Abstract
In this study, we tested whether the human heart possesses a cardiac stem cell (CSC) pool that promotes regeneration after infarction. For this purpose, CSC growth and senescence were measured in 20 hearts with acute infarcts, 20 hearts with end-stage postinfarction cardiomyopathy, and 12 control hearts. CSC number increased markedly in acute and, to a lesser extent, in chronic infarcts. CSC growth correlated with the increase in telomerase-competent dividing CSCs from 1.5% in controls to 28% in acute infarcts and 14% in chronic infarcts. The CSC mitotic index increased 29-fold in acute and 14-fold in chronic infarcts. CSCs committed to the myocyte, smooth muscle, and endothelial cell lineages increased ≈85-fold in acute infarcts and ≈25-fold in chronic infarcts. However, p16INK4a-p53-positive senescent CSCs also increased and were 10%, 18%, and 40% in controls, acute infarcts, and chronic infarcts, respectively. Old CSCs had short telomeres and apoptosis involved 0.3%, 3.8%, and 9.6% of CSCs in controls, acute infarcts, and chronic infarcts, respectively. These variables reduced the number of functionally competent CSCs from ≈26,000/cm3 of viable myocardium in acute to ≈7,000/cm3 in chronic infarcts, respectively. In seven acute infarcts, foci of spontaneous myocardial regeneration that did not involve cell fusion were identified. In conclusion, the human heart possesses a CSC compartment, and CSC activation occurs in response to ischemic injury. The loss of functionally competent CSCs in chronic ischemic cardiomyopathy may underlie the progressive functional deterioration and the onset of terminal failure.
Keywords: cardiac progenitor cells, human heart, myocardial infarction
Myocardial regeneration occurs in humans after ischemic injury (1, 2), and myocyte proliferation appears to be restricted to the viable myocardium adjacent to and remote from the infarct (2). The identification of cardiac stem cells (CSCs) in the adult heart (3-7) suggests that replicating myocytes may constitute a subpopulation of rapidly growing amplifying cells originated from more primitive cells. CSCs are distributed throughout the heart, raising the possibility that those located within the infarct or in its proximity could divide and differentiate reconstituting dead myocardium. If this hypothesis were the case, strategies may be developed to enhance myocardial growth promoting partial restoration of the infarct. This response would reduce infarct size, improve function, and decrease mortality. Myocardial regeneration within the infarct could have escaped earlier observations because the heart was not viewed as a self-renewing organ, and myocyte replacement was considered to be regulated by a subset of cells capable of a few rounds of doubling, located by necessity in the spared portion of the ventricle (2). Alternatively, the lack of myocardial regeneration might reflect CSC death within the infarct and/or the inability of CSCs to migrate and reach the necrotic area. Thus far, no evidence has been presented that CSCs can reconstitute infarcted tissue in the human heart.
Myocyte division is markedly reduced in chronic ischemic cardiomyopathy (1, 2), and the recognition that CSCs modulate cardiac homeostasis (3-6) suggests that a decrease in number and growth of CSCs may underlie the attenuation in cell multiplication in long-term heart failure. In this study, we investigated whether CSCs divide and differentiate in myocytes and vascular cells acutely after infarction and whether defects in CSC number and/or function promote a decline in myocyte and vessel regeneration chronically. CSC activation was evaluated by markers of cell proliferation (8, 9) together with histone H3 phosphorylation (10) as a marker of mitosis. CSC senescence was determined by the expression of aging-associated proteins and the presence of DNA oxidative stress and apoptosis (11, 12). The telomere-telomerase system was investigated because of its effects on cell growth and viability (13).
Materials and Methods
Patients and CSCs. We studied hearts from patients who died acutely after infarction, and hearts from patients who underwent cardiac transplantation after chronic infarcts (Fig. 7 and Table 1, which are published as supporting information on the PNAS web site). CSCs were identified by c-kit, MDR1, and Sca-1-like epitopes (Fig. 8 and Table 2, which are published as supporting information on the PNAS web site). CSCs were negative for transcription factors and structural proteins of cardiac cell lineages and for markers of cells of hemopoietic origin. CSC number and volume were then determined (Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
CSC Death and Growth. Telomere length in CSCs was evaluated by quantitative fluorescence in situ hybridization. In each heart, 50 CSC nuclei were examined for telomeric length, and 100-200 CSC nuclei for p53 and p16INK4a. X and Y chromosomes were detected by fluorescence in situ hybridization with CEP X α-satellite and CEP Y satellite III DNA probes. CSC apoptosis was evaluated by Hairpin 1 and CSC proliferation by Ki67, MCM5, and phospho-histone H3. Western blotting of proteins implicated in CSC senescence, telomere function, and CSC growth was obtained (Supporting Materials and Methods).
Results
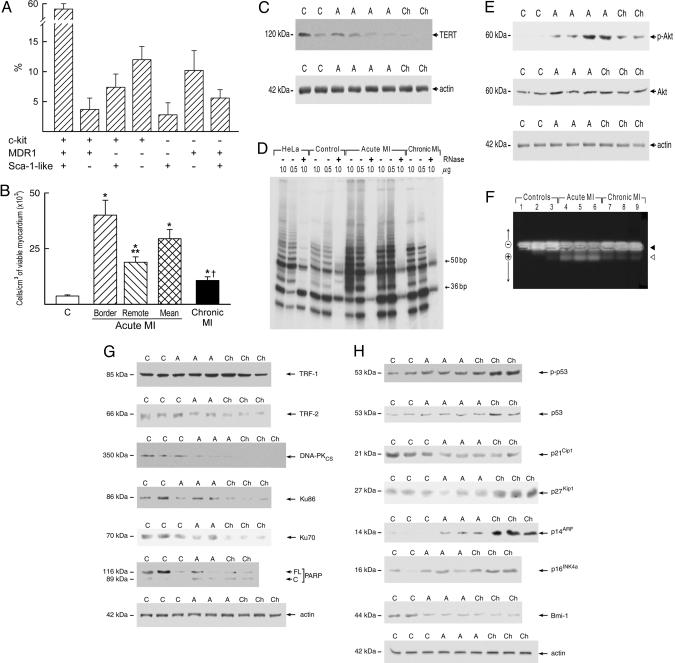
CSC Number. CSCs were lineage-negative cells with a volume of 203 ± 50 μm3. In all hearts, ≈60% of CSCs expressed c-kit, MDR1, and Sca-1-like epitopes together, and only small percentages of CSCs possessed one or two antigens (Fig. 1 A). CSCs were negative for markers of hematopoietic cell lineages, indicating that, at most, a very small fraction of primitive cells could have been of bone marrow origin. Additionally, the Sca-1-like positive CSCs were negative for Ets1, Erg1, flk1, VE-cadherin, and von Willebrand factor excluding the contribution of endothelial cells to the CSC pool. The Sca-1-like protein was detected by Western blotting in human, dog, and rat myocardium and in human bone marrow, endothelial cells, and peripheral blood. The band molecular weight was identical to that identified in mouse heart, kidney, and spleen. Moreover, Sca-1-like positive cells were found in human bone marrow (Fig. 9, which is published as supporting information on the PNAS web site). Because members of the Ly6 protein family were found in humans (14, 15), the Sca-1 antibody may have reacted with an epitope of human CSCs that corresponds to a known or unknown member of the Ly6 family.
Fig. 1.
Heart failure and CSCs. Shown are the expression of surface epitopes (A) in CSCs and the number of CSCs per cm3 of viable myocardium (B). *, P < 0.05 vs. controls (C); **, P < 0.05 between the border and remote myocardium in acute myocardial infarction (MI). †, P < 0.05 between acute and chronic MI. (C) Expression of TERT in human cardiac protein lysates from control (C), acute (A), and chronic (Ch) infarcted hearts. (D) TERT activity measured by the telomeric repeat amplification protocol assay. Products of TERT activity start at 50 bp and display a 6-bp periodicity. Samples treated with RNase (+) were used as a negative control and HeLa cells as a positive control. Serial dilutions of proteins (0.5 and 1.0 μg) were used to confirm the specificity of the reaction. The band at 36 bp corresponds to an internal control for PCR efficiency. (E) Expression of phospho-Akt and total Akt; (F) unphosphorylated (filled arrowhead) and phosphorylated (open arrowhead) forms of TERT in an Akt kinase assay. (G) Expression of the telomere-related proteins, TRF-1, TRF-2, and DNA-PK (DNA-PKcs, Ku86, and Ku70), and full-length and cleaved poly (ADP-ribose) polymerase. (H) Expression of cell cycle inhibitors and markers of cellular senescence. Optical density values are shown in Fig. 10.
The number of CSCs in the viable myocardium of infarcted hearts was increased. However, in acute infarcts, there were 40,000 CSCs per cm3 of tissue in the border zone and <20,000 per cm3 of myocardium in the remote region. In chronic infarcts, in which a border zone was no longer present, the number of CSCs was lower than in acute infarcts (Fig. 1B). Thus, ischemic heart failure led to an increase in the CSC number that was attenuated with time.
Telomerase Function. Telomerase (TERT) protects from the loss of DNA at the chromosomal ends during cell division (13, 16-18). TERT expression and activity provide an index of the growth potential of CSCs and their committed progeny, including amplifying myocytes, endothelial cell (EC), and smooth muscle cell (SMC) progenitors (19). In comparison with controls, TERT did not vary in acute infarcts but decreased 72% in chronic infarcts (Fig. 1C). TERT activity increased 8.6-fold in acute and 2.6-fold in chronic infarcts (Fig. 1D). Because Akt activates TERT (20), the quantity and degree of phosphorylation of Akt were evaluated. Total Akt was similar in control and infarcted hearts, but the phosphorylated form increased 21-fold in acute and 3.8-fold in chronic infarcts (Fig. 1E). To test whether phospho-Akt activated TERT, a peptide containing the Akt-phosphorylation site of human TERT was used in an Akt-kinase assay (20). TERT phosphorylation increased in acute infarcts (Fig. 1F; Fig. 10, which is published as supporting information on the PNAS web site). Phospho-Akt was detected in 60 ± 12% and 13 ± 11% c-kit-positive cells in acute and chronic infarcts, respectively. A value of 9 ± 3% was found in control hearts. Thus, telomerase activity was enhanced acutely and, to a lesser extent, chronically after infarction, and this difference correlated with the difference in active Akt.
Telomeric Shortening and CSC Senescence and Death. Telomeres are protected by a complex of proteins including TRF1, TRF2, uncleaved PARP, and DNA-PK. The levels of TRF2, full-length PARP, and DNA-PK decreased after infarction but significantly more in chronic than in acute infarcts (Figs. 1G and 10). Conversely, cleaved inactive-PARP increased more in chronic than in acute infarcts (Fig. 1G). These molecular modifications were accompanied by increased expression of p14ARF and p16INK4a, phosphoSer-15-p53 and p53. p14ARF inhibits p53 degradation, and p16INK4a inhibits cdk4 and cdk6, blocking cells at the G0-G1 boundary (11). Moreover, p53 inhibits the cell cycle at G1-S or G2-M checkpoint. Additionally, the cyclin-dependent G1 kinase inhibitors p21Cip1 and p27Kip1 decreased acutely after infarction, whereas in chronic infarcts, p21Cip1 decreased but p27Kip1 increased (Figs. 1H and 10). Bmi-1, a negative modulator of the age-associated genes p14ARF and p16INK4a, decreased more in chronic than in acute infarcts. Thus, telomere dysfunction, cellular senescence, and the expression of genes that interfere with cell replication and activate cell death were enhanced predominantly in chronic heart failure.
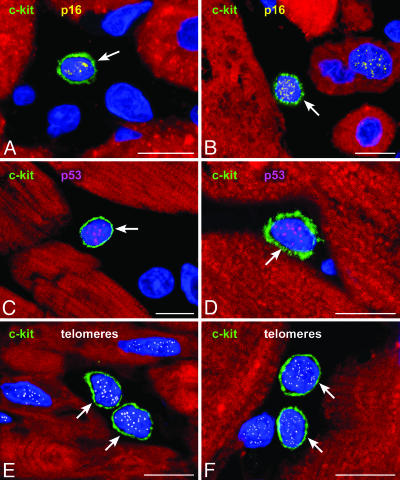
Because of these biochemical alterations, the percentage of CSCs that reached growth arrest and senescence was determined. This finding enabled us to define the pool of functionally competent CSCs. Three markers of cellular senescence were used: p16INK4a, p53, and telomeric shortening (Fig. 2). In comparison with control hearts, the fraction of p16INK4a-positive CSCs increased an average of 1.7-fold in acute and 3.6-fold in chronic infarcts (Fig. 11, which is published as supporting information on the PNAS web site). p53 was present in a smaller fraction of CSCs, and most p53-positive CSCs coexpressed p16INK4a. Moreover, the number of p16INK4a-p53-positive CSCs with short telomeres increased markedly in chronic and to a lesser extent in acute infarcts (Fig. 12, which is published as supporting information on the PNAS web site). Also, CSC apoptosis increased an average of 12-fold in acute and 32-fold in chronic infarcts (Fig. 13, which is published as supporting information on the PNAS web site). Apoptotic CSCs were p16INK4a-p53 positive, and ≈80% exhibited oxidative damage detected by 8-OHdG in the nuclei. As a result, functionally competent CSCs were 73% lower in chronic (6,900 ± 1,100 cells per cm3 myocardium) than in acute (25,600 ± 3,600) infarcts. However, the value in chronic infarcts was higher than in controls (3,500 ± 500). Thus, oxidative stress, telomeric shortening, p16INK4a, p53, and apoptosis coexist in CSCs, reducing their functional pool size in chronic heart failure.
Fig. 2.
Heart failure and CSC senescence. c-kit positive CSCs (green, A-F) express p16INK4a (yellow, A and B) and p53 (magenta, C and D). The detection of telomeres in c-kit-positive CSCs is shown by small white fluorescence dots (E and F). Shown are control myocardium (A), acute infarcts (C and E), and chronic infarcts (B, D, and F). (Scale bars: 10 μm.)
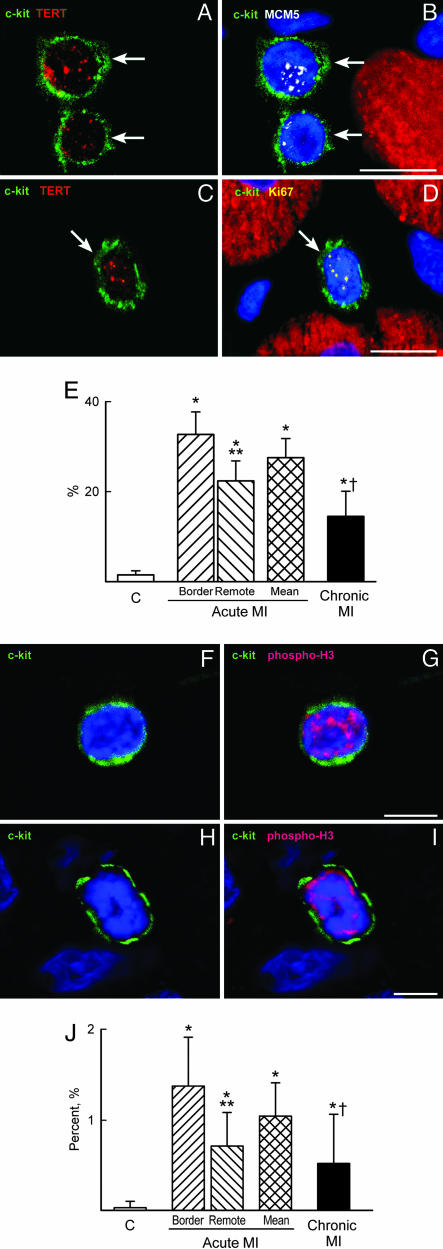
CSC Growth and Differentiation. To assess the magnitude of ongoing CSC growth in infarcted hearts, the number of cycling telomerase-competent CSCs was determined. Two cell-cycle markers were used, Ki67 and MCM5 (Fig. 3 A-D; Fig. 14, which is published as supporting information on the PNAS web site). In comparison with controls, the fraction of telomerase-Ki67-positive CSCs increased an average of 19-fold in acute and 9-fold in chronic infarcts (Fig. 3E). Slightly higher values were obtained with MCM5. Mitotic CSCs, recognized by histone H3 phosphorylation (Fig. 3 F-I), increased 26-fold in acute and 13-fold in chronic infarcts (Fig. 3J). Thus, the activation of CSCs was significantly greater acutely than chronically after infarction.
Fig. 3.
Heart failure and CSC growth. (A-D) c-kit-positive CSCs (green, arrows) express telomerase (red dots, A and C) and MCM5 (white dots, B) or Ki67 (yellow dots, D). Myocytes are labeled by α-sarcomeric actin (red) and nuclei by DAPI (blue). (E) Percentage of telomerase competent CSCs in the cell cycle. (F-I) c-kit-positive CSCs (green) have nuclei labeled by phospho-H3 (red). (J) Mitotic index of CSCs. *, P < 0.05 vs. controls (C); **, P < 0.05 between the border and remote myocardium in acute MI; †, P < 0.05 between acute and chronic MI. A, B, and F-I are acute infarcts. (Scale bars: 10 μm.)
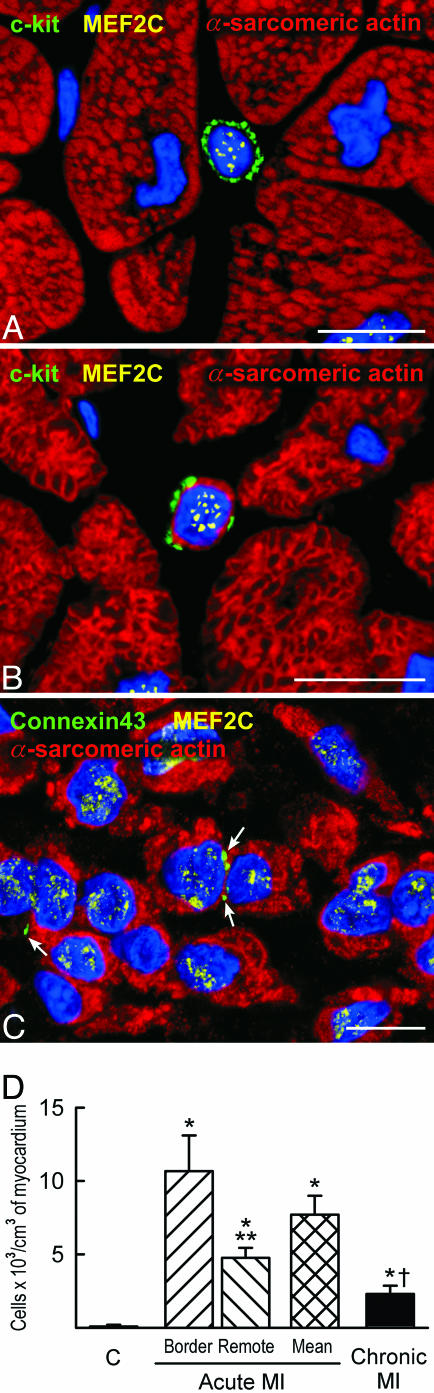
Because of the difficulty to separate in the human heart preexisting myocytes and vascular structures from those generated by activation and differentiation of CSCs, we analyzed the coexpression of stem cell antigens with transcription factors and cytoplasmic proteins specific for cardiomyocytes, SMCs, and ECs. Therefore, the commitment of CSCs to myocytes, SMCs, and ECs was established (Fig. 4 A-C; Fig. 15, which is published as supporting information on the PNAS web site). The more mature phenotype of these cells cannot be easily linked to the CSC population because the stem cell antigens are lost early during differentiation (3). However, maturing myocytes lacking stem cell antigens and newly formed arterioles with a thick wall and small lumen were recognized (Fig. 16, which is published as supporting information on the PNAS web site). The number of myocyte progenitors/precursors increased an average of 90-fold in acute infarcts and 26-fold in chronic infarcts (Fig. 4D). Similar increases were found in SMC and EC progenitors/precursors (Fig. 17, which is published as supporting information on the PNAS web site). Thus, activation and commitment of CSCs was robust in acute infarcts but markedly attenuated in chronic infarcts.
Fig. 4.
Heart failure and lineage commitment of CSCs. (A-C) Myocyte progenitors (A) express c-kit (green) and the myocyte transcription factor MEF2C (yellow dots); myocyte precursors (B) express c-kit (green), MEF2C (yellow dots) and have a thin layer of cytoplasm positive for α-sarcomeric actin (red). (C) Small developing myocytes (α-sarcomeric actin, red) express MEF2C (yellow dots) and have lost the stem cell surface antigen. Connexin 43 is detected between some of these maturing cells (green, arrows). (D) Myocyte progenitors and precursors. *, P < 0.05 vs. controls (C); **, P < 0.05 between the border and remote myocardium in acute MI; †, P < 0.05 between acute and chronic MI. Shown are acute infarcts (A and C) and a chronic infarct (B). (Scale bars: 10 μm.)
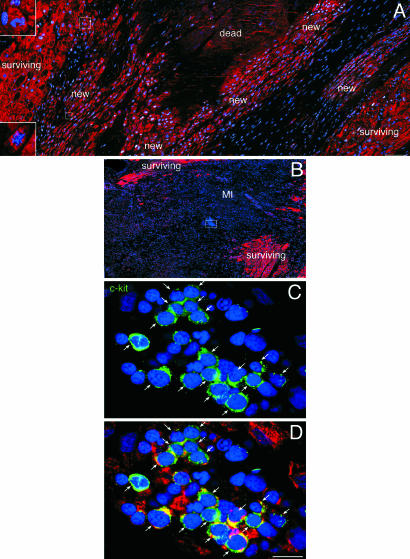
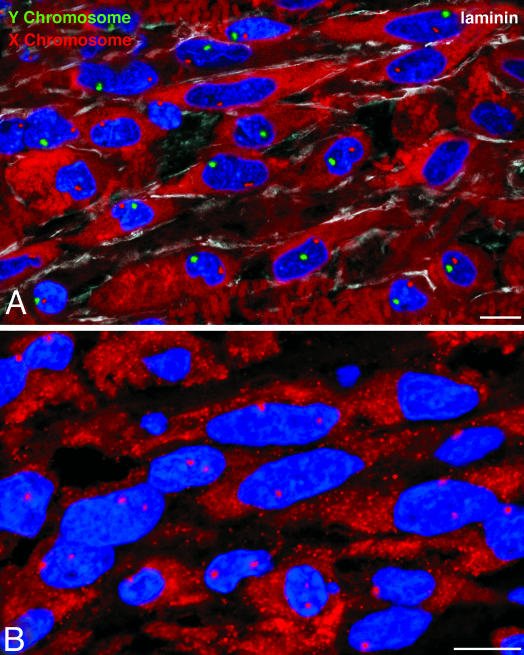
Myocardial Regeneration. The identification of human CSCs raised the possibility that some of these cells located at the border of the infarct or within the infarct may survive the ischemic event and initiate cardiac repair. Seven of the 20 acutely infarcted hearts exhibited areas of spontaneous and intense myocardial regeneration invading the dead tissue (Fig. 5; Fig. 18, which is published as supporting information on the PNAS web site). These foci varied from 500 μm2 to 5 mm2 and consisted of clusters of proliferating myocytes, and SMCs and ECs organized in small new coronary vessels; these regenerated cells were negative for apoptosis. A similar phenomenon was not found in chronic infarcts. Regenerated myocytes were not the product of fusion between CSCs and spared myocytes because, in male hearts, they possessed only one X and one Y chromosome and, in female hearts, only two X chromosomes (Fig. 6). Thus, the heart has an intrinsic growth reserve capable of repairing dead myocardium independently of cell fusion.
Fig. 5.
Heart failure and myocardial regeneration. (A) Area of regenerating myocardium within the infarct. Clusters of highly proliferating small developing myocytes are visible. Myocytes are labeled by cardiac myosin (red) and nuclei by DAPI (blue). Most of these cells are positive for MCM5 (white). Two dividing small myocytes are shown in Insets. (B) Cluster of cells included in a rectangle in the middle of an acute infarct. These cells are shown at higher magnification in C and D. They represent c-kit-positive cells (green, arrows) at times expressing cardiac myosin (red; D). (Scale bars: A and B, 100 μm; C and D, 10 μm.)
Fig. 6.
Myocardial regeneration and cell fusion. Regenerating myocytes in a male (A) and female (B) infarcted heart. In the male heart, only one X (magenta) and one Y (green) chromosome are detected. In the female heart, only two X chromosomes (magenta) are visible. (Scale bars: 10 μm.)
Discussion
The results of this study indicate that the human heart contains a population of CSCs that can divide and differentiate into myocytes, and SMCs and ECs organized in coronary vessels. The CSC pool is enhanced acutely after infarction, but this growth response is attenuated in chronic heart failure. In the latter case, telomerase activity is decreased, and CSC division is impaired by severe telomeric shortening and cellular senescence. Moreover, CSC apoptosis is increased, resulting in a decline of the number of functionally competent CSCs. Therefore, the formation of myocytes and coronary vasculature cannot counteract the chronic loss of parenchymal cells and vascular structures (21-23). We propose that this negative balance between myocardial regeneration and death leads to progressive ventricular dilation and deterioration of ventricular performance (24). Nevertheless, the recognition that the human heart possesses a stem cell compartment that, although compromised, is still present in end-stage failure points to strategies for the treatment of the human disease. It might be possible to isolate CSCs from small samples of myocardium and, after their growth in vitro, the expanded CSCs can be administrated back to the same patient. This therapeutic approach would have all of the advantages of autologous transplantation.
Myocardial regeneration acutely after infarction appears to be mediated by multipotent CSCs, which undergo lineage commitment and form amplifying cells that divide and mature. Whether these cells acquire functional competence and adult characteristics cannot be established at present. However, this possibility is supported by prior observations in animals in which the injection of CSCs in the infarcted myocardium results in the reconstitution of the dead tissue and improvement in cardiac function (5). The newly formed, small, developing myocytes found in experimental animals have the same dimensional and structural characteristics of those detected in the foci of spontaneous cardiac repair in humans. These neonatal-like myocytes are functional and have contractile properties equal or superior to those of the spared hypertrophied myocytes (5). However, acutely after infarction, the magnitude of the infarct may exceed the response of CSCs and tissue reconstitution so that cardiogenic shock supervenes (25). Despite this negative clinical outcome, the identification of spontaneous myocardial regeneration in acute infarcts demonstrates that dead tissue can be replaced partly by new myocardium derived from the activation of endogenous progenitor cells. These data extend previous observations in pressure hypertrophy in humans (3) and point to a critical role of CSCs in cardiac repair.
The mechanism by which viable myocardium can be formed in a region with little or no blood supply and unfavorable environment is unclear. Opening of collateral vessels can provide enough oxygen for cell growth and function and explain the presence of CSCs within the infarct. Similarly, oxygen diffusion from the blood in the left ventricular chamber to the endomyocardium can preserve progenitor cells in the ischemic region. Alternatively, CSCs can migrate from the surviving myocardium to the infarct and differentiate into myocytes and coronary vessels (5, 26).
The exogenous administration of progenitor cells of bone marrow origin in patients with ischemic cardiomyopathy has been found to ameliorate ventricular remodeling and cardiac performance (27). Whether these beneficial effects on the postinfarcted heart are mediated by differentiation of hematopoietic cells into cardiomyocytes and coronary vessels (28) or the injected cells secrete factors that activate the local CSCs has not been resolved yet. As has been suggested, both mechanisms may be involved (27). This positive paracrine loop points to the importance of CSC growth as a therapeutic strategy for the restoration of lost myocardium. The current observations in humans, together with the replacement of infarcted tissue by activation and differentiation of resident CSCs in animals, indicate that the CSC may be the ideal cell for myocardial regeneration. Clones of human CSCs have been published in ref. 26. The presence of stem cells that reside in the heart, and are, therefore, predestined to become cardiac cells, should result in more efficient regeneration and obviate the need for the more complex and time-consuming process involved in the commitment to cell lineages different from the organ of origin (29). A rapid regeneration of irreversibly altered myocardium is crucial with large infarcts in which the immediate reduction of infarct size is critical for survival (25).
Whether CSCs are generated within the myocardium or are continuously supplied to the heart by the bone marrow through the systemic circulation cannot be completely resolved. However, the notion that hematopoietic stem cells (HSCs) migrate and repopulate distant organs such as the heart is not supported by available information. The absence or minimal trafficking of HSCs from the bone marrow to the myocardium has been shown in the normal and pathologic heart (26). Moreover, the lack of comorbidity of heart failure and bone marrow failure in the same patient population does not point to a critical function of the bone marrow in myocardial regeneration. Conversely, the demonstration that human CSCs can acquire the phenotype of cardiomyocytes, SMCs, and ECs establishes a mechanistic link between the morphological findings and the proposed crucial role of this cell population in vivo. In this regard, a large number of developing myocytes (10,000 cells per g), SMCs (1,100 cells per g) and ECs (3,500 cells per g) was measured in the border zone acutely after infarction. The contribution of cell regeneration within the infarct was significantly less and was documented only in a few cases. Cell regeneration decreased >70% chronically after infarction, providing an important element for terminal failure. The reduction in the CSC compartment appeared to be the consequence of replicative senescence and growth arrest together with apoptosis. This view is consistent with the marked shortening of telomeres and the increased expression of p16 and p53. The duration of the cardiac disease, coupled with the compensatory long-term replicative growth of CSCs, may have led to telomere attrition, activation of the p53 and p16 pathways and, ultimately, to the senescent phenotype.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL38132, HL075480, AG15756, HL65577, HL66923, AG17042, HL55757, HL68088, HL70897, HL76794, HL65573, HL081737, AG023071, and HL081737.
Author contributions: K.U., R. Bolli, A.L., J.K., and P.A. designed research; K.U., D.T., F. Sheikh, A.D.A., D.N., F. Silvestri, C.A.B., R. Bussani, A.P.B., F.Q., A.L., and J.K. performed research; K.U., D.T., F. Sheikh, A.D.A., D.N., F. Silvestri, C.A.B., R. Bussani, A.P.B., F.Q., R. Bolli, A.L., J.K., and P.A. analyzed data; and K.U., F.Q., R. Bolli, A.L., J.K., and P.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CSC, cardiac stem cell; EC, endothelial cell; MI, myocardial infarction; SMC, smooth muscle cell; TERT, telomerase.
References
- 1.Kajstura, J., Leri, A., Finato, N., Di Loreto, C., Beltrami, C. A. & Anversa, P. (1998) Proc. Natl. Acad. Sci. USA 95, 8801-8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami, A. P., Urbanek, K., Kajstura, J., Yan, S. M., Finato, N., Bussani, R., Nadal-Ginard, B., Silvestri, F., Leri, A., Beltrami, C. A., et al. (2001) N. Engl. J. Med. 344, 1750-1757. [DOI] [PubMed] [Google Scholar]
- 3.Urbanek, K., Quaini, F., Tasca, G., Torella, D., Castaldo, C., Nadal-Ginard, B., Leri, A., Kajstura, J., Quaini, E. & Anversa, P. (2003) Proc. Natl. Acad. Sci. USA 100, 10440-10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quaini, F., Urbanek, K., Beltrami, A. P., Finato, N., Beltrami, C. A., Nadal-Ginard, B., Kajstura, J., Leri, A. & Anversa, P. (2002) N. Engl. J. Med. 346, 5-15. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami, A. P., Barlucchi, L., Torella, D., Baker, M., Limana, F., Chimenti, S., Kasahara, H., Rota, M., Musso, E., Urbanek, K., et al. (2003) Cell 114, 763-776. [DOI] [PubMed] [Google Scholar]
- 6.Oh, H., Bradfute, S. B., Gallardo, T. D., Nakamura, T., Gaussin, V., Mishina, Y., Pocius, J., Michael, L. H., Behringer, R. R., Garry, D. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12313-12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuura, K., Nagai, T., Nishigaki, N., Oyama, T., Nishi, J., Wada, H., Sano, M., Toko, H., Akazawa, H., Sato, T., et al. (2004) J. Biol. Chem. 279, 11384-11391. [DOI] [PubMed] [Google Scholar]
- 8.Scholzen, T. & Gerdes, J. (2000) J. Cell. Physiol. 182, 311-322. [DOI] [PubMed] [Google Scholar]
- 9.Dutta, A. & Bell, S. P. (1997) Annu. Rev. Cell Dev. Biol. 13, 293-332. [DOI] [PubMed] [Google Scholar]
- 10.Hans, F. & Dimitrov, S. (2001) Oncogene 20, 3021-3027. [DOI] [PubMed] [Google Scholar]
- 11.Sherr, C. J. (2004) Cell 116, 235-246. [DOI] [PubMed] [Google Scholar]
- 12.Nia, A. B., Van Schooten, F. J., Schilderman, P. A., De Kok, T. M., Haenen, G. R., Van Herwijnen, M. H., Van Agen, E., Pachen, D. & Kleinjans, J. C. (2001) Carcinogenesis 22, 395-401. [DOI] [PubMed] [Google Scholar]
- 13.Blasco, M. A. (2002) Nat. Rev. Cancer 2, 627-633. [DOI] [PubMed] [Google Scholar]
- 14.Horie, M., Okutomi, K., Taniguchi, Y., Ohbuchi, Y., Suzuki, M. & Takahashi, E. (1998) Genomics 53, 365-368. [DOI] [PubMed] [Google Scholar]
- 15.Klippel, S., Strunck, E., Busse, C., Behringer, D. & Pahl, H. L. (2002) Blood 100, 2441-2448. [DOI] [PubMed] [Google Scholar]
- 16.Blackburn, E. H. (2001) Cell 106, 661-673. [DOI] [PubMed] [Google Scholar]
- 17.Smogorzewska, A. & de Lange, T. (2002) EMBO J. 21, 4338-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpless, N. E. & DePinho, R. A. (2004) J. Clin. Invest. 113, 160-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haendeler, J., Hoffmann, J., Diehl, J. F., Vasa, M., Spyridopoulos, I., Zeiher, A. M. & Dimmeler, S. (2004) Circ. Res. 94, 703-705. [DOI] [PubMed] [Google Scholar]
- 20.Torella, D., Rota, M., Nurzynska, D., Musso, E., Monsen, A., Shiraishi, I., Zias, E., Walsh, K., Rosenzweig, A., Sussman, M. A., et al. (2004) Circ. Res. 94, 514-524. [DOI] [PubMed] [Google Scholar]
- 21.Beltrami, C. A., Finato, N., Rocco, M., Feruglio, G. A., Puricelli, C., Cigola, E., Quaini, F., Sonnenblick, E. H., Olivetti, G. & Anversa, P. (1994) Circulation 89, 151-163. [DOI] [PubMed] [Google Scholar]
- 22.Narula, J., Haider, N., Virmani, R., DiSalvo, T. G., Kolodgie, F. D., Hajjar, R. J., Schmidt, U., Semigran, M. J., Dec, G. W. & Khaw, B. A. (1996) N. Engl. J. Med. 335, 1182-1189. [DOI] [PubMed] [Google Scholar]
- 23.Olivetti, G., Abbi, R., Quaini, F., Kajstura, J., Cheng, W., Nitahara, J. A., Quaini, E., Di Loreto, C., Beltrami, C. A., Krajewski, S., et al. (1997) N. Engl. J. Med. 336, 1131-1141. [DOI] [PubMed] [Google Scholar]
- 24.Jessup, M. & Brozena, S. (2003) N. Engl. J. Med. 348, 2007-2018. [DOI] [PubMed] [Google Scholar]
- 25.Page, D. L., Caulfield, J. B., Kastor, J. A., DeSanctis, R. W. & Sanders, C. A. (1971) N. Engl. J. Med. 285, 133-137. [DOI] [PubMed] [Google Scholar]
- 26.Anversa, P., Sussman, M. A. & Bolli, R. (2004) Circulation 109, 2832-2838. [DOI] [PubMed] [Google Scholar]
- 27.Britten, M. B., Abolmaali, N. D., Assmus, B., Lehmann, R., Honold, J., Schmitt, J., Vogl, T. J., Martin, H., Schachinger, V., Dimmeler, S., et al. (2003) Circulation 108, 2212-2218. [DOI] [PubMed] [Google Scholar]
- 28.Körbling, M. & Estrov, Z. (2003) N. Engl. J. Med. 349, 570-582. [DOI] [PubMed] [Google Scholar]
- 29.Tosh, D. & Slack, J. M. W. (2002) Nat. Rev. Mol. Cell Biol. 3, 187-194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.