Abstract
Borna disease virus (BDV) is a noncytolytic RNA virus that can replicate in the central nervous system (CNS) of mice. This study shows that BDV multiplication was efficiently blocked in transgenic mice that express mouse alpha-1 interferon (IFN-α1) in astrocytes. To investigate whether endogenous virus-induced IFN might similarly restrict BDV, we used IFNAR0/0 mice, which lack a functional alpha/beta IFN (IFN-α/β) receptor. As would be expected if virus-induced IFN were important to control BDV infection, we found that cultured embryo cells of IFNAR0/0 mice supported viral multiplication, whereas cells from wild-type mice did not. Unexpectedly, however, BDV spread through the CNSs of IFNAR0/0 and wild-type mice with similar kinetics, suggesting that activation of endogenous IFN-α/β genes in BDV-infected brains was too weak or occurred too late to be effective. Surprisingly, Northern blot analysis showed that the levels of the most abundant viral mRNAs in the brains of persistently infected IFNAR0/0 mice were about 20-fold lower than those in wild-type mice. In contrast, genomic viral RNA was produced in about a 10-fold excess in the brains of IFNAR0/0 mice. Human IFN-α2 similarly enhanced transcription and simultaneously repressed replication of the BDV genome in persistently infected Vero cells. Thus, in persistently infected neurons and cultured cells, IFN-α/β appears to freeze the BDV polymerase in the transcriptional mode, resulting in enhanced viral mRNA synthesis and suppressing viral genome replication.
Borna disease virus (BDV) is an enveloped virus with a nonsegmented, negative-stranded RNA genome (13, 34). It is the causative agent of Borna disease, a neurological disorder of horses, sheep, and other farm animals in central Europe (for a review, see reference 37). Successful experimental infection of a broad range of animals has been reported (30). BDV preferentially infects neurons. In rats and probably in most other susceptible animals, it may also replicate in other cell types of the central nervous system (CNS), including astrocytes (6). Nonneuronal cells of several animal species are susceptible to BDV when kept in tissue cultures (7). BDV has been noncytolytic in all cell systems examined to date. Neurological disease in BDV-infected animals results from the antiviral immune response against persistently infected cells of the CNS (38). Therefore, depending on the quality of the host immune response and the kinetics of replication of the virus strain, a persistent infection of the CNS with BDV may or may not result in detectable neurological disease (4, 15). In the mouse, the major histocompatibility complex locus and additional genetic traits determine whether infection with BDV leads to a neurological disorder or asymptomatic viral persistence (18, 20).
Like all other nonsegmented negative-stranded RNA viruses, BDV expresses its genome by synthesizing several subgenomic, capped, and polyadenylated transcripts of plus-strand polarity. This reaction is catalyzed by the virus-encoded RNA-dependent RNA polymerase. Later in the viral multiplication cycle, the same enzyme starts to generate uncapped, plus-strand, genome-length RNA, which it subsequently uses as a template for the synthesis of new viral genomes (33). How the viral polymerase is switched from transcription mode to replication mode is not well understood. From studies performed with the polymerases of vesicular stomatitis virus and human parainfluenza virus type 3, it became clear that these enzymes require the assistance of host cell factors for transcription as well as replication activity (8, 9).
The multiplication of BDV is susceptible to the antiviral action of alpha/beta interferon (IFN-α/β) in monkey Vero cells and some other established cell lines but not in rat C6 glioblastoma cells (19). Whether IFN-α/β can influence the multiplication of BDV in vivo to date has not been clear. With the generation of mutant mice that express a transgene encoding mouse alpha-1 IFN (IFN-α1) in the CNS (2) or that lack functional IFN-α/β receptors (28), appropriate tools to study this question became available. We show here that although transgenically expressed IFN potently inhibits BDV in the CNS, the IFN-α/β system is usually not very effective in vivo, presumably because the BDV-induced IFN response is too weak or occurs too late to restrict viral spread. We further demonstrate that IFN-α/β can change the balance between transcription and replication of the viral genome.
MATERIALS AND METHODS
Mice.
The transgenic mouse line GIFN-12, which expresses mouse IFN-α1 under the control of the astrocyte-specific glial fibrillary acidic protein promoter, has been described elsewhere (2). GIFN-12 males were crossed with B6.A2G-Mx females, which carry a functional Mx1 gene (36). The resulting transgenic and nontransgenic F1 offspring, which are both heterozygous at the Mx1 locus and thus capable of responding to IFN-α/β by synthesizing the Mx1 gene product, were used for the experiments described in this report. IFNAR0/0 (28) and congenic wild-type 129 mice were bred in our local animal facility.
Viruses.
A mouse-adapted strain of BDV was used which was originally assumed to be derived from strain He/80 (18) but later identified as a novel strain, designated RW98 (12). Brains of animals showing extensive neurological disease were collected and used to prepare virus stock as described previously (18, 20). For tissue culture experiments, this virus was grown in a human oligodendrocyte cell line (5). Virus was released from infected cells by high-salt treatment as described previously (12). Viral titers were determined by standard fluorescent-focus assays (21) with Vero cells. Ten-microliter samples of virus stock (corresponding to about 100 focus-forming units [FFU]) were used to infect newborn mice by the intracerebral route with a Hamilton syringe. A multiplicity of infection of about 0.01 was used for the infection of cultured cells with BDV.
Cell cultures.
Embryo cells were prepared from 14-day-old embryos as described previously (3). After infection with BDV, the cell cultures were split twice weekly at a ratio of 1:3. After each passage, a sample of cells was used to seed glass coverslips and processed for indirect immunofluorescence. Vero cells persistently infected with either BDV strain He/80 (19) or strain V (5) were treated with 500 U of human IFN-α2/ml for various times before RNA analysis.
Indirect immunofluorescence.
Cells were fixed with 3% buffered paraformaldehyde in phosphate-buffered saline for 10 min, permeabilized with 0.5% Triton X-100 for 5 min, and stained with a polyclonal antiserum to BDV antigens as described previously (19).
Immunohistochemical analysis.
Paraffin sections were stained for viral antigens with monoclonal antibody Bo18 (17). The technology used was exactly that described previously (18). An identical technique was used to detect Mx1 protein in paraffin sections of brain tissue with rabbit antipeptide serum AP5 (25).
Northern blot analysis.
RNA was prepared from frozen brain hemispheres or from cultured cells using peqGOLD TriFast reagent (PecLab, Erlangen, Germany) according to the manufacturer's protocol. Tissue homogenization was done with 1 ml of TriFast per 100 mg of tissue by passages through 21- and 26-gauge needles. Precipitated RNA samples were dissolved in 0.5 mM EDTA and stored at −70°C. Ten-microgram samples of RNA were subjected to electrophoresis through a 1.2% agarose–formaldehyde gel, transferred to a nylon membrane, and hybridized under standard conditions to radiolabeled cDNA or RNA fragments. To control for possible variations in gel loading, the blots were stripped and rehybridized with a radiolabeled rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (31). After stringent washing, the membranes were exposed to X-ray film. To quantify Northern blot signals, the membranes were further exposed to phosphorimager plates. All values were adjusted for possible variations resulting from unequal loading by normalization against the corresponding GAPDH values.
Western blot analysis.
Lysates were prepared from whole brain hemispheres by Dounce homogenization of tissue in 1 ml of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Ten-microliter samples of the lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and probed with BDV p40-specific monoclonal antibody Bo18 (17). The blots were developed with horseradish peroxidase-conjugated goat anti-mouse antiserum and subsequent incubation with 4-chloro-1-naphthol substrate (Fluka, Buchs, Switzerland).
RESULTS
Astrocytic expression of the IFN-α1 transgene results in Mx1 protein synthesis in neurons of most brain regions.
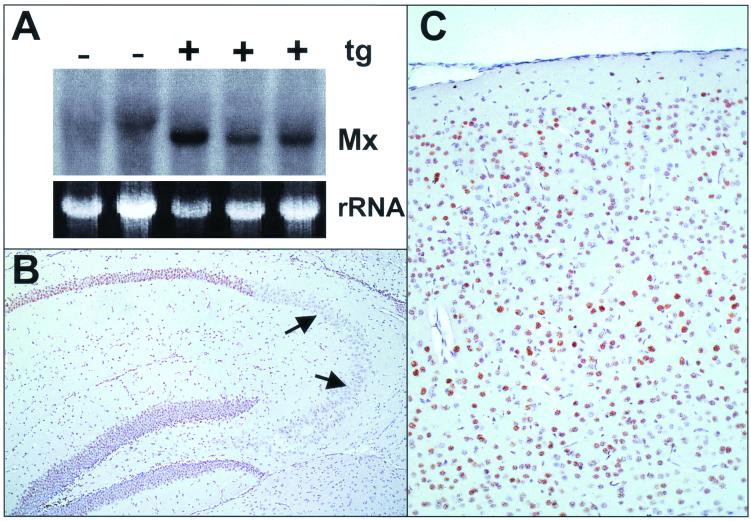
Since IFN-α1 expression in astrocytes of transgenic mouse line GIFN-12 is low (2), direct visualization of the corresponding transgene products in the CNS is difficult. To demonstrate the presence of biologically active IFN-α/β by indirect means, we crossed transgenic GIFN-12 males with B6.A2G-Mx females carrying functional copies of the IFN-regulated Mx1 gene (36). Mx1 is not present in most cell types under normal conditions, but it accumulates to high levels in the nuclei after IFN-α/β treatment (11). Detection of Mx1 thus represents a highly sensitive method to prove the presence of biologically active IFN-α/β. Northern blot analysis of brain RNA from F1 offspring showed that mice carrying the IFN-α1 transgene contained easily detectable levels of Mx1 transcripts, whereas nontransgenic littermates did not (Fig. 1A). Immunohistochemical analysis of paraffin-embedded brain sections with a monospecific antiserum to a C-terminal peptide of Mx1 (29) showed that the nuclei of a large number of neurons from various brain regions were strongly stained (Fig. 1B and C). Interestingly, nuclei of neurons from the CA3 region of the hippocampus stained less strongly for Mx1 than neurons from other brain regions, indicating that these cells might exhibit reduced responsiveness to IFN-α/β.
FIG. 1.
Astrocytic IFN-α1 transgene (tg) expression results in Mx1 protein synthesis in neurons of Mx1-positive mice. (A) RNAs from brains of transgenic (+) and nontransgenic (−) littermates were analyzed by Northern blotting for Mx1 transcripts. Ethidium bromide staining of the gel confirmed that similar amounts of RNAs had been loaded into the slots. (B and C) Immunohistochemical visualization of Mx1 protein (brown staining) in nuclei of hippocampus (B) and neocortex (C) neurons of a transgenic mouse. Note the strong staining of neurons in the dentate gyrus and CA1 and CA2 regions of the hippocampus, which contrasts the weak staining of CA3 neurons (arrows in panel B).
Poor spread of BDV in brains of IFN-α1 transgenic mice.
To test whether IFN can inhibit the multiplication of BDV in the CNS, we infected newborn transgenic and nontransgenic littermates by the intracerebral route with a mouse-adapted BDV variant. We did not expect to observe a BDV-induced neurological disorder in any of these mice because they were of the C57BL/6 genetic background, which permits rapid virus spread but does not promote disease (20). To assess virus susceptibility, the animals were sacrificed at 4 weeks postinfection, and their brains were analyzed for the presence of viral RNA and antigen. The Northern blot probe was designed to detect viral transcripts of 0.8, 1.2, and 1.9 kb. We found that the brains of nontransgenic animals contained much higher levels of all three BDV transcripts than the brains of transgenic littermates (Fig. 2A). Immunohistochemical analysis of paraffin-embedded brain sections with monoclonal antibody Bo18, which specifically stains viral nucleoprotein p40 (17), confirmed the low level of multiplication of BDV in the transgenic mice (Fig. 2B and C). Only a few clusters of antigen-positive cells were detected in the brains of transgenic animals, whereas most brain regions of nontransgenic animals showed large numbers of BDV-infected cells.
FIG. 2.
Viral gene expression is diminished in brains of BDV-infected mice expressing the IFN-α1 transgene (tg). (A) Northern blot analysis of RNA from brains of 4-week-old transgenic (+) and nontransgenic (−) littermates infected with BDV as newborns. Gel positions and sizes of the various viral RNAs are indicated. Equal loading of the gel slots was controlled by hybridizing the membrane to a radiolabeled GAPDH cDNA probe. (B and C) Immunohistochemical visualization of BDV nucleoprotein in the thalamus of transgenic (B) and nontransgenic (C) littermates. Note the small number of infected neurons in transgenic mice compared to the massive viral spread in the brains of nontransgenic animals.
Cultured cells from IFNAR0/0 mice but not from wild-type mice support the replication of BDV.
An unexplained but consistent finding of our previous studies was that primary or permanent mouse cell lines failed to support sustained replication of BDV, whereas most cell lines from other animal species were susceptible (unpublished results). We also observed abortive BDV infections in the mouse neuroblastoma cell line N18-TG2 (16) as well as in two cell lines (HN9.1 and HN25.1) (22) that were generated by fusing N18-TG2 cells with mouse hippocampus neurons (data not shown). We further found that BDV failed to establish permanent infection in the mouse-rat hybrid cell line NG108-15 (data not shown), which resulted from fusing BDV-susceptible rat C6 glioblastoma cells with N18-TG2 cells (24); this result suggested that the BDV resistance phenotype of mouse cells is dominant.
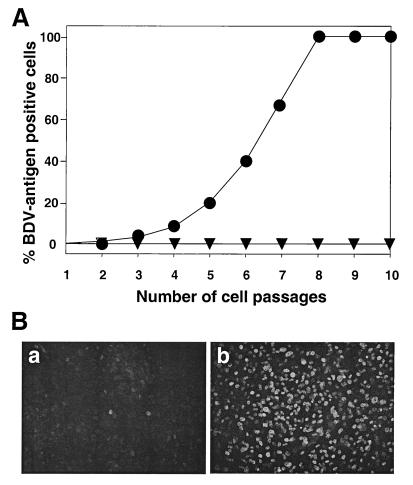
To evaluate the possibility that BDV resistance of mouse cells was mediated by virus-induced IFN, we compared the susceptibilities of cultured primary whole embryo cells from wild-type and IFNAR0/0 mice. We detected a few BDV antigen-positive cells in cultures of wild-type mice at early times postinfection, but these rare cells disappeared rapidly when the cultures were passaged (Fig. 3). In contrast, the number of antigen-positive cells steadily increased in embryo cell cultures from IFNAR0/0 mice (Fig. 3). After eight cell passages, virtually all IFNAR0/0 embryo cells were positive for BDV antigen, as assessed by indirect immunofluorescence, indicating that virus-induced IFN played a critical role in restricting the multiplication of BDV in the wild-type cell culture.
FIG. 3.
Cultured embryo cells from IFNAR0/0 mice support the replication of BDV. (A) Cells from wild-type (triangles) and mutant (circles) mice were infected with BDV and passaged twice weekly at a splitting ratio of 1:3. After each cell passage, a sample of cells was subjected to indirect immunofluorescence analysis in order to determine the percentage of BDV antigen-positive cells. (B) BDV infection induces IFN in mouse embryo cells. Cells from mice carrying a functional version of the IFN-inducible Mx1 gene either were left uninfected (a) or were infected with BDV (b). The cultures were fixed at 72 h postinfection and stained for nuclear Mx1 protein using a specific antiserum.
To further demonstrate that IFN-α/β was involved, we repeated the infection experiment with embryo cells from mice that carry a functional Mx1 gene. We argued that IFN generated during the virus infection would readily lead to the accumulation of nuclear Mx1 protein that could be detected by indirect immunofluorescence. The majority of cells in the uninfected culture yielded no specific Mx1 staining (Fig. 3B, panel a), whereas a large percentage of cells in the infected culture showed strong Mx1 staining at 72 h postinfection (Fig. 3B, panel b); these results indicated that our BDV stocks indeed contained potent IFN-inducing activity.
High levels of BDV antigen in brains of both wild-type and IFNAR0/0 mice.
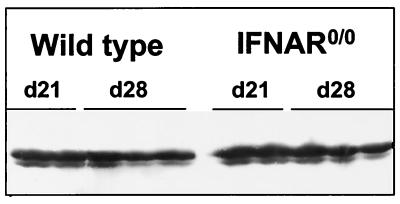
We next examined whether BDV would spread at an increased rate through the CNS of IFNAR0/0 mice and whether it would replicate outside the CNS of such mice. Reverse transcription-PCR analysis of RNA from various organs of infected IFNAR0/0 and wild-type mice yielded no evidence for altered organ tropism of BDV in mutant animals (data not shown). Analysis of brain tissue from infected animals showed that BDV replicated well in the CNSs of both mouse strains. No difference in the amount of viral p40 antigen was evident when samples of whole brain extracts from wild-type and IFNAR0/0 mice were compared at 21 or 28 days postinfection (Fig. 4), and titers of infectious BDV were, on average, about 7 × 107 FFU per brain in both wild-type and IFNAR0/0 mice (Table 1). Immunohistochemical analysis further showed that comparable numbers of brain cells were infected with BDV (data not shown). More detailed analysis of brain sections yielded no evidence for altered cell type specificity of BDV in IFNAR0/0 mice (data not shown).
FIG. 4.
No difference in levels of BDV antigen in brains of wild-type and IFNAR0/0 mice. Infected newborn mice were sacrificed at either 21 or 28 days (d21 or d28, respectively) postinfection, and samples of extracts prepared from whole brain hemispheres were analyzed by Western blotting using BDV p40-specific monoclonal antibody Bo18.
TABLE 1.
BDV titers in the CNS of five individual wild-type and IFNAR0/0mice at day 28 postinfection
| Mice | FFU/brain (107) |
|---|---|
| Wild type | 6.2 |
| 9.5 | |
| 9.9 | |
| 6.0 | |
| 3.6 | |
| IFNAR0/0 | 4.1 |
| 5.0 | |
| 7.1 | |
| 10.6 | |
| 7.6 |
Striking differences in levels of genomic and subgenomic BDV RNAs in brains of infected wild-type and IFNAR0/0 mice.
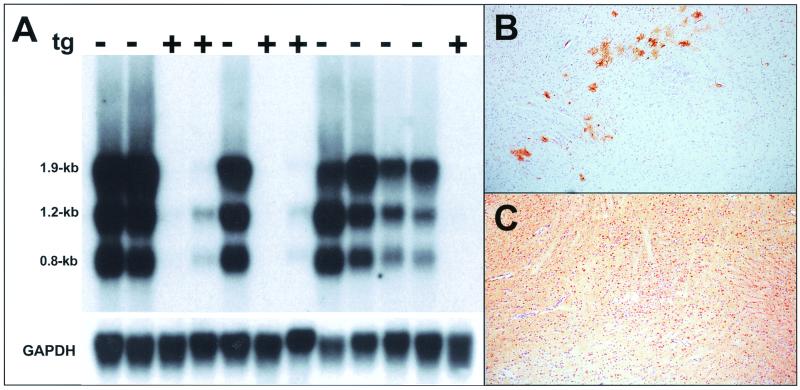
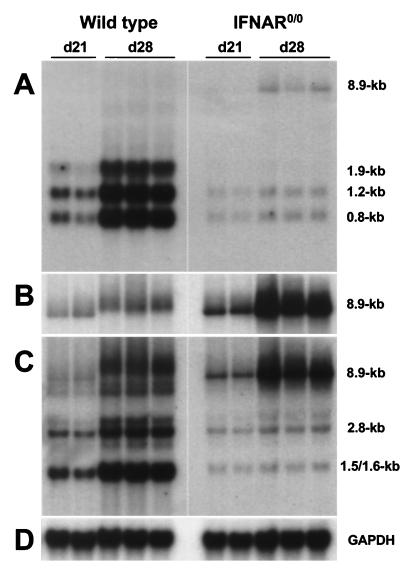
We examined Mx1 expression in the CNS of nontransgenic wild-type mice to determine whether BDV was able to induce the expression of endogenous IFN-α/β genes. Immunohistochemical staining using the Mx1-specific antiserum revealed that large numbers of neurons were positive in brains with a high virus load (data not shown), suggesting that biologically active IFN-α/β was present. To determine whether virus-induced endogenous IFN exhibited a detectable inhibitory effect on the persisting virus, we measured the expression of the various BDV genes in wild-type and IFNAR0/0 mice. Northern blot analysis with a cDNA probe derived from the 3′ end of the BDV genome yielded a surprising result: we found that all subgenomic RNAs of BDV that we analyzed (transcripts of 0.8, 1.2, and 1.9 kb) were at least 25-fold more abundant in brains of wild-type mice than in brains of IFNAR0/0 mice at 28 days postinfection (Fig. 5A). In contrast, genome-size viral RNA (transcripts of 8.9 kb) were present in higher concentrations in brains of IFNAR0/0 mice (Fig. 5A).
FIG. 5.
Striking differences in levels of genomic and subgenomic BDV RNAs in brains of wild-type and IFNAR0/0 mice. Infected newborn mice were sacrificed at either 21 or 28 days (d21 or d28, respectively) postinfection, and samples of brain RNAs were analyzed by Northern blotting using the following hybridization probes: A, radiolabeled cDNA corresponding to the 3′-terminal 1.9 kb of the BDV genome; B, radiolabeled RNA of positive polarity derived from the same part of the viral genome; C, radiolabeled cDNA corresponding to a 2.8-kb transcript from a central fragment of the BDV genome encoding M and G proteins; and D, radiolabeled rat GAPDH cDNA probe. Gel positions and sizes of the various viral RNAs are indicated. The migration differences in genomic viral RNAs are gel artifacts which presumably resulted from traces of salt in some of our RNA preparations.
To determine whether full-length RNA of negative sense was accumulating in the brains of mutant mice, we hybridized the same Northern blot to a radiolabeled RNA probe designed to selectively detect negative-stranded viral RNA. Quantitative analysis of the blots showed that brains of infected IFNAR0/0 mice contained about 10-fold-higher concentrations of virus genomes than those of infected wild-type mice (Fig. 5B). Full-length viral RNA of positive sense could not be detected under these experimental conditions (data not shown). Rehybridization of the same Northern blot with a cDNA probe from the central region of the BDV genome showed that all major transcripts of the third viral transcription unit (transcripts of 1.5, 1.6, and 2.8 kb) were more abundant in brains of wild-type mice than in brains of IFNAR0/0 mice (Fig. 5C). This difference was about 25-fold for the 1.5- and 1.6-kb transcripts and about 7-fold for the 2.8-kb transcript. Thus, although virus-induced endogenous IFN was unable to prevent persistent BDV infection of neurons in wild-type mice, it strongly modulated the activity of the viral polymerase.
IFN-α/β enhances transcription and decreases replication of the BDV genome in persistently infected Vero cells.
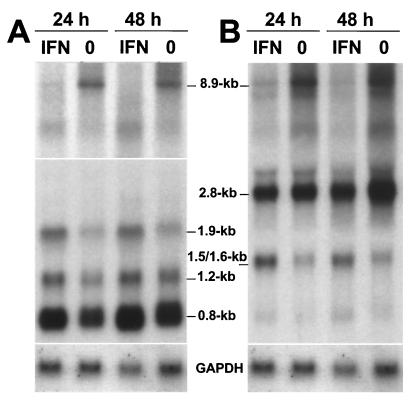
To determine whether the IFN response of neurons is unique, we treated persistently infected Vero cells with human IFN-α2 and analyzed the viral RNA levels at 24 and 48 h after onset of treatment. We chose Vero cells for these experiments because they lack the ability to synthesize IFN-α/β due to a large deletion in the chromosome that carries the necessary gene cluster (10). Quantitative analysis of the Northern blot shown in Fig. 6A revealed that the concentration of the abundant viral 0.8-kb transcript was about twofold higher in IFN-treated Vero cells than in untreated cells. The levels of the 1.2- and 1.9-kb viral RNAs were about 1.5-fold higher in IFN-treated cells. Similarly, the concentrations of the 1.5- and 1.6-kb transcripts of the third transcription unit were enhanced almost twofold in IFN-treated cells (Fig. 6B), whereas the level of the 2.8-kb transcript remained virtually unchanged. Importantly, IFN decreased the concentrations of genome-size (8.9-kb) viral transcripts by at least twofold (Fig. 6A and B). Rehybridization of the same blot with a strand-specific RNA probe showed that genome-size RNA of negative polarity was mainly present in the 8.9-kb band and that its level was reduced in IFN-treated cells (data not shown). Thus, combined stimulatory and inhibitory effects on transcription and replication of the BDV genome characterized the action of IFN-α/β in persistently infected Vero cells. The differential effects closely resembled the above-discussed phenomena in the brains of BDV-infected mice.
FIG. 6.
IFN enhances BDV genome transcription and decreases its replication in persistently infected Vero cells. Semiconfluent monolayers of Vero cells persistently infected with BDV strain He/80 were treated for 24 or 48 h with 500 U of human IFN-α2/ml. Then, RNA was prepared and samples were analyzed on a Northern blot that was sequentially probed with radiolabeled cDNA corresponding to the 3′-terminal 1.9 kb of the BDV genome (A) and radiolabeled cDNA corresponding to a 2.8-kb transcript from a central fragment of the BDV genome encoding M and G proteins (B). Rehybridization of the membrane with radiolabeled rat GAPDH cDNA confirmed that similar amounts of RNAs had been loaded into the slots. Gel positions and sizes of the various viral RNAs are indicated. Note that the upper portion of panel A represents a longer exposure of the membrane to the X-ray film. Lanes 0, no IFN treatment.
DISCUSSION
This study showed that BDV is highly susceptible to the antiviral action of IFN-α/β in vivo when this cytokine is present in the CNS before virus infection as, for example, in transgenic mice that constitutively express IFN-α1 in astrocytes. In contrast, the nonmanipulated IFN-α/β system, which needs to be activated by the infecting virus, is surprisingly ineffective against BDV. This was concluded from experiments with IFNAR0/0 mice, which lack functional surface receptors for virus-induced IFNs. We had expected that BDV would multiply at an enhanced rate in IFNAR0/0 mice, because IFNAR0/0 mice are highly susceptible to a large number of viruses (28, 39). Our analysis demonstrated, however, that BDV spread with comparable efficacies through the CNSs of wild-type and IFNAR0/0 mice. This unexpected phenotype probably resulted from the facts that BDV is a highly neurotropic virus that preferentially infects neurons (15) and that other cells of the CNS are infected less efficiently and at much later times (6). Importantly, neurons are believed to lack the ability to synthesize IFN-α/β in response to viral infection (2), whereas astrocytes and microglia have been shown to produce IFN-α/β in vitro (1, 23). Since other viruses have developed sophisticated mechanisms to counteract the host IFN response (14), it remained possible that BDV similarly uses specific mechanisms in neurons to avoid the antiviral activity of IFN. If true, then BDV would be expected to resist the antiviral effect of transgenically expressed IFN; however, this was clearly not the case. Therefore, we favor the alternative view that BDV simply avoids early infection of cells with effective IFN-α/β-producing capacity, thus using a smart novel viral evasion strategy.
By using Mx1 as a marker for the presence of biologically active IFN-α/β in the CNS of transgenic mice, we observed that neurons from the CA3 region of the hippocampus formation showed a reduced response to transgenically expressed IFN. This particular population of neurons also failed to express the Mx1 marker in response to virus-induced IFN-α/β present in the brains of persistently infected, nontransgenic mice (unpublished observation). We presently do not understand why neurons from different brain regions show differential responses to IFN-α/β. It is possible that CA3 neurons can respond to IFN but that they have a selective defect in Mx1 gene expression. As we have no access to antibodies that would specifically stain other IFN-induced mouse proteins, this hypothesis could not be tested experimentally. We also cannot exclude the possibility that astrocytes in the CA3 region are poor IFN producers, an idea which could explain the lower Mx1 levels in CA3 neurons. It is important to note that neurons from the CA3 region of the hippocampus formation are most frequently positive for viral antigen in animals with natural and experimental Borna disease (15). This is also true for our mouse model system (32 and data not shown), indicating that the apparently low IFN responsiveness of neurons of the CA3 region makes them excellent targets for BDV and possibly other neurotropic viruses.
We were surprised to find that IFN-α/β greatly contributes to BDV resistance of cultured mouse cells. From previous experiments with cultured cells of other species, it was clear that addition of exogenous IFN-α can block the replication of BDV (19), but virus-induced endogenous IFN did not appear to be of great importance. Considering the new data, we speculate that virus-induced IFN might generally limit the multiplication of BDV in cell culture systems but that this restriction becomes apparent only in mouse cells which, for unknown reasons, have a poor intrinsic capacity to support the replication of BDV. It was previously shown (19) that rat C6 glioblastoma cells, which are frequently used for BDV titration experiments, have an uncharacterized genetic defect that reduces the efficacy of IFN toward BDV. It is possible, therefore, that cell lines which readily support the multiplication of BDV either have a reduced ability to produce IFN-α/β in response to BDV exposure or have a reduced ability to mount a potent IFN-mediated antiviral state toward BDV. Since cultured fibroblasts from IFNAR0/0 mice were able to propagate BDV, it was of interest to determine whether BDV has less stringent tissue tropism in IFNAR0/0 mice. This was clearly not the case, indicating that other factors prevent BDV from successfully infecting cells outside the CNS and peripheral nervous system.
The most unexpected finding of this study was that although infectious virus and BDV antigen were present in similar concentrations in brains of wild-type and IFNAR0/0 mice, the levels of viral RNAs were not similar. We found strongly enhanced levels of subgenomic viral RNAs in brains of wild-type mice. On the other hand, the levels of genomic viral RNAs were about 10-fold lower in wild-type mice than in IFNAR0/0 mice. To account for these findings, we speculate that endogenous IFN-α/β, which is presumably produced by BDV-infected astrocytes, may appear too late for effective control of virus spread. We further hypothesize that infectious virus and BDV antigen may have very long half-lives in neurons and other nondividing cells, which could render these viral parameters insensitive to IFN-induced changes in RNA metabolism. Astrocytes of rats are not infected at early times after virus inoculation. Their infection is observed only after most susceptible neurons contain large amounts of viral antigen (6). In fact, the presence of a small number of infected astrocytes in the brains of persistently infected mice has recently also been documented (S. Freude and A. Pagenstecher, unpublished results), indicating that this phenomenon may apply to most animal hosts. Thus, the situation during infection of nontransgenic mice strikingly contrasts the situation for brains of mice that express the IFN-α1 transgene. In the latter mice, IFN is effective presumably because it is present before the virus has spread significantly.
Our results clearly indicate that the action of IFN-α/β in persistently infected, nondividing cells does not lead to the elimination of BDV, but they show that IFN can modulate the program of viral RNA synthesis. Previous data which demonstrated that IFN-α can strongly reduce the levels of BDV antigen and RNA in persistently infected Vero cells (19) are not in conflict with our new results, because long-term effects of IFN on growing cells were analyzed in the previous study. We conclude from the new data presented here that IFN-regulated factors of unknown identity are able to modulate the activity of the BDV polymerase in such a way that it may no longer function as a replicase but instead shows enhanced transcriptase activity. We cannot exclude the alternative view that a more complex mechanism is at work. It is possible that IFN reduces the in vivo stability of genomic RNA of BDV and, at the same time, increase the stability of viral mRNAs. Our experiments with persistently infected Vero cells further indicated that this proposed mode of IFN action is not restricted to neurons. Interestingly, treatment of Vero cells for only 24 h was sufficient to increase the levels of the shorter viral mRNAs by about twofold and to reduce the level of genomic viral RNA by about the same factor.
To our knowledge, this is the first report which demonstrates that the action of IFN can result in promotion rather than inhibition of viral gene transcription, presumably by influencing the ability of the viral polymerase to switch between transcription and replication modes. Since genome replication of negative-stranded RNA viruses is known to depend on host cell factors (9, 26, 27, 35), it is tempting to speculate that an unidentified factor which plays a critical role for the BDV polymerase is down-regulated or functionally altered in cells treated with IFN-α/β. We assume that similar changes in the intracellular environment may be responsible for the IFN-mediated protection of cells from de novo infection with BDV. We cannot exclude the alternative view that the enhanced transcriptional activity of BDV in IFN-treated cells is beneficial for the virus and that it actually represents a viral evasion strategy aimed to overcome the IFN-mediated inhibition of viral mRNA translation.
ACKNOWLEDGMENTS
We thank Iain Campbell, The Scripps Research Institute, San Diego, Calif., for providing the GIFN-12 mice used in this study and Otto Haller, Georg Kochs, Martin Schwemmle, and Urs Schneider for helpful discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft. M.S. was supported by a fellowship from the Ramon Areces Foundation.
REFERENCES
- 1.Akiyama H, Ikeda K, Katoh M, McGeer E G, McGeer P L. Expression of MRP14,27E10, interferon-alpha and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J Neuroimmunol. 1994;50:195–201. doi: 10.1016/0165-5728(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 2.Akwa Y, Hassett D E, Eloranta M L, Sandberg K, Masliah E, Powell H, Whitton J L, Bloom F E, Campbell I L. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. [PubMed] [Google Scholar]
- 3.Arnheiter H, Staeheli P. Expression of interferon dependent resistance to influenza virus in mouse embryo cells. Arch Virol. 1983;76:127–137. doi: 10.1007/BF01311696. [DOI] [PubMed] [Google Scholar]
- 4.Bautista J R, Schwartz G J, De la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 5.Briese T, De la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone K M, Moench T R, Lipkin W I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991;50:205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Danner K, Heubeck D, Mayr A. In vitro studies on Borna virus. I. The use of cell cultures for the demonstration, titration and production of Borna virus. Arch Virol. 1978;57:63–75. doi: 10.1007/BF01315638. [DOI] [PubMed] [Google Scholar]
- 8.De B P, Banerjee A K. Involvement of actin microfilaments in the transcription/replication of human parainfluenza virus type 3: possible role of actin in other viruses. Microsc Res Tech. 1999;47:114–123. doi: 10.1002/(SICI)1097-0029(19991015)47:2<114::AID-JEMT4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.De B P, Banerjee A K. Role of host proteins in gene expression of nonsegmented negative strand RNA viruses. Adv Virus Res. 1997;48:169–204. doi: 10.1016/s0065-3527(08)60288-2. [DOI] [PubMed] [Google Scholar]
- 10.Diaz M O, Ziemin S, Le Beau M M, Pitha P, Smith S D, Chilcote R R, Rowley J D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci USA. 1988;85:5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreiding P, Staeheli P, Haller O. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology. 1985;140:192–196. doi: 10.1016/0042-6822(85)90460-x. [DOI] [PubMed] [Google Scholar]
- 12.Formella S, Jehle C, Sauder C, Staeheli P, Schwemmle M. Sequence variability of Borna disease virus: resistance to superinfection may contribute to high genome stability in persistently infected cells. J Virol. 2000;74:7878–7883. doi: 10.1128/jvi.74.17.7878-7883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Dunia D, Sauder C, De la Torre J C. Borna disease virus and the brain. Brain Res Bull. 1997;44:647–664. doi: 10.1016/S0361-9230(97)00276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodbourn S, Didcock L, Randall R E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 15.Gosztonyi G, Ludwig H. Borna disease - neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995;190:39–73. [PubMed] [Google Scholar]
- 16.Greene L A, Shain W, Chalazonitis A, Breakfield X, Minna J, Coon H G, Nirenberg M. Neuronal properties of hybrid neuroblastoma × sympathetic ganglion cells. Proc Natl Acad Sci USA. 1975;72:4923–4927. doi: 10.1073/pnas.72.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas B, Becht H, Rott R. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J Gen Virol. 1986;67:235–241. doi: 10.1099/0022-1317-67-2-235. [DOI] [PubMed] [Google Scholar]
- 18.Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallensleben W, Staeheli P. Inhibition of Borna disease virus multiplication by interferon: cell line differences in susceptibility. Arch Virol. 1999;144:1209–1216. doi: 10.1007/s007050050580. [DOI] [PubMed] [Google Scholar]
- 20.Hausmann J, Hallensleben W, De la Torre J C, Pagenstecher A, Zimmermann C, Pircher H, Staeheli P. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc Natl Acad Sci USA. 1999;96:9769–9774. doi: 10.1073/pnas.96.17.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog S, Kompter C, Frese K, Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173:171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- 22.Lee H J, Hammond D N, Large T H, Roback J D, Sim J A, Brown D A, Otten U H, Wainer B H. Neuronal properties and trophic activities of immortalized hippocampal cells from embryonic and young adult mice. J Neurosci. 1990;10:1779–1787. doi: 10.1523/JNEUROSCI.10-06-01779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman A P, Pitha P M, Shin H S, Shin M L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGee R, Simpson P, Christian C, Mata M, Nelson P, Nirenberg M. Regulation of acetylcholine release from neuroblastoma × glioma hybrid cells. Proc Natl Acad Sci USA. 1978;75:1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier E, Fah J, Grob M S, End R, Staeheli P, Haller O. A family of interferon-induced Mx-related mRNAs encodes cytoplasmic and nuclear proteins in rat cells. J Virol. 1988;62:2386–2393. doi: 10.1128/jvi.62.7.2386-2393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momose F, Basler C F, O'Neill R E, Iwamatsu A, Palese P, Nagata K. Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. J Virol. 2001;75:1899–1908. doi: 10.1128/JVI.75.4.1899-1908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momose F, Handa H, Nagata K. Identification of host factors that regulate the influenza virus RNA polymerase activity. Biochimie. 1996;78:1103–1108. doi: 10.1016/s0300-9084(97)86736-3. [DOI] [PubMed] [Google Scholar]
- 28.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 29.Pavlovic J, Zurcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 31.Sauder C, Hallensleben W, Pagenstecher A, Schneckenburger S, Biro L, Pertlik D, Hausmann J, Suter M, Staeheli P. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J Virol. 2000;74:9267–9280. doi: 10.1128/jvi.74.19.9267-9280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauder C, Wolfer D P, Lipp H P, Staeheli P, Hausmann J. Learning deficits in mice with persistent Borna disease virus infection of the CNS associated with elevated chemokine expression. Behav Brain Res. 2001;120:189–201. doi: 10.1016/s0166-4328(00)00370-3. [DOI] [PubMed] [Google Scholar]
- 33.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 34.Schwemmle M, Hatalski C G, Lewis A J, Lipkin W I. Borna virus. In: Ahmed R, Chen I, editors. Persistent viral infections. New York, N.Y: John Wiley & Sons, Inc.; 1999. pp. 559–573. [Google Scholar]
- 35.Shimizu K, Handa H, Nakada S, Nagata K. Regulation of influenza virus RNA polymerase activity by cellular and viral factors. Nucleic Acids Res. 1994;22:5047–5053. doi: 10.1093/nar/22.23.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staeheli P, Dreiding P, Haller O, Lindenmann J. Polyclonal and monoclonal antibodies to the interferon-inducible protein Mx of influenza virus-resistant mice. J Biol Chem. 1985;260:1821–1825. [PubMed] [Google Scholar]
- 37.Staeheli P, Sauder C, Hausmann J, Ehrensperger F, Schwemmle M. Epidemiology of Borna disease virus. J Gen Virol. 2000;81:2123–2135. doi: 10.1099/0022-1317-81-9-2123. [DOI] [PubMed] [Google Scholar]
- 38.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 39.van den Broek M F, Muller U, Huang S, Zinkernagel R M, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]