P., Tarantini S., Prodan C.I., Sorond F., Merkely B., Csiszar A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021;17:639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moccia F., Negri S., Faris P., Angelone T. Targeting endothelial ion signalling to rescue cerebral blood flow in cerebral disorders. Vascul. Pharmacol. 2022;145:106997. doi: 10.1016/j.vph.2022.106997. [DOI] [PubMed] [Google Scholar]
- 93.Westhoff T.H., Schubert F., Wirth C., Joppke M., Klar A.A., Zidek W., Gallinat J. The impact of blood pressure on hippocampal glutamate and mnestic function. J. Hum. Hypertens. 2011;25:256–261. doi: 10.1038/jhh.2010.51. [DOI] [PubMed] [Google Scholar]
- 94.Kelly A., Conroy S., Lynch M.A. Evidence that nerve growth factor plays a role in long-term potentiation in the rat dentate gyrus. Neuropharmacology. 1998;37:561–570. doi: 10.1016/S0028-3908(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 95.Kelly A., Mullany P.M., Lynch M.A. Protein synthesis in entorhinal cortex and long-term potentiation in dentate gyrus. Hippocampus. 2000;10:431–437. doi: 10.1002/1098-1063(2000)10:4<431::AID-HIPO9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 96.Hennigan A., Callaghan C.K., Kealy J., Rouine J., Kelly A.M. Deficits in LTP and recognition memory in the genetically hypertensive rat are associated with decreased expression of neurotrophic factors and their receptors in the dentate gyrus. Behav. Brain Res. 2009;197:371–377. doi: 10.1016/j.bbr.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 97.Dai H.L., Hu W.Y., Jiang L.H., Li L., Gaung X.F., Xiao Z.C. p38 MAPK Inhibition Improves Synaptic Plasticity and Memory in Angiotensin II-dependent Hypertensive Mice. Sci. Rep. 2016;6:27600. doi: 10.1038/srep27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noriega-Prieto J.A., Maglio L.E., Perez-Domper P., Davila J.C., Gutierrez A., Torres-Aleman I., Fernandez de Sevilla D. Bidirectional modulation of synaptic transmission by insulin-like growth factor-I. Front. Cell. Neurosci. 2024;18:1390663. doi: 10.3389/fncel.2024.1390663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aleman A., Torres-Aleman I. Circulating insulin-like growth factor I and cognitive function: Neuromodulation throughout the lifespan. Prog. Neurobiol. 2009;89:256–265. doi: 10.1016/j.pneurobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 100.O’Riordan K., Gerstein H., Hullinger R., Burger C. The role of Homer1c in metabotropic glutamate receptor-dependent long-term potentiation. Hippocampus. 2014;24:1–6. doi: 10.1002/hipo.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bridi M., Schoch H., Florian C., Poplawski S.G., Banerjee A., Hawk J.D., Porcari G.S., Lejards C., Hahn C.G., Giese K.P., et al. Transcriptional corepressor SIN3A regulates hippocampal synaptic plasticity via Homer1/mGluR5 signaling. JCI Insight. 2020;5:e92385. doi: 10.1172/jci.insight.92385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramsey M.M., Weiner J.L., Moore T.P., Carter C.S., Sonntag W.E. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Thornton P.L., Ingram R.L., Sonntag W.E. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B106–B112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- 104.Correa S.A., Eales K.L. The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J. Signal Transduct. 2012;2012:649079. doi: 10.1155/2012/649079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jensen V., Rinholm J.E., Johansen T.J., Medin T., Storm-Mathisen J., Sagvolden T., Hvalby O., Bergersen L.H. N-methyl-D-aspartate receptor subunit dysfunction at hippocampal glutamatergic synapses in an animal model of attention-deficit/hyperactivity disorder. Neuroscience. 2009;158:353–364. doi: 10.1016/j.neuroscience.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 106.Medin T., Rinholm J.E., Owe S.G., Sagvolden T., Gjedde A., Storm-Mathisen J., Bergersen L.H. Low dopamine D5 receptor density in hippocampus in an animal model of attention-deficit/hyperactivity disorder (ADHD) Neuroscience. 2013;242:11–20. doi: 10.1016/j.neuroscience.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 107.Lee C.C., Wu D.Y., Chen S.Y., Lin Y.P., Lee T.M. Exercise intensities modulate cognitive function in spontaneously hypertensive rats through oxidative mediated synaptic plasticity in hippocampus. J. Cell. Mol. Med. 2021;25:8546–8557. doi: 10.1111/jcmm.16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perry G.M., Sagvolden T., Faraone S.V. Intraindividual variability (IIV) in an animal model of ADHD—The Spontaneously Hypertensive Rat. Behav. Brain Funct. 2010;6:56. doi: 10.1186/1744-9081-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sagvolden T., Johansen E.B., Woien G., Walaas S.I., Storm-Mathisen J., Bergersen L.H., Hvalby O., Jensen V., Aase H., Russell V.A., et al. The spontaneously hypertensive rat model of ADHD—The importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eckernas D., Hieronymus F., Carlsson T., Bergquist F. Acoustic white noise ameliorates reduced regional brain expression of CaMKII and DeltaFosB in the spontaneously hypertensive rat model of ADHD. IBRO Rep. 2019;6:31–39. doi: 10.1016/j.ibror.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marques D.M., Almeida A.S., Oliveira C.B.A., Machado A.C.L., Lara M.V.S., Porciuncula L.O. Delayed Outgrowth in Response to the BDNF and Altered Synaptic Proteins in Neurons From SHR Rats. Neurochem. Res. 2023;48:2424–2435. doi: 10.1007/s11064-023-03917-9. [DOI] [PubMed] [Google Scholar]
- 112.Zhang-James Y., DasBanerjee T., Sagvolden T., Middleton F.A., Faraone S.V. SLC9A9 mutations, gene expression, and protein-protein interactions in rat models of attention-deficit/hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:835–843. doi: 10.1002/ajmg.b.31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang-James Y., Middleton F.A., Sagvolden T., Faraone S.V. Differential expression of SLC9A9 and interacting molecules in the hippocampus of rat models for attention deficit/hyperactivity disorder. Dev. Neurosci. 2012;34:218–227. doi: 10.1159/000338813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park M., Penick E.C., Edwards J.G., Kauer J.A., Ehlers M.D. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 115.Foster W.J., Taylor H.B.C., Padamsey Z., Jeans A.F., Galione A., Emptage N.J. Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca2+ signaling. Sci. Signal. 2018;11:eaat9093. doi: 10.1126/scisignal.aat9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zuccolo E., Kheder D.A., Lim D., Perna A., Nezza F.D., Botta L., Scarpellino G., Negri S., Martinotti S., Soda T., et al. Glutamate triggers intracellular Ca2+ oscillations and nitric oxide release by inducing NAADP- and InsP3 -dependent Ca2+ release in mouse brain endothelial cells. J. Cell. Physiol. 2019;234:3538–3554. doi: 10.1002/jcp.26953. [DOI] [PubMed] [Google Scholar]
- 117.Pan X., Jiang T., Zhang L., Zheng H., Luo J., Hu X. Physical Exercise Promotes Novel Object Recognition Memory in Spontaneously Hypertensive Rats after Ischemic Stroke by Promoting Neural Plasticity in the Entorhinal Cortex. Front. Behav. Neurosci. 2017;11:185. doi: 10.3389/fnbeh.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng M., Cong J., Wu Y., Xie J., Wang S., Zhao Y., Zang X. Chronic Swimming Exercise Ameliorates Low-Soybean-Oil Diet-Induced Spatial Memory Impairment by Enhancing BDNF-Mediated Synaptic Potentiation in Developing Spontaneously Hypertensive Rats. Neurochem. Res. 2018;43:1047–1057. doi: 10.1007/s11064-018-2515-x. [DOI] [PubMed] [Google Scholar]
- 119.Rossier B.C., Bochud M., Devuyst O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology. 2017;32:112–125. doi: 10.1152/physiol.00026.2016. [DOI] [PubMed] [Google Scholar]
- 120.Barber T.M., Kyrou I., Randeva H.S., Weickert M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021;22:546. doi: 10.3390/ijms22020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanson P., Weickert M.O., Barber T.M. Obesity: Novel and unusual predisposing factors. Ther. Adv. Endocrinol. Metab. 2020;11:2042018820922018. doi: 10.1177/2042018820922018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beilharz J.E., Maniam J., Morris M.J. Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions. Nutrients. 2015;7:6719–6738. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Favieri F., Forte G., Casagrande M. The Executive Functions in Overweight and Obesity: A Systematic Review of Neuropsychological Cross-Sectional and Longitudinal Studies. Front. Psychol. 2019;10:2126. doi: 10.3389/fpsyg.2019.02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trevino S., Aguilar-Alonso P., Flores Hernandez J.A., Brambila E., Guevara J., Flores G., Lopez-Lopez G., Munoz-Arenas G., Morales-Medina J.C., Toxqui V., et al. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 2015;69:421–433. doi: 10.1002/syn.21832. [DOI] [PubMed] [Google Scholar]
- 125.Kendig M.D. Cognitive and behavioural effects of sugar consumption in rodents. A review. Appetite. 2014;80:41–54. doi: 10.1016/j.appet.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 126.Jauch-Chara K., Oltmanns K.M. Obesity--a neuropsychological disease? Systematic review and neuropsychological model. Prog. Neurobiol. 2014;114:84–101. doi: 10.1016/j.pneurobio.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Yeomans M.R. Adverse effects of consuming high fat-sugar diets on cognition: Implications for understanding obesity. Proc. Nutr. Soc. 2017;76:455–465. doi: 10.1017/S0029665117000805. [DOI] [PubMed] [Google Scholar]
- 128.Mackey-Alfonso S.E., Butler M.J., Taylor A.M., Williams-Medina A.R., Muscat S.M., Fu H., Barrientos R.M. Short-term high fat diet impairs memory, exacerbates the neuroimmune response, and evokes synaptic degradation via a complement-dependent mechanism in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2024;121:56–69. doi: 10.1016/j.bbi.2024.07.021. [DOI] [PubMed] [Google Scholar]
- 129.Abbott K.N., Arnott C.K., Westbrook R.F., Tran D.M.D. The effect of high fat, high sugar, and combined high fat-high sugar diets on spatial learning and memory in rodents: A meta-analysis. Neurosci. Biobehav. Rev. 2019;107:399–421. doi: 10.1016/j.neubiorev.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 130.Paulo S.L., Miranda-Lourenco C., Belo R.F., Rodrigues R.S., Fonseca-Gomes J., Tanqueiro S.R., Geraldes V., Rocha I., Sebastiao A.M., Xapelli S., et al. High Caloric Diet Induces Memory Impairment and Disrupts Synaptic Plasticity in Aged Rats. Curr. Issues Mol. Biol. 2021;43:2305–2319. doi: 10.3390/cimb43030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grant W.B. A Brief History of the Progress in Our Understanding of Genetics and Lifestyle, Especially Diet, in the Risk of Alzheimer’s Disease. J. Alzheimer’s. Dis. 2024;100:S165–S178. doi: 10.3233/JAD-240658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le Thuc O., Garcia-Caceres C. Obesity-induced inflammation: Connecting the periphery to the brain. Nat. Metab. 2024;6:1237–1252. doi: 10.1038/s42255-024-01079-8. [DOI] [PubMed] [Google Scholar]
- 133.Naomi R., Teoh S.H., Embong H., Balan S.S., Othman F., Bahari H., Yazid M.D. The Role of Oxidative Stress and Inflammation in Obesity and Its Impact on Cognitive Impairments-A Narrative Review. Antioxidants. 2023;12:1071. doi: 10.3390/antiox12051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moccia F., Negri S., Faris P., Berra-Romani R. Targeting the endothelial Ca2+ toolkit to rescue endothelial dysfunction in obesity associated-hypertension. Curr. Med. Chem. 2019;27:240–257. doi: 10.2174/0929867326666190905142135. [DOI] [PubMed] [Google Scholar]
- 135.Porter D., Faivre E., Flatt P.R., Holscher C., Gault V.A. Actions of incretin metabolites on locomotor activity, cognitive function and in vivo hippocampal synaptic plasticity in high fat fed mice. Peptides. 2012;35:1–8. doi: 10.1016/j.peptides.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 136.Valladolid-Acebes I., Merino B., Principato A., Fole A., Barbas C., Lorenzo M.P., Garcia A., Del Olmo N., Ruiz-Gayo M., Cano V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am. J. Physiol. Endocrinol. Metab. 2012;302:E396–E402. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- 137.Tarantini S., Valcarcel-Ares M.N., Yabluchanskiy A., Tucsek Z., Hertelendy P., Kiss T., Gautam T., Zhang X.A., Sonntag W.E., de Cabo R., et al. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Negri S., Faris P., Moccia F. Reactive Oxygen Species and Endothelial Ca2+ Signaling: Brothers in Arms or Partners in Crime? Int. J. Mol. Sci. 2021;22:9821. doi: 10.3390/ijms22189821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moccia F., Montagna D. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as a Sensor of Oxidative Stress in Cancer Cells. Cells. 2023;12:1261. doi: 10.3390/cells12091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morrison C.D., Pistell P.J., Ingram D.K., Johnson W.D., Liu Y., Fernandez-Kim S.O., White C.L., Purpera M.N., Uranga R.M., Bruce-Keller A.J., et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased Nrf2 signaling. J. Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valcarcel-Ares M.N., Tucsek Z., Kiss T., Giles C.B., Tarantini S., Yabluchanskiy A., Balasubramanian P., Gautam T., Galvan V., Ballabh P., et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:290–298. doi: 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karimi S.A., Komaki A., Salehi I., Sarihi A., Shahidi S. Role of group II metabotropic glutamate receptors (mGluR2/3) blockade on long-term potentiation in the dentate gyrus region of hippocampus in rats fed with high-fat diet. Neurochem. Res. 2015;40:811–817. doi: 10.1007/s11064-015-1531-3. [DOI] [PubMed] [Google Scholar]
- 143.Alzoubi K.H., Aleisa A.M., Alkadhi K.A. Impairment of long-term potentiation in the CA1, but not dentate gyrus, of the hippocampus in Obese Zucker rats: Role of calcineurin and phosphorylated CaMKII. J. Mol. Neurosci. 2005;27:337–346. doi: 10.1385/JMN:27:3:337. [DOI] [PubMed] [Google Scholar]
- 144.Sasaki-Hamada S., Hojyo Y., Mizumoto R., Koyama H., Yanagisawa S., Oka J.I. Cognitive and hippocampal synaptic profiles in monosodium glutamate-induced obese mice. Neurosci. Res. 2021;170:201–207. doi: 10.1016/j.neures.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Sasaki-Hamada S., Hojo Y., Koyama H., Otsuka H., Oka J. Changes in hippocampal synaptic functions and protein expression in monosodium glutamate-treated obese mice during development of glucose intolerance. Eur. J. Neurosci. 2015;41:1393–1401. doi: 10.1111/ejn.12891. [DOI] [PubMed] [Google Scholar]
- 146.Vouimba R.M., Bakoyiannis I., Ducourneau E.G., Maroun M., Ferreira G. Bidirectional modulation of hippocampal and amygdala synaptic plasticity by post-weaning obesogenic diet intake in male rats: Influence of the duration of diet exposure. Hippocampus. 2021;31:117–121. doi: 10.1002/hipo.23278. [DOI] [PubMed] [Google Scholar]
- 147.Lopez-Taboada I., Gonzalez-Pardo H., Conejo N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020;11:564413. doi: 10.3389/fpsyg.2020.564413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boitard C., Maroun M., Tantot F., Cavaroc A., Sauvant J., Marchand A., Laye S., Capuron L., Darnaudery M., Castanon N., et al. Juvenile obesity enhances emotional memory and amygdala plasticity through glucocorticoids. J. Neurosci. 2015;35:4092–4103. doi: 10.1523/JNEUROSCI.3122-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hernandez-Ramirez S., Osorio-Gomez D., Escobar M.L., Rodriguez-Duran L., Velasco M., Bermudez-Rattoni F., Hiriart M., Guzman-Ramos K.R. Catecholaminergic stimulation restores high-sucrose diet-induced hippocampal dysfunction. Psychoneuroendocrinology. 2021;127:105178. doi: 10.1016/j.psyneuen.2021.105178. [DOI] [PubMed] [Google Scholar]
- 150.Wang M., Yoon G., Song J., Jo J. Exendin-4 improves long-term potentiation and neuronal dendritic growth in vivo and in vitro obesity condition. Sci. Rep. 2021;11:8326. doi: 10.1038/s41598-021-87809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chellammal H.S.J., Hasan M.H., Kshirsagar R.P., Musukula V.K.R., Ramachandran D., Diwan P.V. Metformin inhibits cardiometabolic syndrome associated cognitive deficits in high fat diet rats. J. Diabetes Metab. Disord. 2022;21:1415–1426. doi: 10.1007/s40200-022-01074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gonzalez Olmo B.M., Bettes M.N., DeMarsh J.W., Zhao F., Askwith C., Barrientos R.M. Short-term high-fat diet consumption impairs synaptic plasticity in the aged hippocampus via IL-1 signaling. NPJ Sci. Food. 2023;7:35. doi: 10.1038/s41538-023-00211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kharroubi A.T., Darwish H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes. 2015;6:850–867. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagayach A., Bhaskar R., Ghosh S., Singh K.K., Han S.S., Sinha J.K. Advancing the understanding of diabetic encephalopathy through unravelling pathogenesis and exploring future treatment perspectives. Ageing Res. Rev. 2024;100:102450. doi: 10.1016/j.arr.2024.102450. [DOI] [PubMed] [Google Scholar]
- 155.Trudeau F., Gagnon S., Massicotte G. Hippocampal synaptic plasticity and glutamate receptor regulation: Influences of diabetes mellitus. Eur. J. Pharmacol. 2004;490:177–186. doi: 10.1016/j.ejphar.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 156.Reyes Bueno J.A., Sanchez-Guijo G., Raez P.D., Garcia-Arnes J.A., Garzon-Maldonado F.J., Castro V.S., de la Cruz-Cosme C., Alba-Linero C., Gutierrez-Bedmar M., Garcia-Casares N. Effects of Type 2 Diabetes on the Neuropsychological Profile in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2024;99:887–897. doi: 10.3233/JAD-230791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Majimbi M., McLenachan S., Nesbit M., Chen F.K., Lam V., Mamo J., Takechi R. In vivo retinal imaging is associated with cognitive decline, blood-brain barrier disruption and neuroinflammation in type 2 diabetic mice. Front. Endocrinol. 2023;14:1224418. doi: 10.3389/fendo.2023.1224418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nowaczewska M., Kaminska A., Kukulska-Pawluczuk B., Junik R., Pawlak-Osinska K. Effect of hyperglycemia on cerebral blood flow in patients with diabetes. Diabetes Res. Clin. Pract. 2019;153:1–5. doi: 10.1016/j.diabres.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 159.Ansari M.A., Rao M.S., Al-Jarallah A., Babiker F.M. Early time course of oxidative stress in hippocampal synaptosomes and cognitive loss following impaired insulin signaling in rats: Development of sporadic Alzheimer’s disease. Brain Res. 2023;1798:148134. doi: 10.1016/j.brainres.2022.148134. [DOI] [PubMed] [Google Scholar]
- 160.Ishrat T., Khan M.B., Hoda M.N., Yousuf S., Ahmad M., Ansari M.A., Ahmad A.S., Islam F. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav. Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 161.Ishrat T., Parveen K., Hoda M.N., Khan M.B., Yousuf S., Ansari M.A., Saleem S., Islam F. Effects of Pycnogenol and vitamin E on cognitive deficits and oxidative damage induced by intracerebroventricular streptozotocin in rats. Behav. Pharmacol. 2009;20:567–575. doi: 10.1097/FBP.0b013e32832c7125. [DOI] [PubMed] [Google Scholar]
- 162.Shoham S., Bejar C., Kovalev E., Weinstock M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp. Neurol. 2003;184:1043–1052. doi: 10.1016/j.expneurol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 163.Hristov M., Nankova A., Andreeva-Gateva P. Alterations of the glutamatergic system in diabetes mellitus. Metab. Brain Dis. 2024;39:321–333. doi: 10.1007/s11011-023-01299-z. [DOI] [PubMed] [Google Scholar]
- 164.Zhang S., Zhang Y., Wen Z., Yang Y., Bu T., Bu X., Ni Q. Cognitive dysfunction in diabetes: Abnormal glucose metabolic regulation in the brain. Front. Endocrinol. 2023;14:1192602. doi: 10.3389/fendo.2023.1192602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ortiz G.G., Huerta M., Gonzalez-Usigli H.A., Torres-Sanchez E.D., Delgado-Lara D.L., Pacheco-Moises F.P., Mireles-Ramirez M.A., Torres-Mendoza B.M., Moreno-Cih R.I., Velazquez-Brizuela I.E. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes. 2022;13:319–337. doi: 10.4239/wjd.v13.i4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Biessels G.J., Kamal A., Ramakers G.M., Urban I.J., Spruijt B.M., Erkelens D.W., Gispen W.H. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- 167.Chabot C., Massicotte G., Milot M., Trudeau F., Gagne J. Impaired modulation of AMPA receptors by calcium-dependent processes in streptozotocin-induced diabetic rats. Brain Res. 1997;768:249–256. doi: 10.1016/S0006-8993(97)00648-3. [DOI] [PubMed] [Google Scholar]
- 168.Kamal A., Biessels G.J., Urban I.J., Gispen W.H. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: Impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90:737–745. doi: 10.1016/S0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 169.Artola A., Kamal A., Ramakers G.M., Biessels G.J., Gispen W.H. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur. J. Neurosci. 2005;22:169–178. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 170.Biessels G.J., Kamal A., Urban I.J., Spruijt B.M., Erkelens D.W., Gispen W.H. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: Effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/S0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 171.Ansari M.A., Al-Jarallah A., Babiker F.A. Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment. Cells. 2023;12:1728. doi: 10.3390/cells12131728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sasaki-Hamada S., Sacai H., Oka J.I. Diabetes onset influences hippocampal synaptic plasticity in streptozotocin-treated rats. Neuroscience. 2012;227:293–304. doi: 10.1016/j.neuroscience.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 173.Iwai T., Suzuki M., Kobayashi K., Mori K., Mogi Y., Oka J. The influences of juvenile diabetes on memory and hippocampal plasticity in rats: Improving effects of glucagon-like peptide-1. Neurosci. Res. 2009;64:67–74. doi: 10.1016/j.neures.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 174.Otsuka H., Sasaki-Hamada S., Ishibashi H., Oka J.I. Hippocampal acetylcholine receptor activation-dependent long-term depression in streptozotocin-induced diabetic rats. Neurosci. Lett. 2024;822:137650. doi: 10.1016/j.neulet.2024.137650. [DOI] [PubMed] [Google Scholar]
- 175.Wang Y., Yang Y., Liu Y., Guo A., Zhang Y. Cognitive impairments in type 1 diabetes mellitus model mice are associated with synaptic protein disorders. Neurosci. Lett. 2022;777:136587. doi: 10.1016/j.neulet.2022.136587. [DOI] [PubMed] [Google Scholar]
- 176.Di Luca M., Ruts L., Gardoni F., Cattabeni F., Biessels G.J., Gispen W.H. NMDA receptor subunits are modified transcriptionally and post-translationally in the brain of streptozotocin-diabetic rats. Diabetologia. 1999;42:693–701. doi: 10.1007/s001250051217. [DOI] [PubMed] [Google Scholar]
- 177.Gardoni F., Kamal A., Bellone C., Biessels G.J., Ramakers G.M., Cattabeni F., Gispent W.H., Di Luca M. Effects of streptozotocin-diabetes on the hippocampal NMDA receptor complex in rats. J. Neurochem. 2002;80:438–447. doi: 10.1046/j.0022-3042.2001.00713.x. [DOI] [PubMed] [Google Scholar]
- 178.Kamal A., Biessels G.J., Gispen W.H., Ramakers G.M. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006;1073–1074:276–280. doi: 10.1016/j.brainres.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 179.Kamal A., Artola A., Biessels G.J., Gispen W.H., Ramakers G.M. Increased spike broadening and slow afterhyperpolarization in CA1 pyramidal cells of streptozotocin-induced diabetic rats. Neuroscience. 2003;118:577–583. doi: 10.1016/S0306-4522(02)00874-6. [DOI] [PubMed] [Google Scholar]
- 180.Franzon R., Chiarani F., Mendes R.H., Bello-Klein A., Wyse A.T. Dietary soy prevents brain Na+, K(+)-ATPase reduction in streptozotocin diabetic rats. Diabetes Res. Clin. Pract. 2005;69:107–112. doi: 10.1016/j.diabres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 181.Tapella L., Soda T., Mapelli L., Bortolotto V., Bondi H., Ruffinatti F.A., Dematteis G., Stevano A., Dionisi M., Ummarino S., et al. Deletion of calcineurin from GFAP-expressing astrocytes impairs excitability of cerebellar and hippocampal neurons through astroglial Na+/K+ ATPase. Glia. 2020;68:543–560. doi: 10.1002/glia.23737. [DOI] [PubMed] [Google Scholar]
- 182.Valastro B., Cossette J., Lavoie N., Gagnon S., Trudeau F., Massicotte G. Up-regulation of glutamate receptors is associated with LTP defects in the early stages of diabetes mellitus. Diabetologia. 2002;45:642–650. doi: 10.1007/s00125-002-0818-5. [DOI] [PubMed] [Google Scholar]
- 183.Yang J., Song Y., Wang H., Liu C., Li Z., Liu Y., Kong Y. Insulin treatment prevents the increase in D-serine in hippocampal CA1 area of diabetic rats. Am. J. Alzheimer’s Dis. Other Demen. 2015;30:201–208. doi: 10.1177/1533317514545379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li J., Zhang S., Zhang L., Wang R., Wang M. Effects of L-3-n-butylphthalide on cognitive dysfunction and NR2B expression in hippocampus of streptozotocin (STZ)-induced diabetic rats. Cell Biochem. Biophys. 2015;71:315–322. doi: 10.1007/s12013-014-0200-5. [DOI] [PubMed] [Google Scholar]
- 185.Kalalian-Moghaddam H., Baluchnejadmojarad T., Roghani M., Goshadrou F., Ronaghi A. Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2013;698:259–266. doi: 10.1016/j.ejphar.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 186.Ghaderi S., Gholipour P., Komaki A., Shahidi S., Seif F., Bahrami-Tapehebur M., Salehi I., Zarei M., Sarihi A., Rashno M. Underlying mechanisms behind the neuroprotective effect of vanillic acid against diabetes-associated cognitive decline: An in vivo study in a rat model. Phytother. Res. 2024;38:1262–1277. doi: 10.1002/ptr.8111. [DOI] [PubMed] [Google Scholar]
- 187.Davari S., Talaei S.A., Alaei H., Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: Behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 188.Momeni Z., Bautista M., Neapetung J., Urban R., Yamamoto Y., Krishnan A., Campanucci V.A. RAGE signaling is required for AMPA receptor dysfunction in the hippocampus of hyperglycemic mice. Physiol. Behav. 2021;229:113255. doi: 10.1016/j.physbeh.2020.113255. [DOI] [PubMed] [Google Scholar]
- 189.Reisi P., Babri S., Alaei H., Sharifi M.R., Mohaddes G., Noorbakhsh S.M., Lashgari R. Treadmill running improves long-term potentiation (LTP) defects in streptozotocin-induced diabetes at dentate gyrus in rats. Pathophysiology. 2010;17:33–38. doi: 10.1016/j.pathophys.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 190.Poorgholam P., Yaghmaei P., Noureddini M., Hajebrahimi Z. Artemisin and human endometrial-derived stem cells improve cognitive function and synaptic plasticity in a rat model of Alzheimer disease and diabetes. Metab. Brain. Dis. 2023;38:1925–1936. doi: 10.1007/s11011-023-01200-y. [DOI] [PubMed] [Google Scholar]
- 191.Gerges N.Z., Aleisa A.M., Alkadhi K.A. Impaired long-term potentiation in obese zucker rats: Possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/S0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 192.Stranahan A.M., Norman E.D., Lee K., Cutler R.G., Telljohann R.S., Egan J.M., Mattson M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ismail T.R., Yap C.G., Naidu R., Pamidi N. Environmental Enrichment and Metformin Improve Metabolic Functions, Hippocampal Neuron Survival, and Hippocampal-Dependent Memory in High-Fat/High-Sucrose Diet-Induced Type 2 Diabetic Rats. Biology. 2023;12:480. doi: 10.3390/biology12030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Min J.A., Lee H.R., Kim J.I., Ju A., Kim D.J., Kaang B.K. Impairment of long-term potentiation in the hippocampus of alcohol-treated OLETF rats. Neurosci. Lett. 2011;500:52–56. doi: 10.1016/j.neulet.2011.05.239. [DOI] [PubMed] [Google Scholar]
- 195.Krishnan B., Sallam H.S., Tumurbataar B., Saieva S., Baymon D., Tuvdendorj D., Micci M.A., Abate N., Taglialatela G. Amelioration of hippocampal dysfunction by adipose tissue-targeted stem cell transplantation in a mouse model of type 2 diabetes. J. Neurochem. 2020;153:51–62. doi: 10.1111/jnc.14915. [DOI] [PubMed] [Google Scholar]
- 196.Gao M., Ji S., Li J., Zhang S. DL-3-n-butylphthalide (NBP) ameliorates cognitive deficits and CaMKII-mediated long-term potentiation impairment in the hippocampus of diabetic db/db mice. Neurol. Res. 2019;41:1024–1033. doi: 10.1080/01616412.2019.1672387. [DOI] [PubMed] [Google Scholar]
- 197.Tu L.L., Sun Q., Wei L.L., Shi J., Li J.P. Upregulation of GABA receptor promotes long-term potentiation and depotentiation in the hippocampal CA1 region of mice with type 2 diabetes mellitus. Exp. Ther. Med. 2019;18:2429–2436. doi: 10.3892/etm.2019.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yin H., Wang W., Yu W., Li J., Feng N., Wang L., Wang X. Changes in Synaptic Plasticity and Glutamate Receptors in Type 2 Diabetic KK-Ay Mice. J. Alzheimer’s. Dis. 2017;57:1207–1220. doi: 10.3233/JAD-160858. [DOI] [PubMed] [Google Scholar]
- 199.Nistico R., Cavallucci V., Piccinin S., Macri S., Pignatelli M., Mehdawy B., Blandini F., Laviola G., Lauro D., Mercuri N.B., et al. Insulin receptor beta-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. Neuromolecular. Med. 2012;14:262–269. doi: 10.1007/s12017-012-8184-z. [DOI] [PubMed] [Google Scholar]
- 200.Abbas T., Faivre E., Holscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav. Brain Res. 2009;205:265–271. doi: 10.1016/j.bbr.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 201.Khaledi N., Jeddi S., Abbasi S., Eftekharzadeh M., Khodadadi H., Namdari M., Noye Tuplin E. The impact of early-life exercise on CREB-signaling pathway and hippocampus neuroplasticity in diabetic adult male rats; the study of developmental model. Neurol. Res. 2024;46:835–847. doi: 10.1080/01616412.2024.2359265. [DOI] [PubMed] [Google Scholar]
- 202.Wang H., Chen F., Du Y.F., Long Y., Reed M.N., Hu M., Suppiramaniam V., Hong H., Tang S.S. Targeted inhibition of RAGE reduces amyloid-beta influx across the blood-brain barrier and improves cognitive deficits in db/db mice. Neuropharmacology. 2018;131:143–153. doi: 10.1016/j.neuropharm.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 203.Youn Y.J., Kim S., Jeong H.J., Ah Y.M., Yu Y.M. Sodium-glucose cotransporter-2 inhibitors and their potential role in dementia onset and cognitive function in patients with diabetes mellitus: A systematic review and meta-analysis. Front. Neuroendocrinol. 2024;73:101131. doi: 10.1016/j.yfrne.2024.101131. [DOI] [PubMed] [Google Scholar]
- 204.Mei J., Li Y., Niu L., Liang R., Tang M., Cai Q., Xu J., Zhang D., Yin X., Liu X., et al. SGLT2 inhibitors: A novel therapy for cognitive impairment via multifaceted effects on the nervous system. Transl. Neurodegener. 2024;13:41. doi: 10.1186/s40035-024-00431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mone P., Lombardi A., Gambardella J., Pansini A., Macina G., Morgante M., Frullone S., Santulli G. Empagliflozin Improves Cognitive Impairment in Frail Older Adults With Type 2 Diabetes and Heart Failure With Preserved Ejection Fraction. Diabetes Care. 2022;45:1247–1251. doi: 10.2337/dc21-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mone P., Guerra G., Lombardi A., Illario M., Pansini A., Marro A., Frullone S., Taurino A., Sorriento D., Verri V., et al. Effects of SGLT2 inhibition via empagliflozin on cognitive and physical impairment in frail diabetic elders with chronic kidney disease. Pharmacol. Res. 2024;200:107055. doi: 10.1016/j.phrs.2023.107055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sa-Nguanmoo P., Tanajak P., Kerdphoo S., Jaiwongkam T., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017;333:43–50. doi: 10.1016/j.taap.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 208.Guerra G., Lucariello A., Perna A., Botta L., De Luca A., Moccia F. The Role of Endothelial Ca2+ Signaling in Neurovascular Coupling: A View from the Lumen. Int. J. Mol. Sci. 2018;19:938. doi: 10.3390/ijms19040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Longden T.A., Mughal A., Hennig G.W., Harraz O.F., Shui B., Lee F.K., Lee J.C., Reining S., Kotlikoff M.I., Konig G.M., et al. Local IP3 receptor-mediated Ca2+ signals compound to direct blood flow in brain capillaries. Sci. Adv. 2021;7:eabh0101. doi: 10.1126/sciadv.abh0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Freeman K., Sackheim A., Mughal A., Koide M., Bonson G., Ebner G., Hennig G., Lockette W., Nelson M.T. Pathogenic soluble tau peptide disrupts endothelial calcium signaling and vasodilation in the brain microvasculature. bioRxiv. 2023 doi: 10.1101/2023.08.08.552492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Longden T.A., Dabertrand F., Koide M., Gonzales A.L., Tykocki N.R., Brayden J.E., Hill-Eubanks D., Nelson M.T. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Negri S., Faris P., Pellavio G., Botta L., Orgiu M., Forcaia G., Sancini G., Laforenza U., Moccia F. Group 1 metabotropic glutamate receptors trigger glutamate-induced intracellular Ca2+ signals and nitric oxide release in human brain microvascular endothelial cells. Cell. Mol. Life Sci. 2020;77:2235–2253. doi: 10.1007/s00018-019-03284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peters E.C., Gee M.T., Pawlowski L.N., Kath A.M., Polk F.D., Vance C.J., Sacoman J.L., Pires P.W. Amyloid-beta disrupts unitary calcium entry through endothelial NMDA receptors in mouse cerebral arteries. J. Cereb. Blood Flow Metab. 2021;42:145–161. doi: 10.1177/0271678X211039592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.LeMaistre J.L., Sanders S.A., Stobart M.J., Lu L., Knox J.D., Anderson H.D., Anderson C.M. Coactivation of NMDA receptors by glutamate and D-serine induces dilation of isolated middle cerebral arteries. J. Cereb. Blood Flow Metab. 2012;32:537–547. doi: 10.1038/jcbfm.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Negri S., Faris P., Maniezzi C., Pellavio G., Spaiardi P., Botta L., Laforenza U., Biella G., Moccia D.F. NMDA receptors elicit flux-independent intracellular Ca2+ signals via metabotropic glutamate receptors and flux-dependent nitric oxide release in human brain microvascular endothelial cells. Cell Calcium. 2021;99:102454. doi: 10.1016/j.ceca.2021.102454. [DOI] [PubMed] [Google Scholar]
- 216.Moccia F., Brunetti V., Soda T., Berra-Romani R., Scarpellino G. Cracking the Endothelial Calcium (Ca2+) Code: A Matter of Timing and Spacing. Int. J. Mol. Sci. 2023;24:16765. doi: 10.3390/ijms242316765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wilson C., Zhang X., Lee M.D., MacDonald M., Heathcote H.R., Alorfi N.M.N., Buckley C., Dolan S., McCarron J.G. Disrupted Endothelial Cell Heterogeneity and Network Organization Impair Vascular Function in Prediabetic Obesity. Metabolism. 2020;111:154340. doi: 10.1016/j.metabol.2020.154340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Berra-Romani R., Guzman-Silva A., Vargaz-Guadarrama A., Flores-Alonso J.C., Alonso-Romero J., Trevino S., Sanchez-Gomez J., Coyotl-Santiago N., Garcia-Carrasco M., Moccia F. Type 2 Diabetes Alters Intracellular Ca2+ Handling in Native Endothelium of Excised Rat Aorta. Int. J. Mol. Sci. 2019;21:250. doi: 10.3390/ijms21010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alaaeddine R., Elkhatib M.A.W., Mroueh A., Fouad H., Saad E.I., El-Sabban M.E., Plane F., El-Yazbi A.F. Impaired Endothelium-Dependent Hyperpolarization Underlies Endothelial Dysfunction during Early Metabolic Challenge: Increased ROS Generation and Possible Interference with NO Function. J. Pharmacol. Exp. Ther. 2019;371:567–582. doi: 10.1124/jpet.119.262048. [DOI] [PubMed] [Google Scholar]
- 220.Matsumoto T., Kobayashi S., Ando M., Watanabe S., Iguchi M., Taguchi K., Kobayashi T. Impaired endothelium-derived hyperpolarization-type relaxation in superior mesenteric arteries isolated from female Otsuka Long-Evans Tokushima Fatty rats. Eur. J. Pharmacol. 2017;807:151–158. doi: 10.1016/j.ejphar.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 221.Moccia F., Brunetti V., Perna A., Guerra G., Soda T., Berra-Romani R. The Molecular Heterogeneity of Store-Operated Ca2+ Entry in Vascular Endothelial Cells: The Different roles of Orai1 and TRPC1/TRPC4 Channels in the Transition from Ca2+-Selective to Non-Selective Cation Currents. Int. J. Mol. Sci. 2023;24:3259. doi: 10.3390/ijms24043259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hakim M.A., Behringer E.J. Methyl-Beta-Cyclodextrin Restores KIR Channel Function in Brain Endothelium of Female Alzheimer’s Disease Mice. J. Alzheimer’s. Dis. Rep. 2021;5:693–703. doi: 10.3233/ADR-210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hakim M.A., Behringer E.J. K(IR) channel regulation of electrical conduction along cerebrovascular endothelium: Enhanced modulation during Alzheimer’s disease. Microcirculation. 2023;30:e12797. doi: 10.1111/micc.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lacalle-Aurioles M., Trigiani L.J., Bourourou M., Lecrux C., Hamel E. Alzheimer’s disease and cerebrovascular pathology alter brain endothelial inward rectifier potassium (KIR 2.1) channels. Br. J. Pharmacol. 2022;179:2259–2274. doi: 10.1111/bph.15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Taylor J.L., Martin-Aragon Baudel M., Nieves-Cintron M., Navedo M.F. Vascular Function and Ion Channels in Alzheimer’s Disease. Microcirculation. 2024;31:e12881. doi: 10.1111/micc.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hopper R.A., Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J. Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Garthwaite G., Bartus K., Malcolm D., Goodwin D., Kollb-Sielecka M., Dooldeniya C., Garthwaite J. Signaling from blood vessels to CNS axons through nitric oxide. J. Neurosci. 2006;26:7730–7740. doi: 10.1523/JNEUROSCI.1528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang H.Y., Takagi H., Stoney P.N., Echeverria A., Kuhn B., Hsu K.S., Takahashi T. Anoxia-induced hippocampal LTP is regeneratively produced by glutamate and nitric oxide from the neuro-glial-endothelial axis. iScience. 2024;27:109515. doi: 10.1016/j.isci.2024.109515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Soda T., Brunetti V., Berra-Romani R., Moccia F. The Emerging Role of N-Methyl-D-Aspartate (NMDA) Receptors in the Cardiovascular System: Physiological Implications, Pathological Consequences, and Therapeutic Perspectives. Int. J. Mol. Sci. 2023;24:3914. doi: 10.3390/ijms24043914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Doreulee N., Sergeeva O.A., Yanovsky Y., Chepkova A.N., Selbach O., Godecke A., Schrader J., Haas H.L. Cortico-striatal synaptic plasticity in endothelial nitric oxide synthase deficient mice. Brain Res. 2003;964:159–163. doi: 10.1016/S0006-8993(02)04121-5. [DOI] [PubMed] [Google Scholar]
- 231.Fekete M., Liotta E.M., Molnar T., Fulop G.A., Lehoczki A. The role of atrial fibrillation in vascular cognitive impairment and dementia: Epidemiology, pathophysiology, and preventive strategies. GeroScience. 2024 doi: 10.1007/s11357-024-01290-1. [DOI] [PubMed] [Google Scholar]
- 232.Varrias D., Saralidze T., Borkowski P., Pargaonkar S., Spanos M., Bazoukis G., Kokkinidis D. Atrial Fibrillation and Dementia: Pathophysiological Mechanisms and Clinical Implications. Biomolecules. 2024;14:455. doi: 10.3390/biom14040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kogelschatz B., Zenger B., Steinberg B.A., Ranjan R., Jared Bunch T. Atrial fibrillation and the risk of early-onset dementia and cognitive decline: An updated review. Trends Cardiovasc. Med. 2024;34:236–241. doi: 10.1016/j.tcm.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 234.Srinivas S., Vignesh Rk B., Ayinapudi V.N., Govindarajan A., Sundaram S.S., Priyathersini N. Neurological Consequences of Cardiac Arrhythmias: Relationship Between Stroke, Cognitive Decline, and Heart Rhythm Disorders. Cureus. 2024;16:e57159. doi: 10.7759/cureus.57159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lions S., Dragu R., Carsenty Y., Zukermann R., Aronson D. Determinants of cardiac repolarization and risk for ventricular arrhythmias during mild therapeutic hypothermia. J. Crit. Care. 2018;46:151–156. doi: 10.1016/j.jcrc.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 236.Keefe J.A., Hulsurkar M.M., Reilly S., Wehrens X.H.T. Mouse models of spontaneous atrial fibrillation. Mamm. Genome. 2023;34:298–311. doi: 10.1007/s00335-022-09964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nguyen M.N., Kiriazis H., Gao X.M., Du X.J. Cardiac Fibrosis and Arrhythmogenesis. Compr. Physiol. 2017;7:1009–1049. doi: 10.1002/cphy.c160046. [DOI] [PubMed] [Google Scholar]
- 238.Moccia F., Lodola F., Stadiotti I., Pilato C.A., Bellin M., Carugo S., Pompilio G., Sommariva E., Maione A.S. Calcium as a Key Player in Arrhythmogenic Cardiomyopathy: Adhesion Disorder or Intracellular Alteration? Int. J. Mol. Sci. 2019;20:3986. doi: 10.3390/ijms20163986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maione A.S., Faris P., Iengo L., Catto V., Bisonni L., Lodola F., Negri S., Casella M., Guarino A., Polvani G., et al. Ca2+ dysregulation in cardiac stromal cells sustains fibro-adipose remodeling in Arrhythmogenic Cardiomyopathy and can be modulated by flecainide. J. Transl. Med. 2022;20:522. doi: 10.1186/s12967-022-03742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moccia F., Brunetti V., Soda T., Faris P., Scarpellino G., Berra-Romani R. Store-Operated Ca2+ Entry as a Putative Target of Flecainide for the Treatment of Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2023;12:5295. doi: 10.3390/jcm12165295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Marks A.R. Targeting ryanodine receptors to treat human diseases. J. Clin. Investig. 2023;133:e16289. doi: 10.1172/JCI162891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Splawski I., Timothy K.W., Sharpe L.M., Decher N., Kumar P., Bloise R., Napolitano C., Schwartz P.J., Joseph R.M., Condouris K., et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 243.Eisner D.A., Caldwell J.L., Kistamas K., Trafford A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bhat S., Dao D.T., Terrillion C.E., Arad M., Smith R.J., Soldatov N.M., Gould T.D. CACNA1C (Cav1.2) in the pathophysiology of psychiatric disease. Prog. Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kamijo S., Ishii Y., Horigane S.I., Suzuki K., Ohkura M., Nakai J., Fujii H., Takemoto-Kimura S., Bito H. A Critical Neurodevelopmental Role for L-Type Voltage-Gated Calcium Channels in Neurite Extension and Radial Migration. J. Neurosci. 2018;38:5551–5566. doi: 10.1523/JNEUROSCI.2357-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sanderson J.L., Freund R.K., Castano A.M., Benke T.A., Dell’Acqua M.L. The Ca(V)1.2 G406R mutation decreases synaptic inhibition and alters L-type Ca2+ channel-dependent LTP at hippocampal synapses in a mouse model of Timothy Syndrome. Neuropharmacology. 2022;220:109271. doi: 10.1016/j.neuropharm.2022.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Servili E., Trus M., Sajman J., Sherman E., Atlas D. Elevated basal transcription can underlie timothy channel association with autism related disorders. Prog. Neurobiol. 2020;191:101820. doi: 10.1016/j.pneurobio.2020.101820. [DOI] [PubMed] [Google Scholar]
- 248.Servili E., Trus M., Atlas D. Ion occupancy of the channel pore is critical for triggering excitation-transcription (ET) coupling. Cell Calcium. 2019;84:102102. doi: 10.1016/j.ceca.2019.102102. [DOI] [PubMed] [Google Scholar]
- 249.Moccia F., Fiorio Pla A., Lim D., Lodola F., Gerbino A. Intracellular Ca2+ signalling: Unexpected new roles for the usual suspect. Front. Physiol. 2023;14:1210085. doi: 10.3389/fphys.2023.1210085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Greer P.L., Greenberg M.E. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 251.Wild A.R., Sinnen B.L., Dittmer P.J., Kennedy M.J., Sather W.A., Dell’Acqua M.L. Synapse-to-Nucleus Communication through NFAT Is Mediated by L-type Ca2+ Channel Ca2+ Spike Propagation to the Soma. Cell Rep. 2019;26:3537–3550.e3534. doi: 10.1016/j.celrep.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rajgor D., Purkey A.M., Sanderson J.L., Welle T.M., Garcia J.D., Dell’Acqua M.L., Smith K.R. Local miRNA-Dependent Translational Control of GABA(A)R Synthesis during Inhibitory Long-Term Potentiation. Cell Rep. 2020;31:107785. doi: 10.1016/j.celrep.2020.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Horigane S.I., Ozawa Y., Zhang J., Todoroki H., Miao P., Haijima A., Yanagawa Y., Ueda S., Nakamura S., Kakeyama M., et al. A mouse model of Timothy syndrome exhibits altered social competitive dominance and inhibitory neuron development. FEBS Open Bio. 2020;10:1436–1446. doi: 10.1002/2211-5463.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li G., Wang J., Liao P., Bartels P., Zhang H., Yu D., Liang M.C., Poh K.K., Yu C.Y., Jiang F., et al. Exclusion of alternative exon 33 of Ca(V)1.2 calcium channels in heart is proarrhythmogenic. Proc. Natl. Acad. Sci. USA. 2017;114:E4288–E4295. doi: 10.1073/pnas.1617205114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Navakkode S., Zhai J., Wong Y.P., Li G., Soong T.W. Enhanced long-term potentiation and impaired learning in mice lacking alternative exon 33 of Ca(V)1.2 calcium channel. Transl. Psychiatry. 2022;12:1. doi: 10.1038/s41398-021-01683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lim X.R., Abd-Alhaseeb M.M., Ippolito M., Koide M., Senatore A.J., Plante C., Hariharan A., Weir N., Longden T.A., Laprade K.A., et al. Endothelial Piezo1 channel mediates mechano-feedback control of brain blood flow. Nat. Commun. 2024;15:8686. doi: 10.1038/s41467-024-52969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Testai F.D., Gorelick P.B., Chuang P.Y., Dai X., Furie K.L., Gottesman R.F., Iturrizaga J.C., Lazar R.M., Russo A.M., Seshadri S., et al. Cardiac Contributions to Brain Health: A Scientific Statement From the American Heart Association. Stroke. 2024;55 doi: 10.1161/STR.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 258.Xu P., Chen A., Li Y., Xing X., Lu H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019;51:432–442. doi: 10.1152/physiolgenomics.00006.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ku P.H., Yang Y.R., Yeh N.C., Li P.Y., Lu C.F., Wang R.Y. Prefrontal activity and heart rate variability during cognitive tasks may show different changes in young and older adults with and without mild cognitive impairment. Front. Aging Neurosci. 2024;16:1392304. doi: 10.3389/fnagi.2024.1392304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Badaly D., Beers S.R., Ceschin R., Lee V.K., Sulaiman S., Zahner A., Wallace J., Berdaa-Sahel A., Burns C., Lo C.W., et al. Cerebellar and Prefrontal Structures Associated With Executive Functioning in Pediatric Patients With Congenital Heart Defects. Front. Neurol. 2022;13:827780. doi: 10.3389/fneur.2022.827780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sahel A., Ceschin R., Badaly D., Lewis M., Lee V.K., Wallace J., Weinberg J., Schmithorst V., Lo C., Panigrahy A. Increased Cerebello-Prefrontal Connectivity Predicts Poor Executive Function in Congenital Heart Disease. J. Clin. Med. 2023;12:5264. doi: 10.3390/jcm12165264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ichijo Y., Kono S., Yoshihisa A., Misaka T., Kaneshiro T., Oikawa M., Miura I., Yabe H., Takeishi Y. Impaired Frontal Brain Activity in Patients With Heart Failure Assessed by Near-Infrared Spectroscopy. J. Am. Heart Assoc. 2020;9:e014564. doi: 10.1161/JAHA.119.014564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Woo M.A., Palomares J.A., Macey P.M., Fonarow G.C., Harper R.M., Kumar R. Global and regional brain mean diffusivity changes in patients with heart failure. J. Neurosci. Res. 2015;93:678–685. doi: 10.1002/jnr.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bermudez C., Kerley C.I., Ramadass K., Farber-Eger E.H., Lin Y.C., Kang H., Taylor W.D., Wells Q.S., Landman B.A. Volumetric brain MRI signatures of heart failure with preserved ejection fraction in the setting of dementia. Magn. Reson. Imaging. 2024;109:49–55. doi: 10.1016/j.mri.2024.02.016. [DOI] [PubMed] [Google Scholar]
- 265.Cui Y., Tang T.Y., Lu C.Q., Ju S. Insulin Resistance and Cognitive Impairment: Evidence From Neuroimaging. J. Magn. Reson. Imaging. 2022;56:1621–1649. doi: 10.1002/jmri.28358. [DOI] [PubMed] [Google Scholar]
- 266.Gomez-Apo E., Mondragon-Maya A., Ferrari-Diaz M., Silva-Pereyra J. Structural Brain Changes Associated with Overweight and Obesity. J. Obes. 2021;2021:6613385. doi: 10.1155/2021/6613385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kitzbichler M.G., Martins D., Bethlehem R.A.I., Dear R., Romero-Garcia R., Warrier V., Seidlitz J., Dipasquale O., Turkheimer F., Cercignani M., et al. Two human brain systems micro-structurally associated with obesity. Elife. 2023;12:e85175. doi: 10.7554/eLife.85175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Solis-Urra P., Rodriguez-Ayllon M., Verdejo-Roman J., Erickson K.I., Verdejo-Garcia A., Catena A., Ortega F.B., Esteban-Cornejo I. Early life factors and structural brain network in children with overweight/obesity: The ActiveBrains project. Pediatr. Res. 2024;95:1812–1817. doi: 10.1038/s41390-023-02923-5. [DOI] [PubMed] [Google Scholar]
- 269.Silva D.S., Caseli B.G., de Campos B.M., Avelar W.M., Lino A., Balthazar M.L.F., Figueiredo M.J.O., Cendes F., Pegoraro L.F.L., Coan A.C. Cerebral Structure and Function in Stroke-free Patients with Atrial Fibrillation. J. Stroke Cerebrovasc. Dis. 2021;30:105887. doi: 10.1016/j.jstrokecerebrovasdis.2021.105887. [DOI] [PubMed] [Google Scholar]
- 270.Zhuang Y., Shi Y., Zhang J., Kong D., Guo L., Bo G., Feng Y. Neurologic Factors in Patients with Vascular Mild Cognitive Impairment Based on fMRI. World Neurosurg. 2021;149:461–469. doi: 10.1016/j.wneu.2020.11.120. [DOI] [PubMed] [Google Scholar]
- 271.Sachdev P.S., Chen X., Joscelyne A., Wen W., Brodaty H. Amygdala in stroke/transient ischemic attack patients and its relationship to cognitive impairment and psychopathology: The Sydney Stroke Study. Am. J. Geriatr. Psychiatry. 2007;15:487–496. doi: 10.1097/JGP.0b013e3180581fe6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data was created for this manuscript.