Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, and it is currently the seventh leading cause of death worldwide. It is characterized by the extracellular aggregation of the amyloid β-peptide (Aβ) into oligomers and fibrils that cause synaptotoxicity and neuronal death. Aβ exhibits a dual role in promoting oxidative stress and inflammation. This review aims to unravel the intricate connection between these processes and their contribution to AD progression. The review delves into oxidative stress in AD, focusing on the involvement of metals, mitochondrial dysfunction, and biomolecule oxidation. The distinct yet overlapping concept of nitro-oxidative stress is also discussed, detailing the roles of nitric oxide, mitochondrial perturbations, and their cumulative impact on Aβ production and neurotoxicity. Inflammation is examined through astroglia and microglia function, elucidating their response to Aβ and their contribution to oxidative stress within the AD brain. The blood–brain barrier and oligodendrocytes are also considered in the context of AD pathophysiology. We also review current diagnostic methodologies and emerging therapeutic strategies aimed at mitigating oxidative stress and inflammation, thereby offering potential treatments for halting or slowing AD progression. This comprehensive synthesis underscores the pivotal role of Aβ in bridging oxidative stress and inflammation, advancing our understanding of AD and informing future research and treatment paradigms.
Keywords: Alzheimer’s disease, amyloid β-peptide, neurodegeneration, nitro-oxidative stress, BACE1
1. Introduction
Alzheimer’s disease (AD) is a devastating condition that has reached pandemic proportions, with 55 million people affected in 2019 [1] and projections estimating that this number will rise to 139 million by 2050 [2].
AD diagnosis is mainly made by cognitive tests, brain imaging, and genetic analysis. It is classified into two main types: sporadic and familial. Sporadic AD is the most common form, accounting for most cases, and typically occurs in individuals after the age of 65 without a clear genetic link. Its onset is influenced by a combination of genetic, environmental, and lifestyle factors. Familial AD, on the other hand, is rarer and occurs in families with a history of the disease, often appearing at an earlier age (30s or 40s). This form is associated with specific genetic mutations, which will be discussed in this review.
There are no effective treatments for AD, despite the amyloid β-peptide being identified more than 40 years ago [3]. By the end of the 20th century, AD was characterized histopathologically and molecularly as a brain disease marked by extracellular senile plaques, composed mainly of aggregated Aβ, and intracellular neurofibrillary tangles made of tau.
The primary component of senile plaques is a peptide, consisting of 40 to 42 amino acids, termed amyloid-β [3] (from the Greek term amylon, meaning starch) due to its propensity to adopt a β-sheet conformation and aggregate into unbranched, twisted fibrils that have a starch-like affinity for iodine [4]. Similar characteristics are observed in various pathological systemic amyloids, such as transthyretin [5], amylin [5], or ABri and ADan peptides [6,7]. Regarding Aβ, it produces brain damage, increasing oxidative stress and inflammation, which are the main topics of this review.
2. Aβ Production
The amyloid precursor protein (APP) is an integral type I transmembrane glycoprotein present in most human cells. It has three major isoforms, with the largest one, containing 770 amino acids, being the most abundant in the brain [8]. In humans, APP coexists with other APP-like proteins, APLP1 and APLP2, which do not contain the Aβ sequence and appeared earlier in vertebrate evolution. These proteins are reported to regulate cell adhesion [8], among other functions. Specifically, APP has been implicated in synaptogenesis [9], axonal transport [10], and the regulation of the GABA receptor [11].
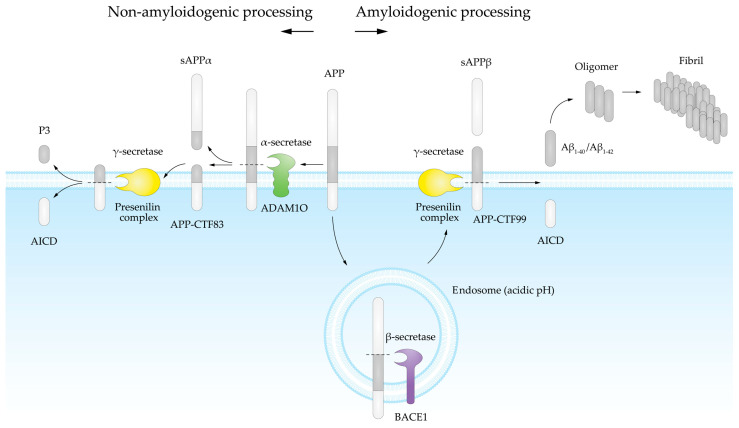
Aβ is produced through the enzymatic processing of APP [12,13] (Figure 1). When APP is cleaved by an α-secretase, Aβ is not produced, as this enzymatic activity cuts APP in the middle of the Aβ sequence (Figure 1). However, when APP is sequentially cleaved by a β-secretase and a γ-secretase, Aβ is released [14].
Figure 1.
Physiological APP cleavage pathways are depicted. The non-amyloidogenic pathway is shown on the left side of the figure. In this pathway, α-secretase cleaves APP, producing sAPPα and CTF83. Subsequently, CTF83 is cleaved by γ-secretase, releasing the P3 peptide extracellularly and AICD intracellularly. The amyloidogenic pathway is shown on the right side of the figure. This pathway involves β-secretase cleavage, which takes place mainly within the intracellular endosome pathway, thus producing sAPPβ and CTF99. CTF99 is then cleaved by γ-secretase at the cell membrane, releasing AICD intracellularly and Aβ extracellularly.
The amyloidogenic pathway begins with the cleavage of APP by β-secretase, specifically the β-site APP cleaving enzyme 1 (BACE1), a transmembrane aspartyl protease enzyme [15,16]. BACE1 has a homolog, BACE2, which exhibits minimal β-secretase activity and primarily functions outside the brain, playing roles in pigmentation and glucose homeostasis [17,18,19]. The β-secretase activity occurs mainly within the intracellular endosome pathway, where the acidic pH activates BACE1 [20,21,22]. Soluble APP-β (sAPPβ) is a byproduct of BACE1 cleavage, which does not induce neural progenitor cell proliferation [23].
Subsequently, the heterogeneous presenilin complex, consisting of presenilin (PSEN), nicastrin (NCT), presenilin enhancer 2 (PEN-2), and anterior pharynx defective 1 (APH1) [24,25,26], executes γ-secretase activity at the plasma membrane, releasing the Aβ fragment and the intracellular fragment APP intracellular domain (AICD). This process is similar to Notch proteolytical cleavage, in which the functional form of Notch is cleaved by the γ-secretase complex, releasing the intracellular fragment Notch intracellular domain (NICD). The fact that NICD has transcriptional regulatory activity in the nucleus [27] has prompted researchers to investigate the potential transcriptional function of AICD [28].
The variability in Aβ length is due to γ-secretase activity, with Aβ1-40 being the most common form and Aβ1-42 occurring to a lesser extent [29]. There are also forms ranging from 38 to 43 amino acids. The human Aβ1-43 amino acid sequence is:
DAEFR5HDSGY10EVHHQ15KLVFF20AEDVG25SNKSA30IIGLM35VGGVV40 IA42T43,
with the last three amino acids being critical for its aggregation tendency. Certain mutations also play a significant role in Aβ aggregation, mainly those located around the amino acid at position 20 [29,30,31,32,33].
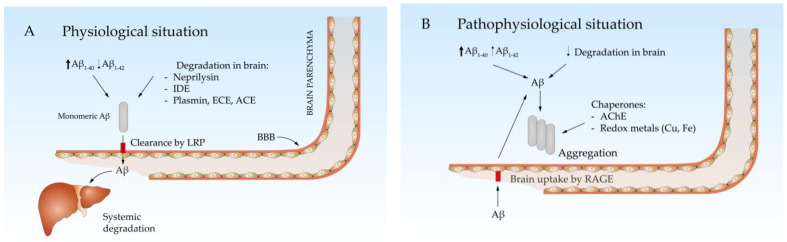
Both APP cleavage pathways occur physiologically from childhood onward. Aβ produced by various tissues circulates in the blood and is degraded mainly by the liver [34,35,36] (Figure 2A). In the brain, Aβ can be degraded by the insulin-degrading enzyme (IDE) [37], neprilysin (NEP) [38], and other enzymes such as plasmin [39], endothelin-converting enzyme (ECE) [40], or angiotensin-converting enzyme (ACE) [41]. The remaining Aβ is released into the blood through the blood–brain barrier (BBB) [42,43]. Aβ is transported from the cerebrospinal fluid (CSF) to the bloodstream via low-density lipoprotein receptor-related protein 1 (LRP1)- or very low-density lipoprotein receptor (VLDLR)-mediated transcytosis [44,45]. Both are membrane receptors that facilitate the movement of Aβ across the BBB by binding to it and transporting it through cells (transcytosis). This process helps clear Aβ from the brain, playing a critical role in maintaining brain homeostasis and preventing its accumulation.
Figure 2.
Physiological (A) and pathophysiological (B) brain Aβ equilibrium. (A) Aβ is predominantly produced as Aβ1-40 and can be degraded within the brain parenchyma or cleared to the blood via LRP, ultimately being degraded in the liver. (B) With age, the production of Aβ1-42 increases, while its degradation within the brain and clearance decrease. This leads to aggregation, facilitated by protein chaperones and redox-active metals.
With age, the concentration of Aβ in the brain increases due to various mechanisms. Aβ production is enhanced because of increased translation and activity of BACE1 [46] and presenilin [47] driven by nitro-oxidative stress, which is strongly associated with aging [48]. Additionally, the expression of NEP and IDE decreases with age [49,50] and LRPs become less efficient at removing Aβ [51]. In this scenario, the BBB acts as a dam that retains Aβ in the brain, leading to increased concentrations and favoring Aβ aggregation (Figure 2B). While monomeric Aβ is not only not neurotoxic but in fact has been proposed to play some physiological roles, such as regulating insulin signaling [52], its β-sheet conformation allows it to form oligomers and fibrils that are neurotoxic to both neurons and vascular cells in the brain [53,54,55]. Some extracellular molecules, such as transthyretin, clusterin (also termed Apolipoprotein J), and albumin, act as chaperones by binding monomeric Aβ and preventing its aggregation [56,57,58]. However, their function is impaired by certain polymorphisms, as seen with clusterin, where specific variants are considered risk factors for late-onset AD [59].
3. Synaptotoxicity and Neuronal Death
Histopathological and experimental evidence shows that oxidative stress [60,61,62,63] and inflammation [64,65] are directly involved in Aβ synaptoxicity and neuronal death. They will be discussed later in this review. It is also known that Aβ oligomers, given their small size and their hydrophobic nature due to their secondary structure of β-sheets, can interact with membrane proteins present in the synaptic cleft, altering their function.
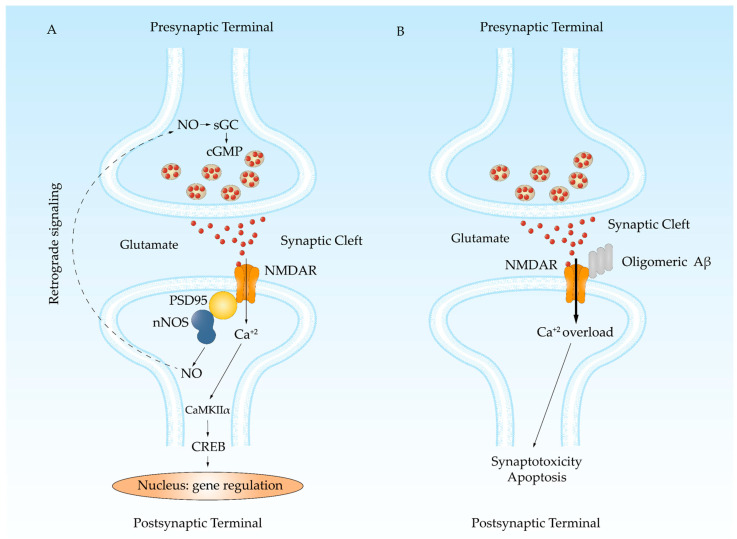
Among the different proteins with which oligomeric Aβ can interact, the NMDA receptor (NMDAR) stands out (Figure 3). It has been demonstrated in cultures of hippocampal neurons that stimulation with NMDA following oligomeric Aβ treatment prolongs the channel open state. This effect was not observed when cells were treated with Aβ oligomers and a high potassium solution, which activates voltage-dependent calcium channels [51]. This suggests that the oligomeric Aβ effect is specific to NMDAR activation, although it may not be entirely exclusive. Ultimately, Aβ oligomers cause greater calcium influx, which affects the reservoirs responsible for calcium homeostasis. It promotes ryanodine receptor (RyR)-mediated calcium release from the endoplasmic reticulum and mitochondrial calcium uptake [66], leading to mitochondrial dysfunction and reactive oxygen species (ROS) production [67,68]. All these processes generate an environment of low amplitude and long-lasting abnormal calcium signaling that promotes synaptotoxicity [69,70,71].
Figure 3.
NMDAR is a target for Aβ oligomers. (A) LTP allows memory formation by the continuous stimulation of the glutamatergic signaling. Glutamate increases calcium entrance activating nNOS. NO induces the release of glutamate by the presynaptic terminal. Calcium also activates CaMKIIα, that phosphorylates CREB, triggering the transcription of genes needed for synaptic spine growth. (B) Aβ oligomers bind to NMDAR impairing a proper closing, which produces a leak of calcium into the cell that induces synaptotoxicity and neuronal death.
Other proteins susceptible to the Aβ effects are the α-7-nicotinic receptors, which present high Aβ affinity, and become activated at picomolar concentrations of Aβ but moderately inhibited at higher concentrations [72,73]. This is supposed to contribute to the cholinergic deficit characteristic of AD [74,75], and the rationale basis for the use of anticholinesterase drugs that can increase the bioavailability of acetylcholine in the synaptic cleft. The degeneration of the cholinergic pathways proposed to be initiated in the nucleus basalis of Meynert (or nucleus basalis magnocellularis) could also be contributing significantly to the cholinergic deficit in AD [74,75].
Other mechanisms of Aβ toxicity have been proposed, such as the regulation of intracellular calcium by SURF4 throughout the store-operated calcium channel impairment [76] or the insertion of oligomers into membranes forming ion-permeable pores [77]. Nonetheless, it is difficult to consider that the possible formation of a pore has a relevant contribution to the general neuronal damage, since pores appear to be quite scarce, and dimers, which have been demonstrated to be highly toxic, cannot produce a transmembrane pore [78].
Finally, GSK-3β, a serine/threonine kinase, contributes to neuronal death in AD. GSK-3β has been extensively studied in the context of AD, and it has been implicated in a variety of cellular processes that are disturbed in AD, including Aβ generation [79], tau phosphorylation [80], synaptic plasticity [81], and inflammation [82]. Furthermore, brain GSK-3β levels increase with age [83], and it is found hyperactive in AD samples [84].
4. Oxidative Stress in AD
Oxidative stress occurs when there is an excess in the production of ROS, surpassing the antioxidant defenses. ROS are highly reactive molecules containing oxygen, including free radicals like superoxide (O₂−) and hydroxyl (OH·), and non-radicals like hydrogen peroxide (H₂O₂) [85].
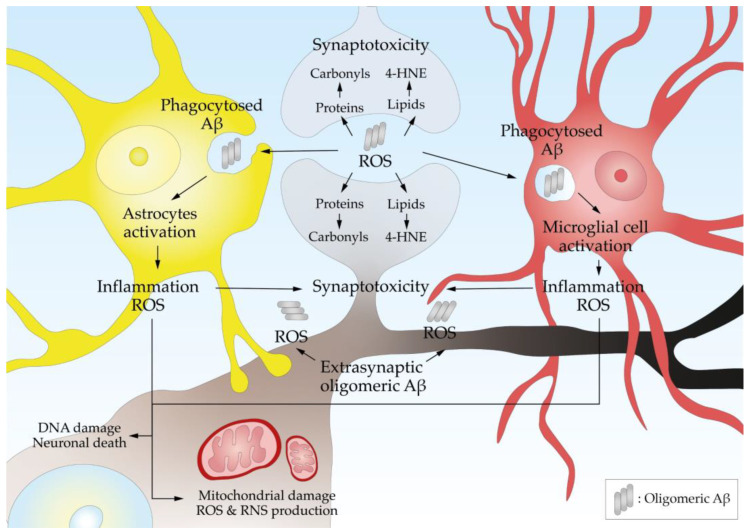
ROS damage DNA, proteins, and lipids, leading to synaptotoxicity and neuronal death (Figure 4). A marker of oxidative DNA damage, 8-Hydroxy-2′-Deoxyguanosine (8-OHdG), is elevated in the brains of AD patients [86] and reflects the extent of oxidative damage to genetic material. Malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are highly reactive byproducts of lipid peroxidation, whose adducts are found at higher levels in AD brains [87,88]. The oxidation of proteins at various residues forming carbonyls has been extensively documented [89,90].
Figure 4.
Aβ oligomers produce oxidative stress and neuroinflammation. Synaptic and extrasynaptic Aβ oligomers produce ROS that damage proteins, lipids, and DNA. The Aβ oligomers attract astrocytes that phagocytose them, which triggers their activation, releasing proinflammatory factors and more ROS. Microglia are attracted by chemokines and, after activation, also release proinflammatory factors. All together, these processes produce synaptotoxicity and neurotoxicity.
The brain is particularly susceptible to oxidative damage due to its high oxygen consumption [91], abundant lipid content [92], and relatively low antioxidant defenses [93]. Elevated levels of oxidative markers have been found in AD patients [94], suggesting a tight link between oxidative stress and disease progression but also that oxidative stress contributed to the onset of the disease [95,96].
The primary sources of oxidative stress in the brain include mitochondrial respiration and enzymatic reactions involving oxidases, peroxidases and oxygenases. Their activities are increased in AD [97]. Furthermore, Aβ oligomers contribute to oxidative stress by generating ROS [98,99]. The aggregation of tau into paired helical filaments (PHFs) can impair cellular processes and exacerbate oxidative damage, further promoting neuronal dysfunction and death [100].
Finally, homocysteine, an amino acid that is an intermediate product in the metabolism of methionine, can lead to increased oxidative stress and inflammation [101], both of which are critical factors in the development and progression of AD. Homocysteine has been proposed to exacerbate the formation of Aβ plaques [102]. It can also induce apoptosis and excitotoxicity in neurons [103] and endothelial dysfunction [104] (further contributing to cognitive decline).
4.1. Metals and Aβ
Aggregated Aβ generates H2O2 and OH-· through the reduction of metal ions such as iron and copper [98,99,105]. These metals are highly concentrated in Aβ deposits and have been proposed as factors contributing to AD etiology due to their redox activity [106]. Copper and iron can catalyze the production of ROS through Fenton reactions, leading to oxidative stress and neuronal damage [107,108,109,110]. Zinc, while essential for brain function, can induce the aggregation of Aβ peptides by stabilizing their oligomeric forms [111]. The imbalance of these metals disrupts cellular processes, increases ROS production, and exacerbates the neuroinflammatory response.
In particular, copper imbalance has been closely linked to AD as it results from a shift in metal ion pools within the brain [112]. Normally, tightly bound copper ions play crucial roles in energy production and antioxidant defense. However, as copper becomes increasingly loosely bound, it exacerbates oxidative stress [112]. This transition, which may be worsened by aging processes, can disrupt mitochondrial function, deplete energy reserves in neurons with high metabolic demands, and lead to enhanced protein misfolding and aggregation [94].
4.2. Mitochondrial Dysfunction in AD
The mitochondria, as the powerhouse of the cell, are the main site for ROS production during ATP synthesis. Aβ-induced ROS lead to mitochondrial dysfunction mainly due to the inhibition of the electron transport chain [113]. Mitochondrial dysfunction is evident in AD, impairing oxidative phosphorylation [114], increasing ROS production [115], reducing ATP levels [116], and impairing mitochondrial dynamics [117]. This dysfunction contributes to neuronal injury and cognitive impairment. The accumulation of oxidative damage within mitochondria further impairs their function, creating a detrimental feedback loop that accelerates the progression of AD [118]. The oxidative damages affect to mitochondrial proteins [119]. Aconitase, which is part of the tricarboxylic acid (TCA) cycle, exhibits high levels of oxidation in AD [120]. It contains an iron-sulphur cluster that is particularly vulnerable to ROS. Oxidation of aconitase disrupts the TCA cycle, leading to reduced energy production and increased mitochondrial dysfunction [120]. VDAC, part of the mitochondrial permeability transition pore, has been also reported to be oxidized, affecting ionic homeostasis and contributing to apoptosis [121].
Mitochondrial DNA (mtDNA) encodes essential mitochondrial proteins, and it is oxidized in AD [122]. ROS-induced damage to mtDNA results in mutations and impaired synthesis of critical ETC components, further exacerbating mitochondrial dysfunction [122,123].
4.3. Oxidation of Biomolecules
Oxidative stress leads to damage to neuronal lipids, proteins, and DNA, contributing to neuronal apoptosis and synaptic dysfunction. Furthermore, ROS and reactive nitrogen species (RNS) stress can activate inflammatory pathways and exacerbate neuroinflammation [124]. The combined effects of oxidative stress, nitro-oxidative stress, and neuroinflammation contribute to the cognitive decline and memory impairment in AD patients due to the damage to neuronal structures and synaptic connections [125].
Oxidative stress causes significant damage to DNA, including base modifications, strand breaks, and the formation of DNA–protein cross-links [126]. 8-OHdG is found at elevated levels in the brains of AD patients [86]. In neurons, which have limited capacity for DNA repair [127,128], such damage can accumulate over time along the life of the individuals and trigger apoptotic pathways [129] and inflammatory responses [130], further promoting neurodegeneration.
Oxidative modification of proteins forms carbonyls, compromising protein physiological functions [131,132], as seen in the former paragraphs. It can also result in the formation of advanced glycation end-products (AGEs), impairing protein function and contributing to cellular dysfunction [133,134,135,136,137,138]. Furthermore, ROS can induce reversible oxidation of thiol groups in sulphur-containing residues (cysteine or methionine). It induces a wide range of oxidative post-translational modifications, including sulfenic, sulfinic, and sulfonic acids, and crosslinking through the formation of disulphide bonds. A particular case of crosslinking is S-glutathionylation, in which sulphur-containing residues bind glutathione (GSH) [139]. However, under high levels of ROS, some of these post-translational modifications can turn irreversible and disrupt protein physiological functions [140]. In fact, AD patients present higher levels of disulphide bond-induced protein crosslinking compared to healthy individuals [141].
In the brain, which is rich in polyunsaturated fatty acids, lipid peroxidation produces major damages in the neuronal membrane. MDA and 4-HNE are highly reactive and can form adducts with proteins and nucleic acids [142,143]. In AD, increased levels of lipid peroxidation have been observed, correlating with the severity of the disease [143]. Lipid peroxidation can impair membrane-bound receptor functioning and enzymes, disrupting neuronal signaling and promoting synaptic dysfunction and neuronal loss [144].
As mentioned before, Aβ can be oxidized in the presence of copper [99], which increases its misfolding into Aβ sheets, forming toxic oligomers. Interestingly, oxidation of methionine 35 has been shown to be a retardant of Aβ aggregation [145]. Protein tau has also been reported to be oxidized [146], contributing to its misfolding [147].
In transgenic mouse models of AD, oxidative stress markers begin to appear at different stages depending on the model. In 5xFAD mice, known for their rapid disease progression, oxidative stress markers appear around 3 to 6 months of age, leading to early and pronounced oxidative damage, characterized by the increased presence of biomarkers such as 8-OHdG in plasma and MDA in brain and liver [148]. In Tg2576 and APP/PS1 mice, oxidative stress markers typically become detectable around 6 months of age [149,150,151], with increased levels as the disease progresses. In all of the models, between 6 and 12 months, higher levels of oxidative stress are generally observed, reflecting increased damage to lipids, proteins, and DNA. Both astrocytes and microglia show increased activation during this period, contributing to elevated oxidative stress [152,153,154]. In the advanced stages (12 to 18 months and beyond), oxidative stress markers are typically at their highest levels, correlating with advanced neurodegeneration and extensive plaque deposition [155,156,157].
4.4. Oxidative Stress and Aβ Production
Oxidative stress contributes to the production [46,95,96] and aggregation of Aβ [158]. Furthermore, Aβ itself can generate ROS [98], creating a vicious cycle of oxidative damage and Aβ production. ROS can promote the amyloidogenic pathway by increasing the activity of BACE1 by activating stress-activated protein kinases, such as c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38-MAPK), which induce the transcription of BACE1 [95,96]. Furthermore, the translation of BACE1 mRNA is dependent on the activation of the kinases of the translation factor eIF2. These kinases phosphorylate eIF2 at its α subunit when challenged with stressful stimuli such as oxidative stress [46], nitric oxide (NO) production [159], or virus infection such as the one produced by the herpes simplex virus 1 (HSV-1) [160].
Oxidative stress can also impair the clearance of Aβ by affecting the function of proteolytic enzymes and the ubiquitin-proteasome system [161], increasing its concentration inside the brain, and contributing significantly to AD onset and progression.
5. Nitro-Oxidative Stress in AD
Nitro-oxidative stress is driven by ONOO-, which is not a free radical but a highly reactive anion formed by the reaction of NO with the superoxide anion [162]. Its reactivity leads to the nitration of biomolecules, mainly some amino acids such as tyrosines, producing nitrotyrosines [163]. This is a pathological post-translational modification that alters and potentially suppresses the biological function of a protein [47,162,164,165]. On the other hand, nitrosative processes are produced by NO directly and are commonly physiological posttranslational modifications, in many cases reversible [162].
5.1. NO and Its Functions
NOSs, which exist in several forms, are not considered isoforms since they are encoded by different genes [162]. They catalyze the production of NO from L-arginine and they play distinct roles in biological systems. The types of NOS are endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS). eNOS is a constitutive enzyme of the endothelial cells, where it plays a key role in regulating vascular tone, blood pressure, and preventing platelet aggregation, but it is also expressed in other cell types like neurons and glia [162,166]. nNOS is a constitutive enzyme expressed in neurons and involved in neurotransmission, playing a key role in brain function, including memory and learning [162,166]. nNOS is also expressed in other cell types [162,166]. iNOS is produced by immune cells and glial cells in response to inflammatory stimuli and is part of the immune defense mechanisms, helping to destroy pathogens [162,166], but potentially contributing to tissue damage if overproduced. This dual behavior is evident since genetic deletion of iNOS in a mouse model of AD promotes neurodegeneration [167], and iNOS inhibition could be an effective approach in treating AD and other neurodegenerative diseases [168].
5.2. Mitochondria and Nitro-Oxidative Stress
Mitochondrial NOS (mtNOS) is an isoform of nNOS [163] expressed in the matrix and the inner membrane of mitochondria. It regulates mitochondrial respiration by modulating the electron transport chain and ATP production by inhibiting cytochrome c oxidase [169]. NO from mtNOS also influences mitochondrial biogenesis and dynamics [117]. Dysregulation of mtNOS activity can lead to mitochondrial dysfunction, contributing to neurodegenerative diseases [170] and cardiovascular diseases [171].
5.3. Nitrosylation and Biomolecules
Nitrosylation is a post-translational modification that involves the covalent attachment of NO to organic molecules without altering the substrate charge, leading to the formation of C-nitroso, N-nitroso, O-nitroso, or S-nitroso derivatives [171]. Most commonly, it binds to the thiol group of cysteine residues on proteins, modulating their function. This process, known as S-nitrosylation, is reversible and plays a regulatory role in cellular signaling [172]. S-nitrosylation regulates the physiological function of numerous proteins [163].
In a pathological scenario, inflammatory factors (TNF-α, IL-1β, IL-6) contribute to an increased production of NO mainly by the activation of the expression of iNOS [173], leading to excessive S-nitrosylation [174]. In AD, this modification becomes dysregulated and impairs the function of proteins involved in synaptic function, such as NMDAR [175], and mitochondrial dynamics, such as dynamin-related protein 1 (Drp1) [176], or even can induce apoptosis by the S-nitrosylation of the caspase cascade [177].
5.4. Nitration and Biomolecules
RNS can modify DNA, proteins, and lipids [162]. 8-Nitroguanine (8-NO2G) reflects the nitration of guanine residues in DNA and is indicative of nitrative stress and DNA damage in AD [178]. The nitration of tyrosine residues, forming 3-nitrotyrosine, is a marker of nitrotyrosination in AD [133,179]. These modifications alter the structure and function of proteins, impairing their normal activity.
The nitrotyrosination of the glycolytic enzyme triosephosphate isomerase induced by Aβ decreases the glycolytic flow [133]. Moreover, it triggers the production of the highly neurotoxic methylglyoxal [133,135], which glycates proteins [138]. Nitrative stress has also been implicated in the formation of neurofibrillary tangles [133,180,181].
Aβ can undergo nitration at the tyrosine in position 10, which enhances its misfolding into Aβ sheets, leading to the formation of toxic oligomers and stabilizing them [182].
RNS also induces the peroxidation and the nitration of lipids, altering the integrity of the membrane and the trigger of intracellular signaling [183,184].
5.5. Nitro-Oxidative Stress and Aβ Production
The nitrotyrosination of presenilin 1 (PS1), the catalytic subunit of γ-secretase, increases the association of the PS1 fragments, PS1-CTF and PS1-NTF, which form the active catalytic center of the γ-secretase complex [47]. Peroxynitrite also shifts Aβ production towards Aβ1-42 and increases the Aβ1-42/Aβ1-40 ratio [47], the pathophysiological situation found in AD patients.
6. Neuroinflammation in AD
Inflammaging refers to the chronic, low-grade inflammation that typically accompanies aging, contributing to the development of age-related diseases such as cardiovascular disease, diabetes, and neurodegenerative disorders [185]. This persistent inflammatory state is believed to result from the cumulative effect of lifelong exposure to various stressors, such as infections, environmental toxins, and lifestyle factors, which gradually impair the immune system’s regulatory functions. Key mechanisms underlying inflammaging include cellular senescence, the production of proinflammatory cytokines, and alterations in the gut microbiome [186]. AD is included in the concept of inflammaging since the major risk factor for AD is aging, it is tightly linked to cardiovascular diseases and diabetes, and there are inflammatory processes in the brain [187].
The accumulation of Aβ triggers an inflammatory response, leading to neuronal loss and synaptic dysfunction that contribute to the cognitive deficits observed in AD patients [188]. The inflammatory response is carried out by astrocytes and microglial cells [189], which migrate toward the Aβ deposits and phagocyte the oligomers and fibrils, producing proinflammatory cytokines, the activation of the complement system in the brains of AD patients, and the production of ROS [190] (Figure 4).
6.1. Astroglia in AD
Astrocytes are essential for maintaining the homeostasis of the central nervous system (CNS). They are star-shaped cells with numerous branching processes that extend throughout the brain and spinal cord. They are classified into two main types: fibrous astrocytes, predominantly found in white matter, and protoplasmic astrocytes, found in grey matter [191]. This morphological diversity allows astrocytes to interact with neurons, blood vessels, and other glial cells, facilitating their functions.
6.1.1. Astrocytic Functions
They support neurons by regulating the extracellular environment, maintaining the BBB, modulating synaptic activity, and responding to injury or disease [192]. They also maintain the ionic and chemical balance [192]. Astrocytes provide metabolic support to neurons by supplying essential nutrients, such as glucose and lactate [193], and also lipidic molecules, such as cholesterol, through ApoE-containing lipoproteins [194,195]. They regulate cerebral blood flow through their interactions with blood vessels [196], ensuring an adequate supply of oxygen and nutrients to active brain regions.
Additionally, astrocytes uptake and recycle neurotransmitters, particularly glutamate [197], which is vital for preventing excitotoxicity and maintaining synaptic health. They release gliotransmitters too, such as the neurodepressor ATP and the NMDAR modulator D-serine, which influence synaptic activity and plasticity [198,199]. Through their interactions with synapses, astrocytes contribute to processes like long-term potentiation (LTP) and long-term depression (LTD) [200], essential for learning and memory.
6.1.2. Aβ and Astrocytes
Triggered by neuronal injury and the presence of Aβ deposits, reactive astrocytes exhibit increased expression of glial fibrillary acidic protein (GFAP) [201,202] and an alteration in calcium regulation [203], resulting in metabolic dysfunction [204,205]. It impairs astrocytes’ ability to regulate neurotransmitters, mainly glutamate, through the glutamate transporter 1 (GLT-1), which increases extracellular glutamate and causes excitotoxicity [206].
In relation to oxidative stress, reactive astrocytes release ROS in response to aggregated Aβ [207]. Moreover, astrocytes express iNOS when challenged with Aβ [208]. ROS and NO production contribute to nitro-oxidative damage in surrounding neurons, with the consequences explained in Section 4 and Section 5 of this review. On the other hand, oxidative stress can impair the function of astrocytes in the maintenance of the BBB [209].
Regarding inflammation, signaling pathways involving nuclear factor-kappa B (NF-κB) and MAPKs are upregulated in reactive astrocytes [210,211], leading to increased production of inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6 [212,213], which are commonly observed in the brains of AD patients. These cytokines contribute to inflammation by promoting the activation of immune cells and the release of additional inflammatory factors. They also induce the release of chemokines [214,215], such as monocyte chemoattractant protein-1 (MCP-1), which play a key role in recruiting immune cells to the site of inflammation [215]. In AD, increased levels of MCP-1 are associated with enhanced microglial activation and inflammation [216]. In summary, proinflammatory mediators and chemokines contribute to an inflammatory environment that exacerbates neuronal damage. Therefore, although initially protective because they phagocytize and degrade Aβ [205,217], reactive astrogliosis becomes detrimental in a vicious cycle of inflammation and oxidative damage.
6.2. Microglia in AD
Microglia are the resident immune cells of the brain [218]. Microglia are highly plastic and can adopt different functional states based on environmental signals. Traditionally classified into two very different phenotypes, called M1 (proinflammatory) and M2 (anti-inflammatory), more recent research has stated the complexity of microglial protein expression and behavior, establishing the need to use more detailed non-polarizing nomenclature [219].
6.2.1. Microglia Functions
Upon activation, microglia adopt an amoeboid shape and engage in various functional responses, including phagocytosis, cytokine release, and ROS production [220].
Moreover, microglia carry out the pruning of the synapses during development and in the adult brain [221,222], which is crucial for synaptic plasticity and function [223]. Microglia also support synaptic health by clearing debris [224] and secreting neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) [225] and insulin-like growth factor 1 (IGF1) [226].
6.2.2. Aβ and Microglia
Aβ interacts with pattern recognition receptors (PRRs) on microglia [227], such as toll-like receptors (TLRs) [228], a scavenger receptor [229], and the receptor for advanced glycation end products (RAGE) [230]. All of these facilitate Aβ internalization and degradation [231]. Moreover, microglia secrete neprilysin and insulin-degrading enzyme (IDE) to break down Aβ [232,233]. However, if the Aβ load is too large, another scenario may arise. The binding of Aβ to these PRRs also activates the NADPH oxidase of the membrane, generating a respiratory burst [234], which is a significant source of ROS, with pathophysiological consequences already described in Section 4 and Section 5 of this review. Furthermore, oxidative stress impairs microglial phagocytic function, reducing their ability to clear Aβ plaques and cellular debris [235]. Once NADPH oxidase is activated, microglia produce proinflammatory cytokines (IL-1β, IL-6, TNF-α) and chemokines [236,237]. All these effects contribute to the accumulation of toxic substances in the brain and promote Aβ aggregation and deposition. Microglia can also produce factors that attract more immune cells, creating a noxious feedback loop of inflammation and neurodegeneration, characterized by increased expression of markers such as Iba1 [238].
Astrocytes and microglia closely interact in both physiological and pathophysiological contexts, influencing each other through the release of glutamate, gliotransmitters, cytokines (TNFα, IL1, and IL6 working as paracrine factors), ATP, NO, and ROS [239]. This interaction can either amplify or mitigate the inflammatory response, depending on the surrounding environment. However, it typically becomes harmful once Aβ deposits appear [240].
6.3. Inflammation and Mitochondria in AD
Microglial and astrocytic cytokines can impair mitochondrial function by disrupting calcium homeostasis, inhibiting mitochondrial respiration, and promoting ROS production [241]. Furthermore, the transcription factor NF-κB is activated by inflammatory cytokines and can induce the expression of genes that promote oxidative stress and mitochondrial dysfunction [242]. Besides NF-κB, inflammation-induced activation of p53 can lead to transcriptional changes that promote apoptosis and mitochondrial dysfunction [243]. Another classical proinflammatory enzyme, the cyclooxygenase-2 (COX-2), increases its expression in response to inflammation and enhances the production of proinflammatory prostaglandins, exacerbating mitochondrial damage and neuronal death [244].
6.4. Specialized Pro-Resolving Mediators
Specialized pro-resolving mediators (SPMs) play a crucial role in resolving inflammation and promoting tissue repair [245,246], and their potential in AD is garnering significant interest [247]. SPMs, including lipoxins, resolvins, protectins, and maresins, are bioactive lipid compounds derived from polyunsaturated fatty acids [246]. In the context of AD, SPMs may help counteract chronic inflammation, a hallmark of the disease, by actively resolving inflammation and facilitating the clearance of Aβ and damaged cells. Emerging research suggests that enhancing SPM pathways could mitigate the progression of AD by restoring homeostasis and protecting neuronal function [248].
6.5. Genetic Links to Inflammation in AD
Genetic studies have further evidenced the role of inflammation in AD. Genome-wide association studies (GWAS) have identified several risk genes associated with immune function and inflammation (Supplementary Tables S1 and S2 [249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264]).
The apolipoprotein E (ApoE) ε4 allele is the strongest genetic risk factor for late-onset AD. ApoE influences Aβ clearance and neuroinflammation, and the ε4 variant is associated with increased Aβ deposition, impaired Aβ clearance, and a heightened inflammatory response [265]. Microglial ApoE ε4 is a disease-associated microglia (DAM) marker, driving immunometabolic changes across the microglial transcriptome, associating with Aβ-independent tau accumulation [266].
Triggering receptor expressed on myeloid cells 2 (TREM2) is crucial for microglial function, including activation, survival, and phagocytosis [267]. TREM2 enhances microglial phagocytosis of Aβ and tau, promotes an anti-inflammatory response to Aβ, and supports lipid metabolism in microglia [267]. TREM2 polymorphisms associated with AD produce a loss of function that increases disease risk by impairing the microglial response to Aβ and tau [257,268,269], leading to increased plaque burden and neuroinflammation. There is a demonstrated interaction between ApoE and TREM2 [270,271]. ApoE seems to facilitate the phagocytosis of dying neurons by activating the TREM2 pathway. However, the R47H variant of TREM2 has been found to lower its binding affinity for ApoE [272]. Moreover, microglial Aβ uptake is enhanced when bound to ApoE, and TREM2-defficient microglia show a reduction in Aβ-ApoE absorption [270]. Although crucial for Aβ clearance, TREM2/ApoE interaction seems to not be essential for phagocytic clearance of dying neural cells, but, interestingly, microglia that lack TREM2 prioritize the phagocytosis of dead cells over Aβ plaques [273].
Complement receptor 1 (CR1) regulates the complement system [274], which is part of the innate immune response. It helps clear immune complexes and cellular debris [275]. In AD, CR1 facilitates Aβ clearance by microglia [276], but genetic variants associated with AD may impair this process [251], leading to increased plaque accumulation and neuroinflammation. CR1 is also involved in synaptic pruning [277], and dysregulation can contribute to synaptic loss and cognitive decline.
CD33/Siglec-3 is an inhibitory receptor on microglia that modulates their immune responses [278]. According to GWAS, CD33 is among the leading genes linked to the risk of developing AD [263]. AD brains show higher levels of CD33 on their microglia, and CD33 presence is correlated with plaque burden and cognitive decline [279,280]. However, CD33 enrichment could be perfectly explained as a result of the neuroinflammation process on those brains. Variants of CD33 that reduce its expression are associated with a lower AD risk [281], as decreased CD33 enhances Aβ clearance and is protective against AD. Conversely, other variants that increase CD33 activity and increase Aβ1-42 phagocytosis have recently been correlated with a decreased risk for AD [282]. An explanation to this has been described: the full human CD33 isoform (hCD33M) is correlated with low microglial Aβ clearance, and the isoform resulting from exon 2 exclusion through splicing is correlated with increased phagocytosis (hCD33m) [280,282,283]. Therefore, SNPs that promote either a lower expression of the hCD33M isoform or an enhanced alternative splicing of the protein confer protection against AD.
7. The BBB in AD
The BBB is a selective permeability barrier formed by endothelial cells lining the brain’s blood vessels, supported by astrocytic end-feet, pericytes, and a basal lamina [284,285]. The functions of the BBB include: (i) selective permeability by which it regulates the passage of substances between the blood and the brain, allowing essential nutrients to enter while preventing harmful substances from crossing, being able to exclude up to 98% of bloodstream molecules [286]; (ii) brain protection from toxins, pathogens, and fluctuations in blood composition [287], maintaining a stable environment for neuronal function; (iii) maintenance of the extracellular environment, including ion balance and neurotransmitter levels, essential for proper neuronal activity [196].
These singular capacities are related to the presence of tight junctions between the endothelial cells, reducing BBB permeability by limiting all transport through cells except non-ionic molecules [288].
In physiological states, the BBB extrudes Aβ as commented in Section 2. Moreover, it has been demonstrated certain codependence with classical P-Glycoprotein (P-gP), a transporter located in the luminal area of the blood vessel, which mediates Aβ efflux [289,290]. In fact, there is a correlation between LRP-1 and P-gP expression profiles in AD [291,292].
AD is characterized by oxidative stress and inflammation that significantly impact the integrity and function of BBB, especially due to the presence of amyloid deposits surrounding brain vessels, which derive in cerebral amyloid angiopathy. Aβ and ROS damage endothelial cells, inducing apoptosis [164,179]. The rupture of the BBB in AD is a pathological event that results in increased presence of albumin in CSF of AD [293] or the increased Braak stage-dependent presence of prothrombin surrounding capillaries and immunoreactive glia in AD patients [294]. Yamazaki et al. [295] compared the BBB integrity between AD patients, individuals with pathological aging and individuals with normal aging, in 12 different brain areas. AD patients showed a reduction in expression of the tight junction proteins claudin-5 and occludin that correlated with the presence of Aβ1-40 aggregates. These findings may be a consequence of ROS activating various signaling pathways via RhoA, PI3K, and PKB, causing a rearrangement of the actin and a reduction of occluding and claudin-5 [296]. Also, expression of LRP-1 is reduced in endothelial and pericytes in AD, while glia and neurons show increased expression. These results suggest a phenotype change from vascular transcytosis to amyloid phagocytosis [297,298], leading to increased permeability and dysfunction of the tight junction [299]. Moreover, ONOO− can damage endothelial cells by nitrotyrosinating their proteins [164,179], contributing to increased permeability and dysfunction of the BBB.
Inflammation compromises the integrity of the BBB, allowing peripheral inflammatory cytokines and toxic substances to enter the CNS [300]. This exacerbates neuronal injury and inflammation. Astrocytes play a key role in maintaining the integrity of the BBB because they release factors that support endothelial cell tight junctions [301,302], which are also crucial for maintaining the BBB’s integrity. Therefore, astrocyte activation by Aβ impairs their ability the maintain a functional BBB. In fact, the release of TNF-α and IL-1β can disrupt tight junctions and increase BBB permeability [303]. Furthermore, inflammatory mediators can activate matrix metalloproteinases (MMPs) that degrade extracellular matrix components, leading to BBB breakdown [304].
8. Oligodendrocytes in AD
While much of the focus in AD research has been on neurons, emerging evidence highlights the significant role of oligodendrocytes, the glial cells responsible for myelinating axons in the brain and supporting neuronal function. Oligodendrocytes are critically affected by oxidative stress and inflammation [305], which collectively contribute to the pathogenesis of AD.
Oligodendrocytes produce myelin, a lipid-rich sheath that insulates axons, facilitating rapid and efficient electrical signal transmission [306], provide metabolic support to neurons by supplying energy substrates and maintaining ionic balance [307], and secrete factors that modulate the inflammatory response and maintain the balance between proinflammatory and anti-inflammatory signals [308].
In AD, oligodendrocytes undergo several pathological changes. Demyelination is a hallmark of AD [309,310,311,312], leading to impaired axonal conduction and cognitive decline. Dysfunctional oligodendrocytes also decrease their neuronal support functions [313]. Activated oligodendrocytes can exacerbate neuroinflammation and contribute to disease progression [314]. Recently, using single-cell RNA sequencing analysis, it has been identified a subpopulation of oligodendrocytes associated with the progression of the disease in both APPNL-G-F and 5xFAD male mice and in AD human brains. They presented an altered Erk1/2 signaling that, when inhibited, rescued impaired axonal myelination and other pathologies [315].
ROS and RNS damage myelin and myelin-producing oligodendrocytes [316], impair the ability of oligodendrocytes to repair damaged myelin [316], and even induce apoptosis in these cells [317,318], impairing axonal conduction and exacerbating disease progression.
Inflammation affects oligodendrocytes since their functions are disrupted by TNF-α and IL-1β [319]. The activation of MMPs contributes to degrading extracellular matrix components of the oligodendrocytes [320], and chronic inflammation impairs the ability of oligodendrocytes to support neuronal function and repair myelin [321,322].
Interestingly, a recent study has pointed out that oligodendrocytes functionally express BACE1, further contributing to the Aβ plaque formation in AD [323].
9. Therapeutic Approaches
There is a lot of literature on the mechanisms that contribute to the onset and development of the disease, but there are no specific treatments further than anti-acetylcholinesterase drugs that ameliorate the cholinergic deficit of AD [324]. Memantine is an inhibitor of the glutamate receptors, and clinical studies have shown that it can provide modest improvements in cognitive function but, when combined with anticholinesterasic drugs, may offer additional benefits and improve overall treatment outcomes [325,326]. In addition, more than 200 clinical trials targeting Aβ production have been run, and none was sufficient to recover from AD, and even some of them showed adverse effects [327].
Antibodies are recognized for dismantling Aβ aggregates into monomeric forms through interaction with their Fab regions [328]. Phagocytic cells, such as microglia, express FcγR receptors on their surface for the Fc region of antibodies, contributing to the elimination of Aβ deposits via phagocytosis. Recently, satisfactory results have been obtained with passive immunization with monoclonal antibodies [329,330].
Other therapeutic approaches have probably failed because patients were in advanced stages of the disease, making it hard to get protective results. For this reason, it is essential to diagnose individuals who are in the early asymptomatic phases to start treatment at that time and avoid further complications.
Antioxidant therapies aim to counteract oxidative stress in AD. Agents such as vitamin E [331,332,333,334], coenzyme Q10 [335], and curcumin [336] have been tested in clinical trials with mixed results, indicating a need for further research. Interestingly, amine oxidases, such as monoamine oxidase B (MAO B) and semicarbazide-sensitive amine oxidase (SSAO), play a pathological role in AD by contributing to oxidative stress and neuroinflammation [337,338]. MAO inhibitors, including tranylcypromine, naphthoquinones, and anthraquinone, have been reported to reduce Aβ-induced toxicity by minimizing oxidative stress and even reducing Aβ aggregation [339,340]. The role of MAO inhibitors as anti-inflammatory drugs is also a relevant therapeutic approach for AD [341]. In addition, other anti-inflammatory drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) [342], cytokine inhibitors [343], and selective COX-2 inhibitors [344,345,346], are being explored for AD treatment. However, their efficacy in altering AD progression remains uncertain, requiring more research.
Adopting a diet rich in antioxidants, like the Mediterranean diet, along with regular exercise and cognitive stimulation, may help reduce oxidative stress and support brain health. It is important to emphasize the publication of data that report a significant decrease in the prevalence of AD in Western countries [347]. This decline is attributed to better control of cardiovascular health and a higher educational level of the population, since it is known that cognitive reserve is a protective factor against AD [348]. Therefore, promotion of good nutritional habits, sport practice [349], and having an acceptable educational level [350] would be a first step in AD prevention until drug therapies become available.
10. Conclusions
AD is a multifactorial condition in which Aβ plays a pivotal role. Effective treatment strategies depend on early and accurate diagnosis, especially during the prodromal stages. Current advancements in technology enable the analysis of numerous variables, potentially leading to complex yet precise diagnostic systems in the near future.
The future of AD treatments should focus on three key areas: first, inhibiting Aβ production or reducing its levels, acknowledging its potential physiological roles; second, preventing Aβ aggregation during the early prodromal phases is crucial since dismantling existing aggregates can lead to an uncontrolled rise in soluble Aβ, which may migrate to blood vessels, causing cerebral amyloid angiopathy; and third, protecting against inflammation and neurotoxicity is essential, and the use of antioxidants and anti-inflammatory drugs should continue to be tested.
In conclusion, a comprehensive approach targeting Aβ production, aggregation, and the associated oxidative stress and inflammation is critical for advancing AD treatment. Future research should focus on early detection and multifaceted intervention strategies to slow or halt disease progression and improve patient outcomes.
Acknowledgments
We would like to thank Manuel Pérez for illustrations.
Abbreviations
4-HNE, 4-Hydroxynonenal; 8-NO2G, 8-Nitroguanine; 8-OHdG, 8-Hydroxy-2′-Deoxyguanosine; ACE, angiotensin-converting enzyme; AD, Alzheimer’s disease; AGEs, advanced glycation end-products; AICD, amyloid precursor protein intracellular domain; APH1, anterior pharynx defective 1; APLP, APP-like proteins; ApoE, apolipoprotein E; APP, amyloid precursor protein; Aβ, amyloid beta-peptide; BACE1, β-site APP cleaving enzyme 1; BBB, blood–brain barrier; BDNF, brain-derived neurotrophic factor; CaMKIIα, calcium/calmodulin-dependent protein kinase II alpha; CNS, central nervous system; COX-2, cyclooxygenase-2; CR1, complement receptor 1; CREB, cAMP response element-binding protein; CTF, C-terminal fragment; DMS-5, Diagnostic and Statistical Manual of Mental Disorders; ECE, endothelin-converting enzyme; eIF2-alpha, eukaryotic translation initiation factor 2-alpha; eNOS, endothelial nitric oxide synthase; ETC, electron transport chain; Fab, fragment antigen-binding; Fc, fragment crystallizable; GFAP, glial fibrillary acidic protein; GLT-1, glutamate transporter 1; GSH, glutathione; GSK-3β, glycogen synthase kinase 3 beta; GWAS, genome-wide association studies; HSV-1, herpes simplex virus 1; IDE, insulin-degrading enzyme; IGF1, insulin-like growth factor 1; IL, interleukin; JNK, c-Jun N-terminal kinase; LRP1, low-density lipoprotein receptor-related protein 1; LTP, long-term potentiation; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; MMPs, matrix metalloproteinases; mtDNA, mitochondrial DNA; mtNOS, mitochondrial nitric oxide synthase; NCT, nicastrin; NEP, neprilysin; NF-κB, nuclear factor-kappa B; NICD, notch intracellular domain; NMDAR, N-methyl-D-aspartate receptor; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; NSAIDs, nonsteroidal anti-inflammatory drugs; NTF, N-terminal fragment; ONOO-, Peroxynitrite; p38-MAPK, p38 mitogen-activated protein kinase; PEN-2, presenilin enhancer 2; PET, positron emission tomography; PHFs, paired helical filaments; PRRs, pattern recognition receptors; PSEN, presenilin; RAGE, receptor for advanced glycation end products; RNS, reactive nitrogen species; ROS, reactive oxygen species; RyR, ryanodine receptor; sAPPβ, soluble amyloid precursor protein-beta; SPMs, specialized pro-resolving mediators; TLRs, toll-like receptors; TNF-α, tumor necrosis factor-alpha; TREM2, triggering receptor expressed on myeloid cells 2; VDAC, voltage-dependent anion channel; VLDLR, very low-density lipoprotein receptor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13101208/s1, Table S1: Polymorphisms that contribute to Alzheimer’s disease onset and progression; Table S2: Polymorphisms identified in a GWAS performed with a South Brazilian population.
Author Contributions
H.F.-U., P.P.-P., V.H.-F., G.I.-R. and F.J.M. wrote and discussed the text of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Institut Municipal d’Investigacions Mèdiques-Parc de Salut Mar (CEIm 2022/10334/I; approved October 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Spanish Ministry of Science, Innovation and Universities (MCIU) and the Agencia Estatal de Investigación (AEI) plus European Social Fund Plus (FSE+) through grant PID2020-117691RB-I00/AEI/10.13039/501100011033 and PID2023-149767OB-I00 with the reference MCIU/AEI/10.13039/501100011033 plus FSE+. This work was also funded by the “María de Maeztu Programme” for Units of Excellence in Research and Development (R&D; award CEX2018-000792-M).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization . Global Status Report on the Public Health Response to Dementia. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 2.Long S., Benoist C., Weidner W. World Alzheimer Report 2023 Reducing Dementia Risk: Never Too Early, Never Too Late. Alzheimer’s Disease International; London, UK: 2023. [Google Scholar]
- 3.Glenner G.G., Wong C.W. Alzheimer’s Disease: Initial Report of the Purification and Characterization of a Novel Cerebrovascular Amyloid Protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 4.Krystal H.L., Ross D.A., Mecca A.P. Amyloid: From Starch to Finish. Biol. Psychiatry. 2020;87:e23–e24. doi: 10.1016/j.biopsych.2020.02.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunde M., Serpell L.C., Bartlam M., Fraser P.E., Pepys M.B., Blake C.C.F. Common Core Structure of Amyloid Fibrils by Synchrotron X-ray Diffraction11Edited by F. E. Cohen. J. Mol. Biol. 1997;273:729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- 6.Vidal R., Frangione B., Rostagno A., Mead S., Révész T., Plant G., Ghiso J. A Stop-Codon Mutation in the BRI Gene Associated with Familial British Dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 7.Ghiso J., Rostagno A., Tomidokoro Y., Lashley T., Bojsen-Møller M., Braendgaard H., Plant G., Holton J., Lal R., Revesz T., et al. Genetic Alterations of the BRI2 Gene: Familial British and Danish Dementias. Brain Pathol. 2006;16:71–79. doi: 10.1111/j.1750-3639.2006.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Toro D., Coma M., Uribesalgo I., Guix F.X., Muñoz F.J. The Amyloid β-Protein Precursor and Alzheimer’s Disease. Therapeutic Approaches. Curr. Med. Chem.—Cent. Nerv. Syst. Agents. 2005;5:271–283. doi: 10.2174/156801505774913053. [DOI] [Google Scholar]
- 9.Wang Z., Wang B., Yang L., Guo Q., Aithmitti N., Songyang Z., Zheng H. Presynaptic and Postsynaptic Interaction of the Amyloid Precursor Protein Promotes Peripheral and Central Synaptogenesis. J. Neurosci. 2009;29:10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues E.M., Weissmiller A.M., Goldstein L.S.B. Enhanced β-Secretase Processing Alters APP Axonal Transport and Leads to Axonal Defects. Hum. Mol. Genet. 2012;21:4587–4601. doi: 10.1093/hmg/dds297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinamarca M.C., Raveh A., Schneider A., Fritzius T., Früh S., Rem P.D., Stawarski M., Lalanne T., Turecek R., Choo M., et al. Complex Formation of APP with GABAB Receptors Links Axonal Trafficking to Amyloidogenic Processing. Nat. Commun. 2019;10:1331. doi: 10.1038/s41467-019-09164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang J., Lemaire H.G., Unterbeck A., Salbaum J.M., Masters C.L., Grzeschik K.H., Multhaup G., Beyreuther K., Müller-Hill B. The Precursor of Alzheimer’s Disease Amyloid A4 Protein Resembles a Cell-Surface Receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 13.Tanzi R.E., Gusella J.F., Watkins P.C., Bruns G.A., St George-Hyslop P., Van Keuren M.L., Patterson D., Pagan S., Kurnit D.M., Neve R.L. Amyloid Beta Protein Gene: CDNA, MRNA Distribution, and Genetic Linkage near the Alzheimer Locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 14.Selkoe D.J. The Cell Biology of Beta-Amyloid Precursor Protein and Presenilin in Alzheimer’s Disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/S0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 15.Anderson J.P., Chen Y., Kim K.S., Robakis N.K. An Alternative Secretase Cleavage Produces Soluble Alzheimer Amyloid Precursor Protein Containing a Potentially Amyloidogenic Sequence. J. Neurochem. 1992;59:2328–2331. doi: 10.1111/j.1471-4159.1992.tb10128.x. [DOI] [PubMed] [Google Scholar]
- 16.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., et al. Beta-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 17.Farzan M., Schnitzler C.E., Vasilieva N., Leung D., Choe H. BACE2, a β-Secretase Homolog, Cleaves at the β Site and within the Amyloid-β Region of the Amyloid-β Precursor Protein. Proc. Natl. Acad. Sci. USA. 2000;97:9712–9717. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochin L., Hurbain I., Serneels L., Fort C., Watt B., Leblanc P., Marks M.S., de Strooper B., Raposo G., van Niel G. BACE2 Processes PMEL to form the Melanosome Amyloid Matrix in Pigment Cells. Proc. Natl. Acad. Sci. USA. 2013;110:10658–10663. doi: 10.1073/pnas.1220748110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S.P., Mukherjee C. Similar Activities of Nerve Growth Factor and Its Homologue Proinsulin in Intracellular Hydrogen Peroxide Production and Metabolism in Adipocytes: Transmembrane Signalling Relative to Insulin-Mimicking Cellular Effects. Biochem. Pharmacol. 1982;31:3163–3172. doi: 10.1016/0006-2952(82)90545-7. [DOI] [PubMed] [Google Scholar]
- 20.Das U., Scott D.A., Ganguly A., Koo E.H., Tang Y., Roy S. Activity-Induced Convergence of APP and BACE-1 in Acidic Microdomains via an Endocytosis-Dependent Pathway. Neuron. 2013;79:447–460. doi: 10.1016/j.neuron.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X., Koelsch G., Wu S., Downs D., Dashti A., Tang J. Human Aspartic Protease Memapsin 2 Cleaves the Beta-Secretase Site of Beta-Amyloid Precursor Protein. Proc. Natl. Acad. Sci. USA. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das U., Wang L., Ganguly A., Saikia J.M., Wagner S.L., Koo E.H., Roy S. Visualizing APP and BACE-1 Approximation in Neurons Yields Insight into the Amyloidogenic Pathway. Nat. Neurosci. 2016;19:55–64. doi: 10.1038/nn.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demars M.P., Hollands C., da Zhao K., Lazarov O. Soluble Amyloid Precursor Protein-α Rescues Age-Linked Decline in Neural Progenitor Cell Proliferation. Neurobiol. Aging. 2013;34:2431–2440. doi: 10.1016/j.neurobiolaging.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y.Q., Rogaeva E., Chen F., Kawarai T., et al. Nicastrin Modulates Presenilin-Mediated Notch/Glp-1 Signal Transduction and BetaAPP Processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 25.Francis R., McGrath G., Zhang J., Ruddy D.A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M.C., et al. Aph-1 and Pen-2 Are Required for Notch Pathway Signaling, Gamma-Secretase Cleavage of BetaAPP, and Presenilin Protein Accumulation. Dev. Cell. 2002;3:85–97. doi: 10.1016/S1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 26.Goutte C., Tsunozaki M., Hale V.A., Priess J.R. APH-1 Is a Multipass Membrane Protein Essential for the Notch Signaling Pathway in Caenorhabditis Elegans Embryos. Proc. Natl. Acad. Sci. USA. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J.S., Schroeter E.H., Schrijvers V., Wolfe M.S., Ray W.J., et al. A Presenilin-1-Dependent Gamma-Secretase-like Protease Mediates Release of Notch Intracellular Domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 28.von Rotz R.C., Kohli B.M., Bosset J., Meier M., Suzuki T., Nitsch R.M., Konietzko U. The APP Intracellular Domain Forms Nuclear Multiprotein Complexes and Regulates the Transcription of Its Own Precursor. J. Cell Sci. 2004;117:4435–4448. doi: 10.1242/jcs.01323. [DOI] [PubMed] [Google Scholar]
- 29.Mori H., Takio K., Ogawara M., Selkoe D.J. Mass Spectrometry of Purified Amyloid Beta Protein in Alzheimer’s Disease. J. Biol. Chem. 1992;267:17082–17086. doi: 10.1016/S0021-9258(18)41896-0. [DOI] [PubMed] [Google Scholar]
- 30.Jarrett J.T., Berger E.P., Lansbury P.T. The Carboxy Terminus of the Beta Amyloid Protein Is Critical for the Seeding of Amyloid Formation: Implications for the Pathogenesis of Alzheimer’s Disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 31.Talafous J., Marcinowski K.J., Klopman G., Zagorski M.G. Solution Structure of Residues 1–28 of the Amyloid Beta-Peptide. Biochemistry. 1994;33:7788–7796. doi: 10.1021/bi00191a006. [DOI] [PubMed] [Google Scholar]
- 32.Yang M., Teplow D.B. Amyloid β-Protein Monomer Folding: Free-Energy Surfaces Reveal Alloform-Specific Differences. J. Mol. Biol. 2008;384:450–464. doi: 10.1016/j.jmb.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T.C., Wang R., et al. Longer Forms of Amyloid Beta Protein: Implications for the Mechanism of Intramembrane Cleavage by Gamma-Secretase. J. Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schiossmacher M., Whaley J., Swindlehurst C., et al. Isolation and Quantification of Soluble Alzheimer’s β-Peptide from Biological Fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 35.Maarouf C.L., Walker J.E., Sue L.I., Dugger B.N., Beach T.G., Serrano G.E. Impaired Hepatic Amyloid-Beta Degradation in Alzheimer’s Disease. PLoS ONE. 2018;13:e0203659. doi: 10.1371/journal.pone.0203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghiso J., Shayo M., Calero M., Ng D., Tomidokoro Y., Gandy S., Rostagno A., Frangione B. Systemic Catabolism of Alzheimer’s Abeta40 and Abeta42. J. Biol. Chem. 2004;279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 37.Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E.A., Frosch M.P., Eckman C.B., Tanzi R.E., Selkoe D.J., Guenette S. Insulin-Degrading Enzyme Regulates the Levels of Insulin, Amyloid Beta-Protein, and the Beta-Amyloid Precursor Protein Intracellular Domain in Vivo. Proc. Natl. Acad. Sci. USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirotani K., Tsubuki S., Iwata N., Takaki Y., Harigaya W., Maruyama K., Kiryu-Seo S., Kiyama H., Iwata H., Tomita T., et al. Neprilysin Degrades Both Amyloid Beta Peptides 1-40 and 1-42 Most Rapidly and Efficiently among Thiorphan- and Phosphoramidon-Sensitive Endopeptidases. J. Biol. Chem. 2001;276:21895–21901. doi: 10.1074/jbc.M008511200. [DOI] [PubMed] [Google Scholar]
- 39.Tucker H.M., Kihiko M., Caldwell J.N., Wright S., Kawarabayashi T., Price D., Walker D., Scheff S., McGillis J.P., Rydel R.E., et al. The Plasmin System Is Induced by and Degrades Amyloid-Beta Aggregates. J. Neurosci. 2000;20:3937–3946. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckman E.A., Reed D.K., Eckman C.B. Degradation of the Alzheimer’s Amyloid Beta Peptide by Endothelin-Converting Enzyme. J. Biol. Chem. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- 41.Hemming M.L., Selkoe D.J. Amyloid Beta-Protein Is Degraded by Cellular Angiotensin-Converting Enzyme (ACE) and Elevated by an ACE Inhibitor. J. Biol. Chem. 2005;280:37644–37650. doi: 10.1074/jbc.M508460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata M., Yamada S., Ram Kumar S., Calero M., Bading J., Frangione B., Holtzman D.M., Miller C.A., Strickland D.K., Ghiso J., et al. Clearance of Alzheimer’s Amyloid-Β1-40 Peptide from Brain by LDL Receptor-Related Protein-1 at the Blood-Brain Barrier. J. Clin. Investig. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMattos R.B., Bales K.R., Cummins D.J., Paul S.M., Holtzman D.M. Brain to Plasma Amyloid-β Efflux: A Measure of Brain Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Science (1979) 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 44.Zlokovic B.V., Deane R., Sagare A.P., Bell R.D., Winkler E.A. Low-Density Lipoprotein Receptor-Related Protein-1: A Serial Clearance Homeostatic Mechanism Controlling Alzheimer’s Amyloid β-Peptide Elimination from the Brain. J. Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deane R., Sagare A., Hamm K., Parisi M., Lane S., Finn M.B., Holtzman D.M., Zlokovic B. V ApoE Isoform-Specific Disruption of Amyloid Beta Peptide Clearance from Mouse Brain. J. Clin. Investig. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picón-Pagès P., Gutiérrez D.A., Barranco-Almohalla A., Crepin G., Tajes M., ILL-Raga G., Guix F.X., Menéndez S., Arumí-Uría M., Vicente R., et al. Amyloid Beta-Peptide Increases BACE1 Translation through the Phosphorylation of the Eukaryotic Initiation Factor-2α. Oxidative Med. Cell. Longev. 2020;2020:2739459. doi: 10.1155/2020/2739459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guix F.X., Wahle T., Vennekens K., Snellinx A., Chávez-Gutiérrez L., Ill-Raga G., Ramos-Fernandez E., Guardia-Laguarta C., Lleó A., Arimon M., et al. Modification of γ-secretase by Nitrosative Stress Links Neuronal Ageing to Sporadic Alzheimer’s Disease. EMBO Mol. Med. 2012;4:660–673. doi: 10.1002/emmm.201200243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luca M., Luca A., Calandra C. Accelerated Aging in Major Depression: The Role of Nitro-Oxidative Stress. Oxidative Med. Cell. Longev. 2013;2013:230797. doi: 10.1155/2013/230797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellström-Lindahl E., Ravid R., Nordberg A. Age-Dependent Decline of Neprilysin in Alzheimer’s Disease and Normal Brain: Inverse Correlation with Aβ Levels. Neurobiol. Aging. 2008;29:210–221. doi: 10.1016/j.neurobiolaging.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Kochkina E.G., Plesneva S.A., Vasilev D.S., Zhuravin I.A., Turner A.J., Nalivaeva N.N. Effects of Ageing and Experimental Diabetes on Insulin-Degrading Enzyme Expression in Male Rat Tissues. Biogerontology. 2015;16:473–484. doi: 10.1007/s10522-015-9569-9. [DOI] [PubMed] [Google Scholar]
- 51.Osgood D., Miller M.C., Messier A.A., Gonzalez L., Silverberg G.D. Aging Alters MRNA Expression of Amyloid Transporter Genes at the Blood-Brain Barrier. Neurobiol. Aging. 2017;57:178–185. doi: 10.1016/j.neurobiolaging.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molina-Fernández R., Picón-Pagès P., Barranco-Almohalla A., Crepin G., Herrera-Fernández V., García-Elías A., Fanlo-Ucar H., Fernàndez-Busquets X., García-Ojalvo J., Oliva B., et al. Differential Regulation of Insulin Signalling by Monomeric and Oligomeric Amyloid Beta-Peptide. Brain Commun. 2022;4:fcac243. doi: 10.1093/braincomms/fcac243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahlgren K.N., Manelli A.M., Blaine Stine W., Baker L.K., Krafft G.A., Ladu M.J. Oligomeric and Fibrillar Species of Amyloid-β Peptides Differentially Affect Neuronal Viability. J. Biol. Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 54.Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C., Cotman C.W., Glabel C.G. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science (1979) 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 55.Lambert M.P., Barlo A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., et al. Diffusible, Nonfibrillar Ligands Derived from Aβ1–42 Are Potent Central Nervous System Neurotoxins.Pdf. Neurobiology. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsubara E., Soto C., Governale S., Frangione B., Ghiso J. Apolipoprotein J and Alzheimer’s Amyloid Beta Solubility. Pt 2Biochem. J. 1996;316:671–679. doi: 10.1042/bj3160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picón-Pagès P., Bonet J., García-García J., Garcia-Buendia J., Gutierrez D., Valle J., Gómez-Casuso C.E.S., Sidelkivska V., Alvarez A., Perálvarez-Marín A., et al. Human Albumin Impairs Amyloid β-Peptide Fibrillation Through Its C-Terminus: From Docking Modeling to Protection against Neurotoxicity in Alzheimer’s Disease. Comput. Struct. Biotechnol. J. 2019;17:963–971. doi: 10.1016/j.csbj.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X., Zhang X., Ladiwala A.R.A., Du D., Yadav J.K., Tessier P.M., Wright P.E., Kelly J.W., Buxbaum J.N. Mechanisms of Transthyretin Inhibition of β-Amyloid Aggregation in Vitro. J. Neurosci. 2013;33:19423–19433. doi: 10.1523/JNEUROSCI.2561-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdul Aziz M., Md Ashraf G., Safiqul Islam M. Link of BIN1, CLU, and IDE Gene Polymorphisms with the Susceptibility of Alzheimer’s Disease: Evidence from a Meta-Analysis. Curr. Alzheimer Res. 2022;19:302–316. doi: 10.2174/1567205019666220511140955. [DOI] [PubMed] [Google Scholar]
- 60.McLellan M.E., Kajdasz S.T., Hyman B.T., Bacskai B.J. In Vivo Imaging of Reactive Oxygen Species Specifically Associated with Thioflavine S-Positive Amyloid Plaques by Multiphoton Microscopy. J. Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hensley K., Carney J.M., Mattson M.P., Aksenova M., Harris M., Wu J.F., Floyd R.A., Butterfield D.A. A Model for β-Amyloid Aggregation and Neurotoxicity Based on Free Radical Generation by the Peptide: Relevance to Alzheimer Disease. Proc. Natl. Acad. Sci. USA. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith C.D., Carney J.M., Starke-Reed P.E., Oliver C.N., Stadtman E.R., Floyd R.A., Markesbery W.R. Excess Brain Protein Oxidation and Enzyme Dysfunction in Normal Aging and in Alzheimer Disease. Proc. Natl. Acad. Sci. USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butterfield D.A., Hensley K., Harris M., Mattson M., Carney J. β-Amyloid Peptide Free Radical Fragments Initiate Synaptosomal Lipoperoxidation in a Sequence-Specific Fashion: Implications to Alzheimer′s Disease. Biochem. Biophys. Res. Commun. 1994;200:710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- 64.Wu J., Wang A., Min Z., Xiong Y., Yan Q., Zhang J., Xu J., Zhang S. Lipoxin A4 Inhibits the Production of Proinflammatory Cytokines Induced by β-Amyloid in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2011;408:382–387. doi: 10.1016/j.bbrc.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Parajuli B., Sonobe Y., Horiuchi H., Takeuchi H., Mizuno T., Suzumura A. Oligomeric Amyloid β Induces IL-1β Processing via Production of ROS: Implication in Alzheimer’s Disease. Cell Death Dis. 2013;4:e975. doi: 10.1038/cddis.2013.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paula-Lima A.C., Adasme T., SanMartín C., Sebollela A., Hetz C., Carrasco M.A., Ferreira S.T., Hidalgo C. Amyloid β-Peptide Oligomers Stimulate RyR-Mediated Ca2+ Release Inducing Mitochondrial Fragmentation in Hippocampal Neurons and Prevent RyR-Mediated Dendritic Spine Remodeling Produced by BDNF. Antioxid. Redox Signal. 2011;14:1209–1223. doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen D., Alavi M.V., Kim K.-Y., Kang T., Scott R.T., Noh Y.H., Lindsey J.D., Wissinger B., Ellisman M.H., Weinreb R.N., et al. A New Vicious Cycle Involving Glutamate Excitotoxicity, Oxidative Stress and Mitochondrial Dynamics. Cell Death Dis. 2011;2:e240. doi: 10.1038/cddis.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.SanMartín C.D., Veloso P., Adasme T., Lobos P., Bruna B., Galaz J., García A., Hartel S., Hidalgo C., Paula-Lima A.C. RyR2-Mediated Ca2+ Release and Mitochondrial ROS Generation Partake in the Synaptic Dysfunction Caused by Amyloid β Peptide Oligomers. Front. Mol. Neurosci. 2017;10:115. doi: 10.3389/fnmol.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khachaturian Z.S., Alzheimer’s Association Calcium Hypothesis Workgroup Calcium Hypothesis of Alzheimer’s Disease and Brain Aging: A Framework for Integrating New Evidence into a Comprehensive Theory of Pathogenesis. Alzheimer’s Dement. 2017;13:178–182.e17. doi: 10.1016/j.jalz.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Texidó L., Martín-Satué M., Alberdi E., Solsona C., Matute C. Amyloid β Peptide Oligomers Directly Activate NMDA Receptors. Cell Calcium. 2011;49:184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Tackenberg C., Grinschgl S., Trutzel A., Santuccione A.C., Frey M.C., Konietzko U., Grimm J., Brandt R., Nitsch R.M. NMDA Receptor Subunit Composition Determines Beta-Amyloid-Induced Neurodegeneration and Synaptic Loss. Cell Death Dis. 2013;4:e608. doi: 10.1038/cddis.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H.Y., Lee D.H., D’Andrea M.R., Peterson P.A., Shank R.P., Reitz A.B. Beta-Amyloid(1-42) Binds to Alpha7 Nicotinic Acetylcholine Receptor with High Affinity. Implications for Alzheimer’s Disease Pathology. J. Biol. Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 73.Lasala M., Fabiani C., Corradi J., Antollini S., Bouzat C. Molecular Modulation of Human A7 Nicotinic Receptor by Amyloid-β Peptides. Front. Cell. Neurosci. 2019;13:37. doi: 10.3389/fncel.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bekdash R.A. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:1273. doi: 10.3390/ijms22031273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Picón-Pagès P., Bosch-Morató M., Subirana L., Rubio-Moscardó F., Guivernau B., Fanlo-Ucar H., Zeylan M.E., Senyuz S., Herrera-Fernández V., Vicente R., et al. A Genome-Wide Functional Screen Identifies Enhancer and Protective Genes for Amyloid Beta-Peptide Toxicity. Int. J. Mol. Sci. 2023;24:1278. doi: 10.3390/ijms24021278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arispe N., Rojas E., Pollard H.B. Alzheimer Disease Amyloid Beta Protein Forms Calcium Channels in Bilayer Membranes: Blockade by Tromethamine and Aluminum. Proc. Natl. Acad. Sci. USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., et al. Amyloid-Beta Protein Dimers Isolated Directly from Alzheimer’s Brains Impair Synaptic Plasticity and Memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ly P.T.T., Wu Y., Zou H., Wang R., Zhou W., Kinoshita A., Zhang M., Yang Y., Cai F., Woodgett J., et al. Inhibition of GSK3β-Mediated BACE1 Expression Reduces Alzheimer-Associated Phenotypes. J. Clin. Investig. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cho J.-H., Johnson G.V.W. Glycogen Synthase Kinase 3beta Phosphorylates Tau at Both Primed and Unprimed Sites. Differential Impact on Microtubule Binding. J. Biol. Chem. 2003;278:187–193. doi: 10.1074/jbc.M206236200. [DOI] [PubMed] [Google Scholar]
- 81.Jaworski T., Banach-Kasper E., Gralec K. GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural Plast. 2019;2019:4209475. doi: 10.1155/2019/4209475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maixner D.W., Weng H.-R. The Role of Glycogen Synthase Kinase 3 Beta in Neuroinflammation and Pain. J. Pharm. Pharmacol. 2013;1:1. doi: 10.13188/2327-204X.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S.J., Chung Y.H., Joo K.M., Lim H.C., Jeon G.S., Kim D., Lee W.B., Kim Y.S., Cha C.I. Age-Related Changes in Glycogen Synthase Kinase 3β (GSK3β) Immunoreactivity in the Central Nervous System of Rats. Neurosci. Lett. 2006;409:134–139. doi: 10.1016/j.neulet.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 84.Leroy K., Yilmaz Z., Brion J.-P. Increased Level of Active GSK-3beta in Alzheimer’s Disease and Accumulation in Argyrophilic Grains and in Neurones at Different Stages of Neurofibrillary Degeneration. Neuropathol. Appl. Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 85.Hammouda S., Ghzaiel I., Picón-Pagès P., Meddeb W., Khamlaoui W., Hammami S., Muñoz F.J., Hammami M., Zarrouk A. Nigella and Milk Thistle Seed Oils: Potential Cytoprotective Effects against 7β-Hydroxycholesterol-Induced Toxicity on SH-SY5Y Cells. Biomolecules. 2021;11:797. doi: 10.3390/biom11060797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nunomura A., Tamaoki T., Motohashi N., Nakamura M., McKeel D.W., Tabaton M., Lee H., Smith M.A., Perry G., Zhu X. The Earliest Stage of Cognitive Impairment in Transition from Normal Aging to Alzheimer Disease Is Marked by Prominent RNA Oxidation in Vulnerable Neurons. J. Neuropathol. Exp. Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dei R., Takeda A., Niwa H., Li M., Nakagomi Y., Watanabe M., Inagaki T., Washimi Y., Yasuda Y., Horie K., et al. Lipid Peroxidation and Advanced Glycation End Products in the Brain in Normal Aging and in Alzheimer’s Disease. Acta Neuropathol. 2002;104:113–122. doi: 10.1007/s00401-002-0523-y. [DOI] [PubMed] [Google Scholar]
- 88.Bose C., Kshirsagar S., Vijayan M., Kumar S., Singh S.P., Hindle A., Reddy P.H. The Role of RLIP76 in Oxidative Stress and Mitochondrial Dysfunction: Evidence Based on Autopsy Brains from Alzheimer’s Disease Patients. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2024;1870:166932. doi: 10.1016/j.bbadis.2023.166932. [DOI] [PubMed] [Google Scholar]
- 89.Castegna A., Aksenov M., Aksenova M., Thongboonkerd V., Klein J.B., Pierce W.M., Booze R., Markesbery W.R., Butterfield D.A. Proteomic Identification of Oxidatively Modified Proteins in Alzheimer’s Disease Brain. Part I: Creatine Kinase BB, Glutamine Synthase, and Ubiquitin Carboxy-Terminal Hydrolase L-1. Free Radic. Biol. Med. 2002;33:562–571. doi: 10.1016/S0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 90.Castegna A., Aksenov M., Thongboonkerd V., Klein J.B., Pierce W.M., Booze R., Markesbery W.R., Butterfield D.A. Proteomic Identification of Oxidatively Modified Proteins in Alzheimer’s Disease Brain. Part II: Dihydropyrimidinase-related Protein 2, A-enolase and Heat Shock Cognate 71. J. Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 91.Halliwell B. Oxidative Stress and Neurodegeneration: Where Are We Now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 92.Montine T.J., Morrow J.D. Fatty Acid Oxidation in the Pathogenesis of Alzheimer’s Disease. Am. J. Pathol. 2005;166:1283–1289. doi: 10.1016/S0002-9440(10)62347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jové M., Mota-Martorell N., Obis È., Sol J., Martín-Garí M., Ferrer I., Portero-Otín M., Pamplona R. Lipid Adaptations against Oxidative Challenge in the Healthy Adult Human Brain. Antioxidants. 2023;12:177. doi: 10.3390/antiox12010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tamagno E., Bardini P., Obbili A., Vitali A., Borghi R., Zaccheo D., Pronzato M.A., Danni O., Smith M.A., Perry G., et al. Oxidative Stress Increases Expression and Activity of BACE in NT2 Neurons. Neurobiol. Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 96.Coma M., Guix F.X., Ill-Raga G., Uribesalgo I., Alameda F., Valverde M.A., Muñoz F.J. Oxidative Stress Triggers the Amyloidogenic Pathway in Human Vascular Smooth Muscle Cells. Neurobiol. Aging. 2008;29:969–980. doi: 10.1016/j.neurobiolaging.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 97.Gutteridge J.M.C. Biological Origin of Free Radicals, and Mechanisms of Antioxidant Protection. Chem. Biol. Interact. 1994;91:133–140. doi: 10.1016/0009-2797(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 98.Behl C. Hydrogen Peroxide Mediates Amyloid β Protein Toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 99.Huang X., Atwood C.S., Hartshorn M.A., Multhaup G., Goldstein L.E., Scarpa R.C., Cuajungco M.P., Gray D.N., Lim J., Moir R.D., et al. The Aβ Peptide of Alzheimer’s Disease Directly Produces Hydrogen Peroxide through Metal Ion Reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 100.Butterfield D.A., Halliwell B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jakubowski H. Pathophysiological Consequences of Homocysteine Excess. J. Nutr. 2006;136:1741S–1749S. doi: 10.1093/jn/136.6.1741S. [DOI] [PubMed] [Google Scholar]
- 102.Li J., Chu J., Barrero C., Merali S., Praticò D. Homocysteine Exacerbates Β-amyloid Pathology, Tau Pathology, and Cognitive Deficit in a Mouse Model of Alzheimer Disease with Plaques and Tangles. Ann. Neurol. 2014;75:851–863. doi: 10.1002/ana.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kruman I.I., Culmsee C., Chan S.L., Kruman Y., Guo Z., Penix L., Mattson M.P. Homocysteine Elicits a DNA Damage Response in Neurons That Promotes Apoptosis and Hypersensitivity to Excitotoxicity. J. Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carey A., Parodi-Rullan R., Vazquez-Torres R., Canepa E., Fossati S. Homocysteine Potentiates Amyloid Β-induced Death Receptor 4- and 5-mediated Cerebral Endothelial Cell Apoptosis, Blood Brain Barrier Dysfunction and Angiogenic Impairment. Aging Cell. 2024;23:e14106. doi: 10.1111/acel.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ill-Raga G., Ramos-Fernández E., Guix F.X., Tajes M., Bosch-Morató M., Palomer E., Godoy J., Belmar S., Cerpa W., Simpkins J.W., et al. Amyloid-β Peptide Fibrils Induce Nitro-Oxidative Stress in Neuronal Cells. J. Alzheimer’s Dis. 2010;22:641–652. doi: 10.3233/JAD-2010-100474. [DOI] [PubMed] [Google Scholar]
- 106.Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/S0022-510X(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 107.Smith D.G., Cappai R., Barnham K.J. The Redox Chemistry of the Alzheimer’s Disease Amyloid β Peptide. Biochim. Biophys. Acta (BBA)—Biomembr. 2007;1768:1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The Role of Iron in Brain Ageing and Neurodegenerative Disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Castro P.A., Ramirez A., SepÃlveda F.J., Peters C., Fierro H., Waldron J., Luza S., Fuentealba J., MuÃoz F.J., De Ferrari G.V., et al. Copper-Uptake Is Critical for the down Regulation of Synapsin and Dynamin Induced by Neocuproine: Modulation of Synaptic Activity in Hippocampal Neurons. Front. Aging Neurosci. 2014;6:319. doi: 10.3389/fnagi.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ayton S., Portbury S., Kalinowski P., Agarwal P., Diouf I., Schneider J.A., Morris M.C., Bush A.I. Regional Brain Iron Associated with Deterioration in Alzheimer’s Disease: A Large Cohort Study and Theoretical Significance. Alzheimer’s Dement. 2021;17:1244–1256. doi: 10.1002/alz.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miller Y., Ma B., Nussinov R. Zinc Ions Promote Alzheimer Aβ Aggregation via Population Shift of Polymorphic States. Proc. Natl. Acad. Sci. USA. 2010;107:9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ionescu-Tucker A., Cotman C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging. 2021;107:86–95. doi: 10.1016/j.neurobiolaging.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 113.Ma T., Hoeffer C.A., Wong H., Massaad C.A., Zhou P., Iadecola C., Murphy M.P., Pautler R.G., Klann E. Amyloid β-Induced Impairments in Hippocampal Synaptic Plasticity Are Rescued by Decreasing Mitochondrial Superoxide. J. Neurosci. 2011;31:5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perez Ortiz J.M., Swerdlow R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharmacol. 2019;176:3489–3507. doi: 10.1111/bph.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhatia S., Rawal R., Sharma P., Singh T., Singh M., Singh V. Mitochondrial Dysfunction in Alzheimer’s Disease: Opportunities for Drug Development. Curr. Neuropharmacol. 2022;20:675–692. doi: 10.2174/1570159X19666210517114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patro S., Ratna S., Yamamoto H.A., Ebenezer A.T., Ferguson D.S., Kaur A., McIntyre B.C., Snow R., Solesio M.E. ATP Synthase and Mitochondrial Bioenergetics Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:11185. doi: 10.3390/ijms222011185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godoy J.A., Rios J.A., Picón-Pagès P., Herrera-Fernández V., Swaby B., Crepin G., Vicente R., Fernández-Fernández J.M., Muñoz F.J. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules. 2021;11:1012. doi: 10.3390/biom11071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Misrani A., Tabassum S., Yang L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021;13:617588. doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Islam M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017;39:73–82. doi: 10.1080/01616412.2016.1251711. [DOI] [PubMed] [Google Scholar]
- 120.Khodagholi F., Shaerzadeh F., Montazeri F. Mitochondrial Aconitase in Neurodegenerative Disorders: Role of a Metabolism- Related Molecule in Neurodegeneration. Curr. Drug Targets. 2018;19:973–985. doi: 10.2174/1389450118666170816124203. [DOI] [PubMed] [Google Scholar]
- 121.Sultana R., Boyd-Kimball D., Poon H.F., Cai J., Pierce W.M., Klein J.B., Merchant M., Markesbery W.R., Butterfield D.A. Redox Proteomics Identification of Oxidized Proteins in Alzheimer’s Disease Hippocampus and Cerebellum: An Approach to Understand Pathological and Biochemical Alterations in AD. Neurobiol. Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 122.Mecocci P., MacGarvey U., Beal M.F. Oxidative Damage to Mitochondrial DNA Is Increased in Alzheimer’s Disease. Ann. Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 123.Aran K.R., Singh S. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease—A Step towards Mitochondria Based Therapeutic Strategies. Aging Health Res. 2023;3:100169. doi: 10.1016/j.ahr.2023.100169. [DOI] [Google Scholar]
- 124.Solleiro-Villavicencio H., Rivas-Arancibia S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018;12:114. doi: 10.3389/fncel.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hendrickx J.O., Martinet W., Van Dam D., De Meyer G.R.Y. Inflammation, Nitro-Oxidative Stress, Impaired Autophagy, and Insulin Resistance as a Mechanistic Convergence between Arterial Stiffness and Alzheimer’s Disease. Front. Mol. Biosci. 2021;8:651215. doi: 10.3389/fmolb.2021.651215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Behrouzi A., Kelley M.R., Fehrenbacher J.C. Oxidative DNA Damage: A Role in Altering Neuronal Function. J. Cell. Signal. 2022;3:160. doi: 10.33696/Signaling.3.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shadfar S., Brocardo M., Atkin J.D. The Complex Mechanisms by Which Neurons Die Following DNA Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022;23:2484. doi: 10.3390/ijms23052484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nelson T.J., Xu Y. Sting and P53 DNA Repair Pathways Are Compromised in Alzheimer’s Disease. Sci. Rep. 2023;13:8304. doi: 10.1038/s41598-023-35533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ranganathan R., Sapozhnikov G., Ni W., Li S., Song Y. Recent Developments in the Role of DNA Damage Response and Understanding Its Implications for New Therapeutic Approaches in Alzheimer’s Disease. Transl. Med. Aging. 2023;7:52–65. doi: 10.1016/j.tma.2023.06.004. [DOI] [Google Scholar]
- 130.Song X., Ma F., Herrup K. DNA Damage-induced Neuroinflammation in Neurodegenerative Disease. Alzheimer’s Dement. 2021;17:e055175. doi: 10.1002/alz.055175. [DOI] [Google Scholar]
- 131.Sultana R., Perluigi M., Newman S.F., Pierce W.M., Cini C., Coccia R., Butterfield D.A. Redox Proteomic Analysis of Carbonylated Brain Proteins in Mild Cognitive Impairment and Early Alzheimer’s Disease. Antioxid. Redox Signal. 2010;12:327–336. doi: 10.1089/ars.2009.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Picón-Pagès P., Fanlo-Ucar H., Herrera-Fernández V., Ausellé-Bosch S., Galera-López L., Gutiérrez D.A., Ozaita A., Álvarez A.R., Oliva B., Muñoz F.J. Amyloid β-Peptide Causes the Permanent Activation of CaMKIIα through Its Oxidation. Int. J. Mol. Sci. 2022;23:15169. doi: 10.3390/ijms232315169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guix F.X., Ill-Raga G., Bravo R., Nakaya T., de Fabritiis G., Coma M., Miscione G.P., Villà-Freixa J., Suzuki T., Fernàndez-Busquets X., et al. Amyloid-Dependent Triosephosphate Isomerase Nitrotyrosination Induces Glycation and Tau Fibrillation. Brain. 2009;132:1335–1345. doi: 10.1093/brain/awp023. [DOI] [PubMed] [Google Scholar]
- 134.Tajes M., Guivernau B., Ramos-Fernández E., Bosch-Morató M., Palomer E., Guix F.X., Muñoz F.J. The Pathophysiology of Triose Phosphate Isomerase Dysfunction in Alzheimer’s Disease. Histol. Histopathol. 2013;28:43–51. doi: 10.14670/HH-28.43. [DOI] [PubMed] [Google Scholar]
- 135.Tajes M., Eraso-Pichot A., Rubio-Moscardó F., Guivernau B., Ramos-Fernández E., Bosch-Morató M., Guix F.X., Clarimón J., Miscione G.P., Boada M., et al. Methylglyoxal Produced by Amyloid-β Peptide-Induced Nitrotyrosination of Triosephosphate Isomerase Triggers Neuronal Death in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014;41:273–288. doi: 10.3233/JAD-131685. [DOI] [PubMed] [Google Scholar]
- 136.Tajes M., Eraso-Pichot A., Rubio-Moscardó F., Guivernau B., Bosch-Morató M., Valls-Comamala V., Muñoz F.J. Methylglyoxal Reduces Mitochondrial Potential and Activates Bax and Caspase-3 in Neurons: Implications for Alzheimer’s Disease. Neurosci. Lett. 2014;580:78–82. doi: 10.1016/j.neulet.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 137.Sharma A., Weber D., Raupbach J., Dakal T.C., Fließbach K., Ramirez A., Grune T., Wüllner U. Advanced Glycation End Products and Protein Carbonyl Levels in Plasma Reveal Sex-Specific Differences in Parkinson’s and Alzheimer’s Disease. Redox Biol. 2020;34:101546. doi: 10.1016/j.redox.2020.101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramos-Fernández E., Tajes M., Palomer E., ILL-Raga G., Bosch-Morató M., Guivernau B., Román-Dégano I., Eraso-Pichot A., Alcolea D., Fortea J., et al. Posttranslational Nitro-Glycative Modifications of Albumin in Alzheimer’s Disease: Implications in Cytotoxicity and Amyloid-β Peptide Aggregation. J. Alzheimer’s Dis. 2014;40:643–657. doi: 10.3233/JAD-130914. [DOI] [PubMed] [Google Scholar]
- 139.Lennicke C., Cochemé H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell. 2021;81:3691–3707. doi: 10.1016/j.molcel.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 140.Conway M.E., Lee C. The Redox Switch That Regulates Molecular Chaperones. Biomol. Concepts. 2015;6:269–284. doi: 10.1515/bmc-2015-0015. [DOI] [PubMed] [Google Scholar]
- 141.Gumusyayla S., Vural G., Bektas H., Deniz O., Neselioglu S., Erel O. A Novel Oxidative Stress Marker in Patients with Alzheimer’s Disease: Dynamic Thiol–Disulphide Homeostasis. Acta Neuropsychiatr. 2016;28:315–320. doi: 10.1017/neu.2016.13. [DOI] [PubMed] [Google Scholar]
- 142.Cioffi F., Adam R.H.I., Bansal R., Broersen K. A Review of Oxidative Stress Products and Related Genes in Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2021;83:977–1001. doi: 10.3233/JAD-210497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Y., Zhao T., Li J., Xia M., Li Y., Wang X., Liu C., Zheng T., Chen R., Kan D., et al. Oxidative Stress and 4-Hydroxy-2-Nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-Related Diseases. J. Immunol. Res. 2022;2022:2233906. doi: 10.1155/2022/2233906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Butterfield D.A. Brain Lipid Peroxidation and Alzheimer Disease: Synergy between the Butterfield and Mattson Laboratories. Ageing Res. Rev. 2020;64:101049. doi: 10.1016/j.arr.2020.101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Friedemann M., Helk E., Tiiman A., Zovo K., Palumaa P., Tõugu V. Effect of Methionine-35 Oxidation on the Aggregation of Amyloid-β Peptide. Biochem. Biophys. Rep. 2015;3:94–99. doi: 10.1016/j.bbrep.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alquezar C., Arya S., Kao A.W. Tau Post-Translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 2020;11:595532. doi: 10.3389/fneur.2020.595532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hatters D.M. Flipping the Switch: How Cysteine Oxidation Directs Tau Amyloid Conformations. J. Biol. Chem. 2021;297:101309. doi: 10.1016/j.jbc.2021.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jeong Y.A., Yun H.S., Kim Y., Jang C.H., Lim J.S., Kim H.J., Choi M.B., Jung J.W., Oh J., Kim J.-S. Long-Term Administration of Vespa Velutina Nigrithorax Venom Ameliorates Alzheimer’s Phenotypes in 5xFAD Transgenic Mice. Toxins. 2023;15:203. doi: 10.3390/toxins15030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang H., Jin B., Faber J.E. Mouse Models of Alzheimer’s Disease Cause Rarefaction of Pial Collaterals and Increased Severity of Ischemic Stroke. Angiogenesis. 2019;22:263–279. doi: 10.1007/s10456-018-9655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boonruamkaew P., Chonpathompikunlert P., Vong L.B., Sakaue S., Tomidokoro Y., Ishii K., Tamaoka A., Nagasaki Y. Chronic Treatment with a Smart Antioxidative Nanoparticle for Inhibition of Amyloid Plaque Propagation in Tg2576 Mouse Model of Alzheimer’s Disease. Sci. Rep. 2017;7:3785. doi: 10.1038/s41598-017-03411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ren P., Chen J., Li B., Zhang M., Yang B., Guo X., Chen Z., Cheng H., Wang P., Wang S., et al. Nrf2 Ablation Promotes Alzheimer’s Disease-like Pathology in APP/PS1 Transgenic Mice: The Role of Neuroinflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2020;2020:3050971. doi: 10.1155/2020/3050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jin P., Kim J.-A., Choi D.-Y., Lee Y.-J., Jung H.S., Hong J.T. Anti-Inflammatory and Anti-Amyloidogenic Effects of a Small Molecule, 2,4-Bis(p-Hydroxyphenyl)-2-Butenal in Tg2576 Alzheimer’s Disease Mice Model. J. Neuroinflamm. 2013;10:767. doi: 10.1186/1742-2094-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abbink M.R., Kotah J.M., Hoeijmakers L., Mak A., Yvon-Durocher G., van der Gaag B., Lucassen P.J., Korosi A. Characterization of Astrocytes throughout Life in Wildtype and APP/PS1 Mice after Early-Life Stress Exposure. J. Neuroinflamm. 2020;17:91. doi: 10.1186/s12974-020-01762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marino K.M., Squirrell J.M., Chacko J.V., Watters J.W., Eliceiri K.W., Ulland T.K. Metabolic Response of Microglia to Amyloid Deposition during Alzheimer’s Disease Progression in a Mouse Model. bioRxiv. 2023 doi: 10.1101/2023.05.12.540407. [DOI] [Google Scholar]
- 155.Pao P.-C., Patnaik D., Watson L.A., Gao F., Pan L., Wang J., Adaikkan C., Penney J., Cam H.P., Huang W.-C., et al. HDAC1 Modulates OGG1-Initiated Oxidative DNA Damage Repair in the Aging Brain and Alzheimer’s Disease. Nat. Commun. 2020;11:2484. doi: 10.1038/s41467-020-16361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gendron W.H., Fertan E., Pelletier S., Roddick K.M., O’Leary T.P., Anini Y., Brown R.E. Age Related Weight Loss in Female 5xFAD Mice from 3 to 12 Months of Age. Behav. Brain Res. 2021;406:113214. doi: 10.1016/j.bbr.2021.113214. [DOI] [PubMed] [Google Scholar]
- 157.Abdelmoaty M.M., Yeapuri P., Machhi J., Lu Y., Namminga K.L., Kadry R., Lu E., Bhattarai S., Mosley R.L., Gendelman H.E. Immune Senescence in Aged APP/PS1 Mice. NeuroImmune Pharmacol. Ther. 2023;2:317–330. doi: 10.1515/nipt-2023-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang X., Atwood C.S., Moir R.D., Hartshorn M.A., Tanzi R.E., Bush A.I. Trace Metal Contamination Initiates the Apparent Auto-Aggregation, Amyloidosis, and Oligomerization of Alzheimer’s Aβ Peptides. JBIC J. Biol. Inorg. Chem. 2004;9:954–960. doi: 10.1007/s00775-004-0602-8. [DOI] [PubMed] [Google Scholar]
- 159.ILL-Raga G., Tajes M., Busquets-García A., Ramos-Fernández E., Vargas L.M., Bosch-Morató M., Guivernau B., Valls-Comamala V., Eraso-Pichot A., Guix F.X., et al. Physiological Control of Nitric Oxide in Neuronal BACE1 Translation by Heme-Regulated EIF2α Kinase HRI Induces Synaptogenesis. Antioxid. Redox Signal. 2015;22:1295–1307. doi: 10.1089/ars.2014.6080. [DOI] [PubMed] [Google Scholar]
- 160.ILL-Raga G., Palomer E., Wozniak M.A., Ramos-Fernández E., Bosch-Morató M., Tajes M., Guix F.X., Galán J.J., Clarimón J., Antúnez C., et al. Activation of PKR Causes Amyloid SS-Peptide Accumulation via De-Repression of BACE1 Expression. PLoS ONE. 2011;6:e21456. doi: 10.1371/journal.pone.0021456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Somin S., Kulasiri D., Samarasinghe S. Alleviating the Unwanted Effects of Oxidative Stress on Aβ Clearance: A Review of Related Concepts and Strategies for the Development of Computational Modelling. Transl. Neurodegener. 2023;12:11. doi: 10.1186/s40035-023-00344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guix F.X., Uribesalgo I., Coma M., Muñoz F.J. The Physiology and Pathophysiology of Nitric Oxide in the Brain. Prog. Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 163.Picón-Pagès P., Garcia-Buendia J., Muñoz F.J. Functions and Dysfunctions of Nitric Oxide in Brain. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019;1865:1949–1967. doi: 10.1016/j.bbadis.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 164.Muñoz F.J., Opazo C., Gil-Gómez G., Tapia G., Fernández V., Valverde M.A., Inestrosa N.C. Vitamin E but not 17β-Estradiol Protects against Vascular Toxicity Induced by β-Amyloid Wild Type and the Dutch Amyloid Variant. J. Neurosci. 2002;22:3081–3089. doi: 10.1523/JNEUROSCI.22-08-03081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.ILL-Raga G., Palomer E., Ramos-Fernández E., Guix F.X., Bosch-Morató M., Guivernau B., Tajes M., Valls-Comamala V., Jiménez-Conde J., Ois A., et al. Fibrinogen Nitrotyrosination after Ischemic Stroke Impairs Thrombolysis and Promotes Neuronal Death. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015;1852:421–428. doi: 10.1016/j.bbadis.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 166.Tajes M., ILL-Raga G., Palomer E., Ramos-Fernández E., Guix F.X., Bosch-Morató M., Guivernau B., Jiménez-Conde J., Ois A., Pérez-Asensio F., et al. Nitro-Oxidative Stress after Neuronal Ischemia Induces Protein Nitrotyrosination and Cell Death. Oxidative Med. Cell. Longev. 2013;2013:826143. doi: 10.1155/2013/826143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Colton C.A., Vitek M.P., Wink D.A., Xu Q., Cantillana V., Previti M.L., Van Nostrand W.E., Weinberg J.B., Dawson H. NO Synthase 2 (NOS2) Deletion Promotes Multiple Pathologies in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA. 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Minhas R., Bansal Y., Bansal G. Inducible Nitric Oxide Synthase Inhibitors: A Comprehensive Update. Med. Res. Rev. 2020;40:823–855. doi: 10.1002/med.21636. [DOI] [PubMed] [Google Scholar]
- 169.Sarti P., Forte E., Mastronicola D., Giuffrè A., Arese M. Cytochrome c Oxidase and Nitric Oxide in Action: Molecular Mechanisms and Pathophysiological Implications. Biochim. Biophys. Acta (BBA)—Bioenerg. 2012;1817:610–619. doi: 10.1016/j.bbabio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 170.Peng Y., Gao P., Shi L., Chen L., Liu J., Long J. Central and Peripheral Metabolic Defects Contribute to the Pathogenesis of Alzheimer’s Disease: Targeting Mitochondria for Diagnosis and Prevention. Antioxid. Redox Signal. 2020;32:1188–1236. doi: 10.1089/ars.2019.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luan Y., Luan Y., Yuan R.-X., Feng Q., Chen X., Yang Y. Structure and Function of Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) and Their Role in Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2021;2021:4578809. doi: 10.1155/2021/4578809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stamler J.S., Toone E.J., Lipton S.A., Sucher N.J. (S)NO Signals: Translocation, Regulation, and a Consensus Motif. Neuron. 1997;18:691–696. doi: 10.1016/S0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 173.di Penta A., Moreno B., Reix S., Fernandez-Diez B., Villanueva M., Errea O., Escala N., Vandenbroeck K., Comella J.X., Villoslada P. Oxidative Stress and Proinflammatory Cytokines Contribute to Demyelination and Axonal Damage in a Cerebellar Culture Model of Neuroinflammation. PLoS ONE. 2013;8:e54722. doi: 10.1371/journal.pone.0054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chatterji A., Banerjee D., Billiar T.R., Sengupta R. Understanding the Role of S-Nitrosylation/Nitrosative Stress in Inflammation and the Role of Cellular Denitrosylases in Inflammation Modulation: Implications in Health and Diseases. Free Radic. Biol. Med. 2021;172:604–621. doi: 10.1016/j.freeradbiomed.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 175.Ghatak S., Nakamura T., Lipton S.A. Aberrant Protein S-Nitrosylation Contributes to Hyperexcitability-Induced Synaptic Damage in Alzheimer’s Disease: Mechanistic Insights and Potential Therapies. Front. Neural Circuits. 2023;17:1099467. doi: 10.3389/fncir.2023.1099467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cho D.-H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-Nitrosylation of Drp1 Mediates Beta-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nakamura T., Tu S., Akhtar M.W., Sunico C.R., Okamoto S.-I., Lipton S.A. Aberrant Protein S-Nitrosylation in Neurodegenerative Diseases. Neuron. 2013;78:596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gupta S., You P., SenGupta T., Nilsen H., Sharma K. Crosstalk between Different DNA Repair Pathways Contributes to Neurodegenerative Diseases. Biology. 2021;10:163. doi: 10.3390/biology10020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Coma M., Guix F.X., Uribesalgo I., Espuña G., Solé M., Andreu D., Muñoz F.J. Lack of Oestrogen Protection in Amyloid-Mediated Endothelial Damage Due to Protein Nitrotyrosination. Brain. 2005;128:1613–1621. doi: 10.1093/brain/awh492. [DOI] [PubMed] [Google Scholar]
- 180.Zhang Y.J., Xu Y.F., Chen X.Q., Wang X.C., Wang J.-Z. Nitration and Oligomerization of Tau Induced by Peroxynitrite Inhibit Its Microtubule-binding Activity. FEBS Lett. 2005;579:2421–2427. doi: 10.1016/j.febslet.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 181.Reynolds M.R., Reyes J.F., Fu Y., Bigio E.H., Guillozet-Bongaarts A.L., Berry R.W., Binder L.I. Tau Nitration Occurs at Tyrosine 29 in the Fibrillar Lesions of Alzheimer’s Disease and Other Tauopathies. J. Neurosci. 2006;26:10636–10645. doi: 10.1523/JNEUROSCI.2143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guivernau B., Bonet J., Valls-Comamala V., Bosch-Morató M., Godoy J.A., Inestrosa N.C., Perálvarez-Marín A., Fernández-Busquets X., Andreu D., Oliva B., et al. Amyloid-β Peptide Nitrotyrosination Stabilizes Oligomers and Enhances NMDAR-Mediated Toxicity. J. Neurosci. 2016;36:11693–11703. doi: 10.1523/JNEUROSCI.1081-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Williamson K.S., Prasad Gabbita S., Mou S., West M., Pye Q.N., Markesbery W.R., Cooney R.V., Grammas P., Reimann-Philipp U., Floyd R.A., et al. The Nitration Product 5-Nitro-γ-Tocopherol Is Increased in the Alzheimer Brain. Nitric Oxide. 2002;6:221–227. doi: 10.1006/niox.2001.0399. [DOI] [PubMed] [Google Scholar]
- 184.Pérez de la Lastra J.M., Juan C.A., Plou F.J., Pérez-Lebeña E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses. 2022;2:53–64. doi: 10.3390/stresses2010005. [DOI] [Google Scholar]
- 185.Zuo L., Prather E.R., Stetskiv M., Garrison D.E., Meade J.R., Peace T.I., Zhou T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019;20:4472. doi: 10.3390/ijms20184472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ferrucci L., Fabbri E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kosyreva A., Sentyabreva A., Tsvetkov I., Makarova O. Alzheimer’s Disease and Inflammaging. Brain Sci. 2022;12:1237. doi: 10.3390/brainsci12091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hampel H., Hardy J., Blennow K., Chen C., Perry G., Kim S.H., Villemagne V.L., Aisen P., Vendruscolo M., Iwatsubo T., et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry. 2021;26:5481–5503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu Y., Eisel U.L.M. Microglia-Astrocyte Communication in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023;95:785–803. doi: 10.3233/JAD-230199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meraz-Ríos M.A., Toral-Rios D., Franco-Bocanegra D., Villeda-Hernández J., Campos-Peña V. Inflammatory Process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lazic A., Balint V., Stanisavljevic Ninkovic D., Peric M., Stevanovic M. Reactive and Senescent Astroglial Phenotypes as Hallmarks of Brain Pathologies. Int. J. Mol. Sci. 2022;23:4995. doi: 10.3390/ijms23094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Verkhratsky A., Nedergaard M. Physiology of Astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Alberini C.M., Cruz E., Descalzi G., Bessières B., Gao V. Astrocyte Glycogen and Lactate: New Insights into Learning and Memory Mechanisms. Glia. 2018;66:1244–1262. doi: 10.1002/glia.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pfrieger F.W., Ungerer N. Cholesterol Metabolism in Neurons and Astrocytes. Prog. Lipid Res. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 195.Dietschy J.M. Central Nervous System: Cholesterol Turnover, Brain Development and Neurodegeneration. Biol. Chem. 2009;390:287–293. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tajes M., Ramos-Fernández E., Weng-Jiang X., Bosch-Morató M., Guivernau B., Eraso-Pichot A., Salvador B., Fernàndez-Busquets X., Roquer J., Muñoz F.J. The Blood-Brain Barrier: Structure, Function and Therapeutic Approaches to Cross It. Mol. Membr. Biol. 2014;31:152–167. doi: 10.3109/09687688.2014.937468. [DOI] [PubMed] [Google Scholar]
- 197.Calvetti D., Somersalo E. Ménage à Trois: The Role of Neurotransmitters in the Energy Metabolism of Astrocytes, Glutamatergic, and GABAergic Neurons. J. Cereb. Blood Flow Metab. 2012;32:1472–1483. doi: 10.1038/jcbfm.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Covelo A., Araque A. Neuronal Activity Determines Distinct Gliotransmitter Release from a Single Astrocyte. eLife. 2018;7:e32237. doi: 10.7554/eLife.32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Araque A., Carmignoto G., Haydon P.G., Oliet S.H.R., Robitaille R., Volterra A. Gliotransmitters Travel in Time and Space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Falcón-Moya R., Pérez-Rodríguez M., Prius-Mengual J., Andrade-Talavera Y., Arroyo-García L.E., Pérez-Artés R., Mateos-Aparicio P., Guerra-Gomes S., Oliveira J.F., Flores G., et al. Astrocyte-Mediated Switch in Spike Timing-Dependent Plasticity during Hippocampal Development. Nat. Commun. 2020;11:4388. doi: 10.1038/s41467-020-18024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pike C.J., Cummings B.J., Monzavi R., Cotman C.W. β-Amyloid-Induced Changes in Cultured Astrocytes Parallel Reactive Astrocytosis Associated with Senile Plaques in Alzheimer’s Disease. Neuroscience. 1994;63:517–531. doi: 10.1016/0306-4522(94)90547-9. [DOI] [PubMed] [Google Scholar]
- 202.Wyssenbach A., Quintela T., Llavero F., Zugaza J.L., Matute C., Alberdi E. Amyloid Β-induced Astrogliosis Is Mediated by Β1-integrin via NADPH Oxidase 2 in Alzheimer’s Disease. Aging Cell. 2016;15:1140–1152. doi: 10.1111/acel.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sobolczyk M., Boczek T. Astrocytic Calcium and CAMP in Neurodegenerative Diseases. Front. Cell. Neurosci. 2022;16:889939. doi: 10.3389/fncel.2022.889939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Le Douce J., Maugard M., Veran J., Matos M., Jégo P., Vigneron P.-A., Faivre E., Toussay X., Vandenberghe M., Balbastre Y., et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020;31:503–517.e8. doi: 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 205.Shao J., Deng Q., Feng S., Wu C., Liu X., Yang L. Role of Astrocytes in Alzheimer’s Disease Pathogenesis and the Impact of Exercise-Induced Remodeling. Biochem. Biophys. Res. Commun. 2024;732:150418. doi: 10.1016/j.bbrc.2024.150418. [DOI] [PubMed] [Google Scholar]
- 206.Mookherjee P., Green P.S., Watson G.S., Marques M.A., Tanaka K., Meeker K.D., Meabon J.S., Li N., Zhu P., Olson V.G., et al. GLT-1 Loss Accelerates Cognitive Deficit Onset in an Alzheimer’s Disease Animal Model. J. Alzheimer’s Dis. 2011;26:447–455. doi: 10.3233/JAD-2011-110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Abramov A.Y., Canevari L., Duchen M.R. β-Amyloid Peptides Induce Mitochondrial Dysfunction and Oxidative Stress in Astrocytes and Death of Neurons through Activation of NADPH Oxidase. J. Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sukachev V.A., Gritsaĭ N.P. Planar Displacement of the Mandible at the Level of a Bilateral Fracture in a Patient with a Bite Anomaly. Stomatologiia. 1976;55:95–96. [PubMed] [Google Scholar]
- 209.Chen Y., Qin C., Huang J., Tang X., Liu C., Huang K., Xu J., Guo G., Tong A., Zhou L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020;53:e12781. doi: 10.1111/cpr.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lian H., Yang L., Cole A., Sun L., Chiang A.C.-A., Fowler S.W., Shim D.J., Rodriguez-Rivera J., Taglialatela G., Jankowsky J.L., et al. NFκB-Activated Astroglial Release of Complement C3 Compromises Neuronal Morphology and Function Associated with Alzheimer’s Disease. Neuron. 2015;85:101–115. doi: 10.1016/j.neuron.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Saha P., Guha S., Biswas S.C. P38K and JNK Pathways Are Induced by Amyloid-β in Astrocyte: Implication of MAPK Pathways in Astrogliosis in Alzheimer’s Disease. Mol. Cell. Neurosci. 2020;108:103551. doi: 10.1016/j.mcn.2020.103551. [DOI] [PubMed] [Google Scholar]
- 212.Hu J., Akama K.T., Krafft G.A., Chromy B.A., Van Eldik L.J. Amyloid-β Peptide Activates Cultured Astrocytes: Morphological Alterations, Cytokine Induction and Nitric Oxide Release. Brain Res. 1998;785:195–206. doi: 10.1016/S0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 213.Frost G.R., Li Y.-M. The Role of Astrocytes in Amyloid Production and Alzheimer’s Disease. Open Biol. 2017;7:170228. doi: 10.1098/rsob.170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.LaRocca T.J., Cavalier A.N., Roberts C.M., Lemieux M.R., Ramesh P., Garcia M.A., Link C.D. Amyloid Beta Acts Synergistically as a Pro-Inflammatory Cytokine. Neurobiol. Dis. 2021;159:105493. doi: 10.1016/j.nbd.2021.105493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Smits H.A., Rijsmus A., van Loon J.H., Wat J.W.Y., Verhoef J., Boven L.A., Nottet H.S.L.M. Amyloid-β-Induced Chemokine Production in Primary Human Macrophages and Astrocytes. J. Neuroimmunol. 2002;127:160–168. doi: 10.1016/S0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- 216.Wang T., Yao Y., Han C., Li T., Du W., Xue J., Han Y., Cai Y. MCP-1 Levels in Astrocyte-Derived Exosomes Are Changed in Preclinical Stage of Alzheimer’s Disease. Front. Neurol. 2023;14:1119298. doi: 10.3389/fneur.2023.1119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jones R.S., Minogue A.M., Connor T.J., Lynch M.A. Amyloid-β-Induced Astrocytic Phagocytosis Is Mediated by CD36, CD47 and RAGE. J. Neuroimmune Pharmacol. 2013;8:301–311. doi: 10.1007/s11481-012-9427-3. [DOI] [PubMed] [Google Scholar]
- 218.Alliot F., Godin I., Pessac B. Microglia Derive from Progenitors, Originating from the Yolk Sac, and Which Proliferate in the Brain. Dev. Brain Res. 1999;117:145–152. doi: 10.1016/S0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 219.Paolicelli R.C., Sierra A., Stevens B., Tremblay M.-E., Aguzzi A., Ajami B., Amit I., Audinat E., Bechmann I., Bennett M., et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron. 2022;110:3458–3483. doi: 10.1016/j.neuron.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Davis B.M., Salinas-Navarro M., Cordeiro M.F., Moons L., De Groef L. Characterizing Microglia Activation: A Spatial Statistics Approach to Maximize Information Extraction. Sci. Rep. 2017;7:1576. doi: 10.1038/s41598-017-01747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Monsorno K., Ginggen K., Ivanov A., Buckinx A., Lalive A.L., Tchenio A., Benson S., Vendrell M., D’Alessandro A., Beule D., et al. Loss of Microglial MCT4 Leads to Defective Synaptic Pruning and Anxiety-like Behavior in Mice. Nat. Commun. 2023;14:5749. doi: 10.1038/s41467-023-41502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bitzer-Quintero O.K., González-Burgos I. Immune System in the Brain: A Modulatory Role on Dendritic Spine Morphophysiology? Neural Plast. 2012;2012:348642. doi: 10.1155/2012/348642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kurematsu C., Sawada M., Ohmuraya M., Tanaka M., Kuboyama K., Ogino T., Matsumoto M., Oishi H., Inada H., Ishido Y., et al. Synaptic Pruning of Murine Adult-Born Neurons by Microglia Depends on Phosphatidylserine. J. Exp. Med. 2022;219:e20202304. doi: 10.1084/jem.20202304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Du Y., Brennan F.H., Popovich P.G., Zhou M. Microglia Maintain the Normal Structure and Function of the Hippocampal Astrocyte Network. Glia. 2022;70:1359–1379. doi: 10.1002/glia.24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Komori T., Okamura K., Ikehara M., Yamamuro K., Endo N., Okumura K., Yamauchi T., Ikawa D., Ouji-Sageshima N., Toritsuka M., et al. Brain-Derived Neurotrophic Factor from Microglia Regulates Neuronal Development in the Medial Prefrontal Cortex and Its Associated Social Behavior. Mol. Psychiatry. 2024;29:1338–1349. doi: 10.1038/s41380-024-02413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rusin D., Vahl Becirovic L., Lyszczarz G., Krueger M., Benmamar-Badel A., Vad Mathiesen C., Sigurðardóttir Schiöth E., Lykke Lambertsen K., Wlodarczyk A. Microglia-Derived Insulin-like Growth Factor 1 Is Critical for Neurodevelopment. Cells. 2024;13:184. doi: 10.3390/cells13020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wendimu M.Y., Hooks S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells. 2022;11:2091. doi: 10.3390/cells11132091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sebastian Monasor L., Müller S.A., Colombo A.V., Tanrioever G., König J., Roth S., Liesz A., Berghofer A., Piechotta A., Prestel M., et al. Fibrillar Aβ Triggers Microglial Proteome Alterations and Dysfunction in Alzheimer Mouse Models. eLife. 2020;9:e54083. doi: 10.7554/eLife.54083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Coraci I.S., Husemann J., Berman J.W., Hulette C., Dufour J.H., Campanella G.K., Luster A.D., Silverstein S.C., El Khoury J.B. CD36, a Class B Scavenger Receptor, Is Expressed on Microglia in Alzheimer’s Disease Brains and Can Mediate Production of Reactive Oxygen Species in Response to β-Amyloid Fibrils. Am. J. Pathol. 2002;160:101–112. doi: 10.1016/S0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Deane R., Singh I., Sagare A.P., Bell R.D., Ross N.T., LaRue B., Love R., Perry S., Paquette N., Deane R.J., et al. A Multimodal RAGE-Specific Inhibitor Reduces Amyloid β–Mediated Brain Disorder in a Mouse Model of Alzheimer Disease. J. Clin. Investig. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Feng J., Wang J., Du Y., Liu Y., Zhang W., Chen J., Liu Y., Zheng M., Wang K., He G. Dihydromyricetin Inhibits Microglial Activation and Neuroinflammation by Suppressing NLRP3 Inflammasome Activation in APP/PS1 Transgenic Mice. CNS Neurosci. Ther. 2018;24:1207–1218. doi: 10.1111/cns.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kong Y., Ruan L., Qian L., Liu X., Le Y. Norepinephrine Promotes Microglia to Uptake and Degrade Amyloid β Peptide through Upregulation of Mouse Formyl Peptide Receptor 2 and Induction of Insulin-Degrading Enzyme. J. Neurosci. 2010;30:11848–11857. doi: 10.1523/JNEUROSCI.2985-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Haslund-Vinding J., McBean G., Jaquet V., Vilhardt F. NADPH Oxidases in Oxidant Production by Microglia: Activating Receptors, Pharmacology and Association with Disease. Br. J. Pharmacol. 2017;174:1733–1749. doi: 10.1111/bph.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gao C., Jiang J., Tan Y., Chen S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023;8:359. doi: 10.1038/s41392-023-01588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Town T., Nikolic V., Tan J. The Microglial “Activation” Continuum: From Innate to Adaptive Responses. J. Neuroinflamm. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Simpson D.S.A., Oliver P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants. 2020;9:743. doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Imai Y., Kohsaka S. Intracellular Signaling in M-CSF-induced Microglia Activation: Role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- 239.Matejuk A., Ransohoff R.M. Crosstalk between Astrocytes and Microglia: An Overview. Front. Immunol. 2020;11:1416. doi: 10.3389/fimmu.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mallach A., Zielonka M., van Lieshout V., An Y., Khoo J.H., Vanheusden M., Chen W.-T., Moechars D., Arancibia-Carcamo I.L., Fiers M., et al. Microglia-Astrocyte Crosstalk in the Amyloid Plaque Niche of an Alzheimer’s Disease Mouse Model, as Revealed by Spatial Transcriptomics. Cell Rep. 2024;43:114216. doi: 10.1016/j.celrep.2024.114216. [DOI] [PubMed] [Google Scholar]
- 241.López-Armada M.J., Riveiro-Naveira R.R., Vaamonde-García C., Valcárcel-Ares M.N. Mitochondrial Dysfunction and the Inflammatory Response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 242.Peggion C., Calì T., Brini M. Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First? Antioxidants. 2024;13:240. doi: 10.3390/antiox13020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gudkov A.V., Gurova K.V., Komarova E.A. Inflammation and P53: A Tale of Two Stresses. Genes Cancer. 2011;2:503–516. doi: 10.1177/1947601911409747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kang X., Qiu J., Li Q., Bell K.A., Du Y., Jung D.W., Lee J.Y., Hao J., Jiang J. Cyclooxygenase-2 Contributes to Oxidopamine-Mediated Neuronal Inflammation and Injury via the Prostaglandin E2 Receptor EP2 Subtype. Sci. Rep. 2017;7:9459. doi: 10.1038/s41598-017-09528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Natami M., Hosseini S.M., Khaleel R.A., Addulrahman T.S., Zarei M., Asadi S., Gholami S., Mehrvar A. The Role of Specialized Pro-Resolving Mediators (SPMs) in Inflammatory Arthritis: A Therapeutic Strategy. Prostaglandins Other Lipid Mediat. 2024;170:106798. doi: 10.1016/j.prostaglandins.2023.106798. [DOI] [PubMed] [Google Scholar]
- 246.Basil M.C., Levy B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miyazawa K., Fukunaga H., Tatewaki Y., Takano Y., Yamamoto S., Mutoh T., Taki Y. Alzheimer’s Disease and Specialized Pro-Resolving Lipid Mediators: Do MaR1, RvD1, and NPD1 Show Promise for Prevention and Treatment? Int. J. Mol. Sci. 2020;21:5783. doi: 10.3390/ijms21165783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ponce J., Ulu A., Hanson C., Cameron-Smith E., Bertoni J., Wuebker J., Fisher A., Siu K.-C., Marmelat V., Adamec J., et al. Role of Specialized Pro-Resolving Mediators in Reducing Neuroinflammation in Neurodegenerative Disorders. Front. Aging Neurosci. 2022;14:780811. doi: 10.3389/fnagi.2022.780811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Saunders A.M., Strittmatter W.J., Schmechel D., St. George-Hyslop P.H., Pericak-Vance M.A., Joo S.H., Rosi B.L., Gusella J.F., Crapper-MacLachlan D.R., Alberts M.J., et al. Association of Apolipoprotein E Allele Ε4 with Late-onset Familial and Sporadic Alzheimer’s Disease. Neurology. 1993;43:1467. doi: 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 250.Kretzschmar G.C., Alencar N.M., da Silva S.S.L., Sulzbach C.D., Meissner C.G., Petzl-Erler M.L., Souza R.L.R., Boldt A.B.W. GWAS-Top Polymorphisms Associated with Late-Onset Alzheimer Disease in Brazil: Pointing Out Possible New Culprits among Non-Coding RNAs. Front. Mol. Biosci. 2021;8:632314. doi: 10.3389/fmolb.2021.632314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lambert J.-C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., et al. Genome-Wide Association Study Identifies Variants at CLU and CR1 Associated with Alzheimer’s Disease. Nat. Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 252.Seshadri S., Fitzpatrick A.L., Ikram M.A., DeStefano A.L., Gudnason V., Boada M., Bis J.C., Smith A.V., Carrasquillo M.M., Lambert J.C., et al. Genome-Wide Analysis of Genetic Loci Associated with Alzheimer Disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Naj A.C., Jun G., Beecham G.W., Wang L.-S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., et al. Common Variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 Are Associated with Late-Onset Alzheimer’s Disease. Nat. Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.-C., Carrasquillo M.M., Abraham R., Hamshere M.L., Pahwa J.S., Moskvina V., et al. Common Variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP Are Associated with Alzheimer’s Disease. Nat. Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., et al. Genome-Wide Association Study Identifies Variants at CLU and PICALM Associated with Alzheimer’s Disease. Nat. Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cummings A.C., Jiang L., Edwards D.R.V., McCauley J.L., Laux R., McFarland L.L., Fuzzell D., Knebusch C., Caywood L., Reinhart-Mercer L., et al. Genome-Wide Association and Linkage Study in the Amish Detects a Novel Candidate Late-Onset Alzheimer Disease Gene. Ann. Hum. Genet. 2012;76:342–351. doi: 10.1111/j.1469-1809.2012.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2012;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lambert J.-C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., Jun G., DeStefano A.L., Bis J.C., Beecham G.W., et al. Meta-Analysis of 74,046 Individuals Identifies 11 New Susceptibility Loci for Alzheimer’s Disease. Nat. Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mez J., Marden J.R., Mukherjee S., Walter S., Gibbons L.E., Gross A.L., Zahodne L.B., Gilsanz P., Brewster P., Nho K., et al. Alzheimer’s Disease Genetic Risk Variants beyond APOE Ε4 Predict Mortality. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017;8:188–195. doi: 10.1016/j.dadm.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sims R., van der Lee S.J., Naj A.C., Bellenguez C., Badarinarayan N., Jakobsdottir J., Kunkle B.W., Boland A., Raybould R., Bis J.C., et al. Rare Coding Variants in PLCG2, ABI3, and TREM2 Implicate Microglial-Mediated Innate Immunity in Alzheimer’s Disease. Nat. Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Miron J., Picard C., Nilsson N., Frappier J., Dea D., Théroux L., Poirier J. CDK5RAP2 Gene and Tau Pathophysiology in Late-Onset Sporadic Alzheimer’s Disease. Alzheimer’s Dement. 2018;14:787–796. doi: 10.1016/j.jalz.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 262.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wightman D.P., Jansen I.E., Savage J.E., Shadrin A.A., Bahrami S., Holland D., Rongve A., Børte S., Winsvold B.S., Drange O.K., et al. A Genome-Wide Association Study with 1,126,563 Individuals Identifies New Risk Loci for Alzheimer’s Disease. Nat. Genet. 2021;53:1276–1282. doi: 10.1038/s41588-021-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kang S., Gim J., Lee J., Gunasekaran T.I., Choi K.Y., Lee J.J., Seo E.H., Ko P.-W., Chung J.Y., Choi S.-M., et al. Potential Novel Genes for Late-Onset Alzheimer’s Disease in East-Asian Descent Identified by APOE-Stratified Genome-Wide Association Study. J. Alzheimer’s Dis. 2021;82:1451–1460. doi: 10.3233/JAD-210145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tai L.M., Ghura S., Koster K.P., Liakaite V., Maienschein-Cline M., Kanabar P., Collins N., Ben-Aissa M., Lei A.Z., Bahroos N., et al. APOE-modulated Aβ-induced Neuroinflammation in Alzheimer’s Disease: Current Landscape, Novel Data, and Future Perspective. J. Neurochem. 2015;133:465–488. doi: 10.1111/jnc.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ferrari-Souza J.P., Lussier F.Z., Leffa D.T., Therriault J., Tissot C., Bellaver B., Ferreira P.C.L., Malpetti M., Wang Y.-T., Povala G., et al. APOE Ε4 Associates with Microglial Activation Independently of Aβ Plaques and Tau Tangles. Sci. Adv. 2023;9:eade1474. doi: 10.1126/sciadv.ade1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Colonna M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023;23:580–594. doi: 10.1038/s41577-023-00837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S.K., Younkin S., et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lin C., Kong Y., Chen Q., Zeng J., Pan X., Miao J. Decoding STREM2: Its Impact on Alzheimer’s Disease—A Comprehensive Review of Mechanisms and Implications. Front. Aging Neurosci. 2024;16:1420731. doi: 10.3389/fnagi.2024.1420731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yeh F.L., Wang Y., Tom I., Gonzalez L.C., Sheng M. TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron. 2016;91:328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 271.Bailey C.C., DeVaux L.B., Farzan M. The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J. Biol. Chem. 2015;290:26033–26042. doi: 10.1074/jbc.M115.677286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Atagi Y., Liu C.-C., Painter M.M., Chen X.-F., Verbeeck C., Zheng H., Li X., Rademakers R., Kang S.S., Xu H., et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) J. Biol. Chem. 2015;290:26043–26050. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Damisah E.C., Rai A., Hill R.A., Tong L., Grutzendler J. TREM2 and APOE Do Not Modulate Phagocytic Clearance of Dying Cells in the Live Mammalian Brain. bioRxiv. 2023 doi: 10.1101/2023.03.17.533222. [DOI] [Google Scholar]
- 274.Khera R., Das N. Complement Receptor 1: Disease Associations and Therapeutic Implications. Mol. Immunol. 2009;46:761–772. doi: 10.1016/j.molimm.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Warwick C.A., Keyes A.L., Woodruff T.M., Usachev Y.M. The Complement Cascade in the Regulation of Neuroinflammation, Nociceptive Sensitization, and Pain. J. Biol. Chem. 2021;297:101085. doi: 10.1016/j.jbc.2021.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rogers J., Li R., Mastroeni D., Grover A., Leonard B., Ahern G., Cao P., Kolody H., Vedders L., Kolb W.P., et al. Peripheral Clearance of Amyloid β Peptide by Complement C3-Dependent Adherence to Erythrocytes. Neurobiol. Aging. 2006;27:1733–1739. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 277.Gomez-Arboledas A., Acharya M.M., Tenner A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. Immunotargets Ther. 2021;10:373–386. doi: 10.2147/ITT.S305420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gonzalez-Gil A., Porell R.N., Fernandes S.M., Maenpaa E., Li T.A., Li T., Wong P.C., Aoki K., Tiemeyer M., Yu Z.J., et al. Human Brain Sialoglycan Ligand for CD33, a Microglial Inhibitory Siglec Implicated in Alzheimer’s Disease. J. Biol. Chem. 2022;298:101960. doi: 10.1016/j.jbc.2022.101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Karch C.M., Jeng A.T., Nowotny P., Cady J., Cruchaga C., Goate A.M. Expression of Novel Alzheimer’s Disease Risk Genes in Control and Alzheimer’s Disease Brains. PLoS ONE. 2012;7:e50976. doi: 10.1371/journal.pone.0050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Eskandari-Sedighi G., Jung J., Macauley M.S. CD33 Isoforms in Microglia and Alzheimer’s Disease: Friend and Foe. Mol. Aspects Med. 2023;90:101111. doi: 10.1016/j.mam.2022.101111. [DOI] [PubMed] [Google Scholar]
- 282.Bhattacherjee A., Jung J., Zia S., Ho M., Eskandari-Sedighi G., St. Laurent C.D., McCord K.A., Bains A., Sidhu G., Sarkar S., et al. The CD33 Short Isoform Is a Gain-of-Function Variant That Enhances Aβ1–42 Phagocytosis in Microglia. Mol. Neurodegener. 2021;16:19. doi: 10.1186/s13024-021-00443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bradshaw E.M., Chibnik L.B., Keenan B.T., Ottoboni L., Raj T., Tang A., Rosenkrantz L.L., Imboywa S., Lee M., Von Korff A., et al. CD33 Alzheimer’s Disease Locus: Altered Monocyte Function and Amyloid Biology. Nat. Neurosci. 2013;16:848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yao Y., Chen Z.-L., Norris E.H., Strickland S. Astrocytic Laminin Regulates Pericyte Differentiation and Maintains Blood Brain Barrier Integrity. Nat. Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.He Y., Yao Y., Tsirka S.E., Cao Y. Cell-Culture Models of the Blood–Brain Barrier. Stroke. 2014;45:2514–2526. doi: 10.1161/STROKEAHA.114.005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pardridge W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Louveau A., Harris T.H., Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Huang Z., Wong L.-W., Su Y., Huang X., Wang N., Chen H., Yi C. Blood-Brain Barrier Integrity in the Pathogenesis of Alzheimer’s Disease. Front. Neuroendocrinol. 2020;59:100857. doi: 10.1016/j.yfrne.2020.100857. [DOI] [PubMed] [Google Scholar]
- 289.Wang D., Chen F., Han Z., Yin Z., Ge X., Lei P. Relationship between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell Neurosci. 2021;15:695479. doi: 10.3389/fncel.2021.695479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lam F.C., Liu R., Lu P., Shapiro A.B., Renoir J.M., Sharom F.J., Reiner P.B. Beta-Amyloid Efflux Mediated by p-Glycoprotein. J. Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- 291.Erickson M.A., Hansen K., Banks W.A. Inflammation-Induced Dysfunction of the Low-Density Lipoprotein Receptor-Related Protein-1 at the Blood-Brain Barrier: Protection by the Antioxidant N-Acetylcysteine. Brain Behav. Immun. 2012;26:1085–1094. doi: 10.1016/j.bbi.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Storck S.E., Meister S., Nahrath J., Meißner J.N., Schubert N., Di Spiezio A., Baches S., Vandenbroucke R.E., Bouter Y., Prikulis I., et al. Endothelial LRP1 Transports Amyloid-β(1-42) across the Blood-Brain Barrier. J. Clin. Investig. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bowman G.L., Kaye J.A., Moore M., Waichunas D., Carlson N.E., Quinn J.F. Blood–Brain Barrier Impairment in Alzheimer Disease. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zipser B.D., Johanson C.E., Gonzalez L., Berzin T.M., Tavares R., Hulette C.M., Vitek M.P., Hovanesian V., Stopa E.G. Microvascular Injury and Blood-Brain Barrier Leakage in Alzheimer’s Disease. Neurobiol. Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 295.Yamazaki Y., Shinohara M., Shinohara M., Yamazaki A., Murray M.E., Liesinger A.M., Heckman M.G., Lesser E.R., Parisi J.E., Petersen R.C., et al. Selective Loss of Cortical Endothelial Tight Junction Proteins during Alzheimer’s Disease Progression. Brain. 2019;142:1077–1092. doi: 10.1093/brain/awz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Schreibelt G., Kooij G., Reijerkerk A., Doorn R., Gringhuis S.I., Pol S., Weksler B.B., Romero I.A., Couraud P., Piontek J., et al. Reactive Oxygen Species Alter Brain Endothelial Tight Junction Dynamics via RhoA, PI3 Kinase, and PKB Signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- 297.Gali C.C., Fanaee-Danesh E., Zandl-Lang M., Albrecher N.M., Tam-Amersdorfer C., Stracke A., Sachdev V., Reichmann F., Sun Y., Avdili A., et al. Amyloid-Beta Impairs Insulin Signaling by Accelerating Autophagy-Lysosomal Degradation of LRP-1 and IR-β in Blood-Brain Barrier Endothelial Cells in Vitro and in 3XTg-AD Mice. Mol. Cell Neurosci. 2019;99:103390. doi: 10.1016/j.mcn.2019.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Arélin K., Kinoshita A., Whelan C.M., Irizarry M.C., Rebeck G.W., Strickland D.K., Hyman B.T. LRP and Senile Plaques in Alzheimer’s Disease: Colocalization with Apolipoprotein E and with Activated Astrocytes. Brain Res. Mol. Brain Res. 2002;104:38–46. doi: 10.1016/S0169-328X(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 299.Lehner C., Gehwolf R., Tempfer H., Krizbai I., Hennig B., Bauer H.-C., Bauer H. Oxidative Stress and Blood–Brain Barrier Dysfunction Under Particular Consideration of Matrix Metalloproteinases. Antioxid. Redox Signal. 2011;15:1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Huang X., Hussain B., Chang J. Peripheral Inflammation and Blood–Brain Barrier Disruption: Effects and Mechanisms. CNS Neurosci. Ther. 2021;27:36–47. doi: 10.1111/cns.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Alvarez J.I., Katayama T., Prat A. Glial Influence on the Blood Brain Barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Verkhratsky A., Pivoriūnas A. Astroglia Support, Regulate and Reinforce Brain Barriers. Neurobiol. Dis. 2023;179:106054. doi: 10.1016/j.nbd.2023.106054. [DOI] [PubMed] [Google Scholar]
- 303.Versele R., Sevin E., Gosselet F., Fenart L., Candela P. TNF-α and IL-1β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-β Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022;23:10235. doi: 10.3390/ijms231810235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kanda H., Shimamura R., Koizumi-Kitajima M., Okano H. Degradation of Extracellular Matrix by Matrix Metalloproteinase 2 Is Essential for the Establishment of the Blood-Brain Barrier in Drosophila. iScience. 2019;16:218–229. doi: 10.1016/j.isci.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kim J.Y., Kim J.-H., Kim Y.-D., Seo J.H. High Vulnerability of Oligodendrocytes to Oxidative Stress Induced by Ultrafine Urban Particles. Antioxidants. 2020;10:4. doi: 10.3390/antiox10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jackman N., Ishii A., Bansal R. Oligodendrocyte Development and Myelin Biogenesis: Parsing Out the Roles of Glycosphingolipids. Physiology. 2009;24:290–297. doi: 10.1152/physiol.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Philips T., Rothstein J.D. Oligodendroglia: Metabolic Supporters of Neurons. J. Clin. Investig. 2017;127:3271–3280. doi: 10.1172/JCI90610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Madsen P.M., Desu H.L., de Rivero Vaccari J.P., Florimon Y., Ellman D.G., Keane R.W., Clausen B.H., Lambertsen K.L., Brambilla R. Oligodendrocytes Modulate the Immune-Inflammatory Response in EAE via TNFR2 Signaling. Brain Behav. Immun. 2020;84:132–146. doi: 10.1016/j.bbi.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tse K., Cheng A., Ma F., Herrup K. DNA Damage-associated Oligodendrocyte Degeneration Precedes Amyloid Pathology and Contributes to Alzheimer’s Disease and Dementia. Alzheimer’s Dement. 2018;14:664–679. doi: 10.1016/j.jalz.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Huang Z., Jordan J.D., Zhang Q. Myelin Pathology in Alzheimer’s Disease: Potential Therapeutic Opportunities. Aging Dis. 2024;15:698. doi: 10.14336/AD.2023.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Depp C., Sun T., Sasmita A.O., Spieth L., Berghoff S.A., Nazarenko T., Overhoff K., Steixner-Kumar A.A., Subramanian S., Arinrad S., et al. Myelin Dysfunction Drives Amyloid-β Deposition in Models of Alzheimer’s Disease. Nature. 2023;618:349–357. doi: 10.1038/s41586-023-06120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Vanzulli I., Papanikolaou M., De-La-Rocha I.C., Pieropan F., Rivera A.D., Gomez-Nicola D., Verkhratsky A., Rodríguez J.J., Butt A.M. Disruption of Oligodendrocyte Progenitor Cells Is an Early Sign of Pathology in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Aging. 2020;94:130–139. doi: 10.1016/j.neurobiolaging.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Han S., Gim Y., Jang E.-H., Hur E.-M. Functions and Dysfunctions of Oligodendrocytes in Neurodegenerative Diseases. Front. Cell Neurosci. 2022;16:1083159. doi: 10.3389/fncel.2022.1083159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhou Y., Song W.M., Andhey P.S., Swain A., Levy T., Miller K.R., Poliani P.L., Cominelli M., Grover S., Gilfillan S., et al. Human and Mouse Single-Nucleus Transcriptomics Reveal TREM2-Dependent and TREM2-Independent Cellular Responses in Alzheimer’s Disease. Nat. Med. 2020;26:131–142. doi: 10.1038/s41591-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Park H., Cho B., Kim H., Saito T., Saido T.C., Won K.-J., Kim J. Single-Cell RNA-Sequencing Identifies Disease-Associated Oligodendrocytes in Male APP NL-G-F and 5XFAD Mice. Nat. Commun. 2023;14:802. doi: 10.1038/s41467-023-36519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Smith K.J., Kapoor R., Felts P.A. Demyelination: The Role of Reactive Oxygen and Nitrogen Species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jana A., Pahan K. Oxidative Stress Kills Human Primary Oligodendrocytes Via Neutral Sphingomyelinase: Implications for Multiple Sclerosis. J. Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zarrouk A., Nury T., Karym E.-M., Vejux A., Sghaier R., Gondcaille C., Andreoletti P., Trompier D., Savary S., Cherkaoui-Malki M., et al. Attenuation of 7-Ketocholesterol-Induced Overproduction of Reactive Oxygen Species, Apoptosis, and Autophagy by Dimethyl Fumarate on 158 N Murine Oligodendrocytes. J. Steroid Biochem. Mol. Biol. 2017;169:29–38. doi: 10.1016/j.jsbmb.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 319.Sochocka M., Diniz B.S., Leszek J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gorter R.P., Baron W. Matrix Metalloproteinases Shape the Oligodendrocyte (Niche) during Development and upon Demyelination. Neurosci. Lett. 2020;729:134980. doi: 10.1016/j.neulet.2020.134980. [DOI] [PubMed] [Google Scholar]
- 321.Rupnik M., Baker D., Selwood D.L. Oligodendrocytes, BK Channels and the Preservation of Myelin. F1000Research. 2021;10:781. doi: 10.12688/f1000research.53422.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kalafatakis I., Karagogeos D. Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair. Biomolecules. 2021;11:1058. doi: 10.3390/biom11071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sasmita A.O., Depp C., Nazarenko T., Sun T., Siems S.B., Ong E.C., Nkeh Y.B., Böhler C., Yu X., Bues B., et al. Oligodendrocytes Produce Amyloid-β and Contribute to Plaque Formation alongside Neurons in Alzheimer’s Disease Model Mice. Nat. Neurosci. 2024;27:1668–1674. doi: 10.1038/s41593-024-01730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hampel H., Mesulam M.-M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain. 2018;141:1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Folch J., Busquets O., Ettcheto M., Sánchez-López E., Castro-Torres R.D., Verdaguer E., Garcia M.L., Olloquequi J., Casadesús G., Beas-Zarate C., et al. Memantine for the Treatment of Dementia: A Review on Its Current and Future Applications. J. Alzheimer’s Dis. 2018;62:1223–1240. doi: 10.3233/JAD-170672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Karimi Tari P., Parsons C.G., Collingridge G.L., Rammes G. Memantine: Updating a Rare Success Story in pro-Cognitive Therapeutics. Neuropharmacology. 2024;244:109737. doi: 10.1016/j.neuropharm.2023.109737. [DOI] [PubMed] [Google Scholar]
- 327.Schneider L.S., Mangialasche F., Andreasen N., Feldman H., Giacobini E., Jones R., Mantua V., Mecocci P., Pani L., Winblad B., et al. Clinical Trials and Late-Stage Drug Development for Alzheimer’s Disease: An Appraisal from 1984 to 2014. J. Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Valls-Comamala V., Guivernau B., Bonet J., Puig M., Perálvarez-Marín A., Palomer E., Fernàndez-Busquets X., Altafaj X., Tajes M., Puig-Pijoan A., et al. The Antigen-Binding Fragment of Human Gamma Immunoglobulin Prevents Amyloid β-Peptide Folding into β-Sheet to Form Oligomers. Oncotarget. 2017;8:41154–41165. doi: 10.18632/oncotarget.17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., Kanekiyo M., Li D., Reyderman L., Cohen S., et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2022;388:9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 330.Marciani D.J. Promising Results from Alzheimer’s Disease Passive Immunotherapy Support the Development of a Preventive Vaccine. Research. 2019;2019:5341375. doi: 10.34133/2019/5341375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Morris M.C., Beckett L.A., Scherr P.A., Hebert L.E., Bennett D.A., Field T.S., Evans D.A. Vitamin E and Vitamin C Supplement Use and Risk of Incident Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 332.Zandi P.P. Reduced Risk of Alzheimer Disease in Users of Antioxidant Vitamin Supplements. Arch. Neurol. 2004;61:82. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 333.Basambombo L.L., Carmichael P.-H., Côté S., Laurin D. Use of Vitamin E and C Supplements for the Prevention of Cognitive Decline. Ann. Pharmacother. 2017;51:118–124. doi: 10.1177/1060028016673072. [DOI] [PubMed] [Google Scholar]
- 334.Morris M.C. Dietary Intake of Antioxidant Nutrients and the Risk of Incident Alzheimer Disease in a Biracial Community Study. JAMA. 2002;287:3230. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 335.Jiménez-Jiménez F.J., Alonso-Navarro H., García-Martín E., Agúndez J.A.G. Coenzyme Q10 and Dementia: A Systematic Review. Antioxidants. 2023;12:533. doi: 10.3390/antiox12020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Voulgaropoulou S.D., van Amelsvoort T.A.M.J., Prickaerts J., Vingerhoets C. The Effect of Curcumin on Cognition in Alzheimer’s Disease and Healthy Aging: A Systematic Review of Pre-Clinical and Clinical Studies. Brain Res. 2019;1725:146476. doi: 10.1016/j.brainres.2019.146476. [DOI] [PubMed] [Google Scholar]
- 337.Behl T., Kaur D., Sehgal A., Singh S., Sharma N., Zengin G., Andronie-Cioara F.L., Toma M.M., Bungau S., Bumbu A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules. 2021;26:3724. doi: 10.3390/molecules26123724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Unzeta M., Hernàndez-Guillamon M., Sun P., Solé M. SSAO/VAP-1 in Cerebrovascular Disorders: A Potential Therapeutic Target for Stroke and Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:3365. doi: 10.3390/ijms22073365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Caraci F., Pappalardo G., Basile L., Giuffrida A., Copani A., Tosto R., Sinopoli A., Giuffrida M.L., Pirrone E., Drago F., et al. Neuroprotective Effects of the Monoamine Oxidase Inhibitor Tranylcypromine and Its Amide Derivatives against Aβ(1-42)-Induced Toxicity. Eur. J. Pharmacol. 2015;764:256–263. doi: 10.1016/j.ejphar.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 340.Campora M., Canale C., Gatta E., Tasso B., Laurini E., Relini A., Pricl S., Catto M., Tonelli M. Multitarget Biological Profiling of New Naphthoquinone and Anthraquinone-Based Derivatives for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2021;12:447–461. doi: 10.1021/acschemneuro.0c00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ostadkarampour M., Putnins E.E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front. Pharmacol. 2021;12:676239. doi: 10.3389/fphar.2021.676239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rivers-Auty J., Mather A.E., Peters R., Lawrence C.B., Brough D. Anti-Inflammatories in Alzheimer’s Disease—Potential Therapy or Spurious Correlate? Brain Commun. 2020;2:fcaa109. doi: 10.1093/braincomms/fcaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Decourt B., Lahiri D.K., Sabbagh M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer’s Disease. Curr. Alzheimer Res. 2017;14:412–425. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Thal L.J., Ferris S.H., Kirby L., Block G.A., Lines C.R., Yuen E., Assaid C., Nessly M.L., Norman B.A., Baranak C.C., et al. A Randomized, Double-Blind, Study of Rofecoxib in Patients with Mild Cognitive Impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 345.Stopschinski B.E., Weideman R.A., McMahan D., Jacob D.A., Little B.B., Chiang H.-S., Saez Calveras N., Stuve O. Microglia as a Cellular Target of Diclofenac Therapy in Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2023;16:175628642311566. doi: 10.1177/17562864231156674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Villa V., Thellung S., Bajetto A., Gatta E., Robello M., Novelli F., Tasso B., Tonelli M., Florio T. Novel Celecoxib Analogues Inhibit Glial Production of Prostaglandin E2, Nitric Oxide, and Oxygen Radicals Reverting the Neuroinflammatory Responses Induced by Misfolded Prion Protein Fragment 90-231 or Lipopolysaccharide. Pharmacol. Res. 2016;113:500–514. doi: 10.1016/j.phrs.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 347.Roehr S., Pabst A., Luck T., Riedel-Heller S.G. Is Dementia Incidence Declining in High-Income Countries? A Systematic Review and Meta-Analysis. Clin. Epidemiol. 2018;10:1233–1247. doi: 10.2147/CLEP.S163649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lee D.H., Seo S.W., Roh J.H., Oh M., Oh J.S., Oh S.J., Kim J.S., Jeong Y. Effects of Cognitive Reserve in Alzheimer’s Disease and Cognitively Unimpaired Individuals. Front. Aging Neurosci. 2022;13:784054. doi: 10.3389/fnagi.2021.784054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.George E.K., Reddy P.H. Can Healthy Diets, Regular Exercise, and Better Lifestyle Delay the Progression of Dementia in Elderly Individuals? J. Alzheimers Dis. 2019;72:S37–S58. doi: 10.3233/JAD-190232. [DOI] [PubMed] [Google Scholar]
- 350.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.