Abstract
Morchella spp. (true morels) are precious edible mushrooms consumed around the world, with a delicious taste, rich nutritional value, and unique healthcare effects. Various fungi and bacteria have been reported to colonize the ascocarps of Morchella, damaging their fruiting bodies and leading to serious economic losses in cultivation. The species identification of these colonizing organisms is crucial for understanding their colonization mechanisms on morels. Slime molds, which have characteristics of both “fungi” and “animals”, can occasionally colonize crops and edible fungi. However, there have been no reports of dictyostelid cellular slime molds (dictyostelids) colonizing plants and fungi to date. In this study, we discovered that dictyostelids colonized the surface of one wild ascoma of Morchella in the forest of Chongqing, China, with the tissues being black and rotten. Macro- and micro-morphological observations, along with molecular phylogenetic analyses, identified the specimens investigated in this study as Dictyostelium implicatum and Morchella sp. Mel-21. The results provide new knowledge of dictyostelid colonization on organisms and contribute to the diversity of species colonizing true morels. Moreover, this is also the first report of dictyostelids distributed in Chongqing, China. This study enhances our insights into the life history and potential ecological significance of dictyostelids and updates their distribution area in China. Further research will be conducted to uncover the mechanisms behind the colonization observed in this study.
Keywords: Dictyostelium, 18S rRNA, true morel, multi-gene, distribution, sorocarp
1. Introduction
True morels (Morchella spp., phylum Ascomycota), a group of the world’s most prized edible and medicinal mushrooms, are of very important economic and scientific value [1]. They are rich in protein, carbohydrate compounds, vitamins, minerals, and other nutrients [2], which have many health benefits, and abundant microorganisms are present on the fruiting bodies [3,4]. Due to the high demand for true morels and their increasing economic importance, morel cultivation has been a global research focus for more than 100 years [5,6]. In recent years, the outdoor cultivation of morels has been successful and greatly expanded in China [6,7]. However, the occurrence of fungi and bacteria colonizing the fruiting bodies of Morchella at cultivation sites has been increasingly and commonly reported [8,9,10,11,12,13,14,15] and causes the development of white plaques, dark-black lesions, wrinkled and rotten apothecia, and even perforation symptoms [16,17,18,19,20,21], resulting in decreased harvest yields, declined commodity quality, and reduced final profits [6,22,23,24,25].
Among these harmful organisms colonizing the ascomata of Morchella, Pseudodiploospora longispora (Matsush.) Jing Z. Sun, X.Z. Liu & H.W. Liu [17,18,26,27] can colonize both the caps and stipes of true morels and are recognized as serious pathogens, which produce numerous conidia spreading rapidly around the cultivation areas, resulting in up to 80% of morel yield losses every year [12]. The Fusarium incarnatum–F. equiseti species complex [16] is a group of fungal pathogens distributed worldwide that mainly colonize the stipes of Morchella importuna M. Kuo, O’Donnell & T.J. Volk and develop spindle, dark brown, sunken patches with sparse white hyphae on their surfaces. Similar symptoms have also been reported in Morchella sextelata M. Kuo due to the colonization of Clonostachys solani (Harting) Schroers & W. Gams [21]. Additionally, Alternaria alternata (Fr.) Keissl., an opportunistic pathogen noted in economically important fruit crops [9], was found to invade the hymenia of M. importuna, resulting in halted fruiting body growth and abnormal morphology [13]. Furthermore, Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson has been observed to colonize the ascocarps of Morchella rufobrunnea Guzmán & F. Tapia [10], while Trichoderma atroviride P. Karst [8]., Pseudomonas chlororaphis subsp. aureofaciens Peix and Bacillus subtilis (Ehrenberg) Cohn [15], and Penicillium raperi G. Sm. [14] have been documented as pathogens colonizing M. sextelata. Unstable environmental conditions provide opportunities for bacteria [15] and fungi [28] to proliferate and colonize morels, damaging their fruiting bodies and causing various diseases [6,21,29]. However, there have been no reports that protists can colonize Morchella.
Slime molds, characterized by features of “fungi” and “animals” during their life cycle [30], include endoparasitic slime molds (Phytomyxea), acrasid cellular slime molds (acrasids), dictyostelid cellular slime molds (dictyostelids), plasmodial slime molds (Myxogastrea), and other heterotypic slime molds [31,32,33,34,35]. Most slime molds are saprophytic without significant economic value, and only Myxogastrea can colonize crops and edible fungi in the form of plasmodia or sporangia, affecting the growth of crops and fungi and even causing severe decay and death [36,37,38,39]. For example, Polymyxa graminis Ledingham, as a lower eukaryote, obligatorily colonizes plant roots and transmits nine kinds of wheat viruses, specifically Bymovirus sp. and Furovirus sp. [40]. Plasmodiophora brassicae Woronin can colonize plants and damage the roots of most cruciferous plants [41]. In addition, Stemonitis splendens Rostaf [42]., Physarella oblonga (Berk. & M.A. Curtis) Morgan [43], and Stemonaria longa (Peck) Nann.-Bremek., Y. Yamam. & R. Sharma [44] have also been reported to colonize mushrooms. Dictyostelid cellular slime molds (dictyostelids) predominantly inhabit the soil and leaf litter layer, along with animal dung, where they feed mostly on bacteria [45,46,47,48]. Because of their crucial evolutionary status, unique life cycle, and significant interactions with the environment and human health, dictyostelids have become model organisms with significant research value in biological characters, genetics, and applications [49,50]. However, to our knowledge, there have been no reports of dictyostelids colonizing plants or fungi.
In this study, we found that dictyostelids colonized the surface of one wild Morchella ascoma in a forest in Chongqing, China, with the tissue observed to be black and rotten. The Morchella sample was identified to be Morchella sp. Mel-21, and the dictyostelids were recognized as Dictyostelium implicatum H. Hagiw. based on morphological and molecular phylogenetic studies. Our results contribute to expanding the knowledge about species colonizing Morchella, especially wild ascomata, and offer novel perspectives on the potential ecological significance of dictyostelids as well as their distribution in China.
2. Materials and Methods
2.1. Specimens
The specimens of Morchella and dictyostelids were collected from Chongqing, China, in March 2023, then dried with silica gel, and finally deposited in Chongqing Normal University, Chongqing, China. The strain numbers of Morchella and dictyostelids are FCNU1120 and H1054, respectively.
2.2. Morphological Study of Dictyostelids
2.2.1. Macroscopic Morphological Observation
The macroscopic morphological characteristics of the dictyostelids were observed under a stereo microscope (Leica S9 Series, Shanghai, China), including the size and morphology of aggregations and pseudoplasmodia, the length of sorocarps, the color and branching pattern of sorophores, and the color of sori.
2.2.2. Microscopic Morphological Observation
Several intact and complete dictyostelids were selected and placed on a microscope slide. Before the morphological observation, the specimens were stained with 1% aqueous Congo red solution. Microscopic features were observed using an Optec BK-FL light microscope (Optec, Chongqing, China), including the spore shape and size, the presence of polar granules, macrocysts and microcysts, the width of the sorophores, the number of stalk cell columns, and the characteristics of the top and base of the sorophores. Then, images were captured with an Optec CCD TP510 digital camera (Optec, Chongqing, China) and processed using Adobe Photoshop CC 2019 v.20.0.4.
2.3. DNA Extraction, Sequencing, and Phylogenetic Analyses
Under the stereo microscope, ten sorocarps of dictyostelids growing on the cap surface of a single Morchella ascoma were randomly selected and transferred to a clean centrifuge tube, and a few tissues from the stipe of Morchella where no dictyostelids were observed to colonize were placed in another centrifuge tube. Methods for genomic DNA extraction and Sanger sequencing followed Du et al. [51]. The 18S ribosomal RNA (18S rRNA) [52] gene for dictyostelids [53] and translation elongation factor 1-a (EF1-a) [51,54,55], internal transcribed spacers 1 and 2 within 5.8S rDNA (ITS) [52,56], RNA polymerase II largest subunit (RPB1) [51], and RNA polymerase II second largest subunit (RPB2) [51] genes for Morchella [57] were selected. The primers used for the PCR amplification and sequencing of the five genes are given in Table 1. Each PCR reaction contained 22 μL of T3 Super PCR Mix (Beijing Tsingke Biotech Co., Ltd., Beijing, China), 1 μL of each primer (Sangon Co., Ltd., Shanghai, China), and 1 μL of template DNA; the final volume was 25 μL. PCRs were conducted in a T1000 Thermal Cycle (Bio-Rad, Singapore) using the cycling parameters shown in Table 2. Amplicons were electrophoresed in 1.5% agarose (Sangon Co., Ltd., Shanghai, China) in 1× TAE, stained with Gold View™ (Chongqing Siding Biotech Ltd., Chongqing, China), and then photographed over an ultraviolet transilluminator (Beijing Labgic Technology Co., Ltd., Beijing, China). Then, the PCR products were sequenced with an ABI 3730 capillary sequencer (Sangon Co., Ltd., Shanghai, China). Newly generated sequences were assembled and edited using SeqMan v.7.1.0 (DNA STAR package; DNAStar Inc., Madison, WI, United States). In addition, 54 sequences of EF1-a, ITS, RPB1, and RPB2 genes from 28 species previously reported in Morchella [57] and 61 sequences of 18S rRNA from 57 species of dictyostelids [53] were retrieved from GenBank and included in the following phylogenetic analysis. Their accession numbers are, respectively, given in Table 3 and Table 4.
Table 1.
Detailed information on PCR and sequencing primers.
| Locus | Primer | Sequence (5′-3′) | Taxon | Reference |
|---|---|---|---|---|
| 18S rRNA | NS1 | GTAGTCATATGCTTGTCTC | Dictyostelium | [52] |
| NS2 | GGCTGCTGGCACCAGACTTGC | |||
| EF1-a | EF-595F | CGTGACTTCATCAAGAACATG | Morchella | [54] |
| EF-1R | GGARGGAAYCATCTTGACGA | [51] | ||
| ITS rDNA | ITS1F | CTTGGTCATTTAGAGGAAGTAA | Morchella | [56] |
| ITS4 | TCCTCCGCTTATTGATATGC | [52] | ||
| RPB1 | RPB1B-F | AACCGGTATATCACGTYGGTAT | Morchella | [51] |
| RPB1B-R | GCCTCRAATTCGTTGACRACGT | |||
| RPB2 | RPB2B-F | TAGGTAGGTCCCAAGAACACC | Morchella | [51] |
| RPB2B-R | GATACCATGGCGAACATTCTG |
Table 2.
PCR programs used for amplification of 18S rRNA, EF1-a, ITS, RPB1, and RPB2 in this study.
| Gene | PCR Program |
|---|---|
| 18S rRNA | 2′ −98 °C, 35× (10″ −98 °C, 10″ −45 °C, 20″ −72 °C), 10′ −72 °C |
| EF1-a | 2′ −98 °C, 35× (10″ −98 °C, 10″ −50 °C, 90″ −72 °C), 10′ −72 °C |
| ITS | 2′ −98 °C, 35× (10″ −98 °C, 10″ −50 °C, 20″ −72 °C), 10′ −72 °C |
| RPB1 | 2′ −98 °C, 35× (10″ −98 °C, 10″ −50 °C, 90″ −72 °C), 10′ −72 °C |
| RPB2 | 2′ −98 °C, 35× (10″ −98 °C, 10″ −50 °C, 90″ −72 °C), 10′ −72 °C |
Table 3.
Detailed information on the retrieved sequences of dictyostelids used in this study. Newly generated sequence information indicated in bold.
| Species | Voucher | Locality | GenBank Accession Number |
|---|---|---|---|
| 18S rRNA | |||
| Acytostelium anastomosans | PP1 | America | AM168115 |
| A. amazonicum | HN1B1 | Honduras | HQ141511 |
| A. digitatum | OH517 | America | AM168114 |
| A. leptosomum | 212rjb | Portugal | HQ141512 |
| A. longisorophorum | DB10A | America | AM168109 |
| A. magnisorum | 08A | America | HQ141513 |
| A. serpentarium | SAB3A | America | AM168113 |
| A. singulare | FDIB | America | HQ141514 |
| A. subglobosum | LB1 | America | AM168110 |
| Dictyostelium aureum | SL1 | America | AM168028 |
| D. australe | NZ80B | New Zealand | AM168029 |
| D. bifurcatum | UK5 | America | AM168084 |
| D. brefeldianum | TNS-C-115 | Japan | AM168030 |
| D. brunneum | WS700 | America | AM168031 |
| D. capitatum | 91HO-50 | Japan | AM168032 |
| D. caveatum | WS695 | America | AM168077 |
| D. coeruleo-stipes | CRLC53B | America | AM168036 |
| D. crassicaule | 93HO-33 | Japan | AM168037 |
| D. delicatum | TNS-C-226 | Japan | AM168093 |
| D. deminutivum | MexM19A | America | AM168092 |
| D. discoideum | V34 | America | AM168039 |
| M1A | Costa Rica | KJ394476 | |
| D. exiguum | TNS-C-199 | Japan | AM168085 |
| D. gloeosporum | TCK52 | Japan | AM168074 |
| D. gracile | TNS-C-183 | Japan | AM168078 |
| D. implicatum | 93HO-1 | Japan | AM168043 |
| H1054 | China | PP658424 | |
| D. lacteum | / 1 | France | AM168045 |
| D. laterosorum | AE4 | America | AM168046 |
| D. longosporum | TNS-C-109 | Japan | AM168048 |
| D. macrocephalum | B33 | Japan | AM168049 |
| D. medium | TNS-C-205 | Japan | AM168050 |
| D. medusoides | OH592 | America | AM168088 |
| D. microsporum | TNS-C-38 | Japan | AM168090 |
| D. minutum | Boots_07_A1 | America | JN590753 |
| Boots_07_B1 | America | JN590758 | |
| D. monochasioides | HAG653 | Japan | AM168052 |
| D. mucoroides | Ice211A1 | Sweden | KC865597 |
| D. polycarpum | OhioWILDS | America | AM168058 |
| D. polycephalum | AP | India | GU562439 |
| D. potamoides | FP1A | America | AM168069 |
| D. pseudobrefeldianum | 91HO-8 | Japan | AM168059 |
| D. purpureum | cavender | America | HQ141481 |
| D. rhizopodium | AusKY-4 | Japan | AM168063 |
| D. rosarium | M45 | America | AM168065 |
| D. septentrionalis | AK2 | America | AM168067 |
| D. sphaerocephalum | Ice241A1 | America | KC865595 |
| Boots_14_A2 | America | JN590756 | |
| Boots_07_A2 | America | JN590754 | |
| Lamproderma puncticulatum | 162 | Switzerland | HQ687202 |
| Polysphondylium anisocaule | NZ47B | New Zealand | AM168096 |
| P. asymetricum | HN20C | Honduras | HQ141503 |
| P. australicum | NB1AP | Australia | HQ141508 |
| P. colligatum | HN13C1 | Honduras | HQ141505 |
| P. equisetoides | B7JB | America | AM168099 |
| P. filamentosum | SU-1 | America | AM168100 |
| P. luridum | LR-2 | America | AM168101 |
| P. multicystogenum | AS2 | Africa | HQ141506 |
| P. patagonicum | /1 | Argentina | GQ496156 |
| P. pseudocandidum | TNS-C-91 | America | AM168107 |
| P. stolonicoideum | K12A | Australia | HQ141507 |
| P. tikalense | HN1C1 | Honduras | HQ141509 |
| P. tikaliensis | OH595 | America | AM168106 |
1 The voucher information of this sample unavailable.
Table 4.
Detailed information on the retrieved sequences of Morchella used in this study. Newly generated sequences information indicated in bold.
Newly generated sequences of dictyostelids and Morchella were separately combined in an alignment with downloaded sequences from each genus. In addition, Lamproderma puncticulatum and M. importuna were chosen, respectively, as the outgroups of dictyostelids and Morchella. Sequence alignments were performed separately for each gene dataset with MAFFT v.7.475 using the E-INS-i strategy [58] and then manually checked with BioEdit v.7.0.9 [59]. Maximum likelihood (ML) and Bayesian inference (BI) phylogenetic analyses were conducted for the combined four-gene dataset (ITS-EF1a-RPB1-RPB2) and the 18S rRNA dataset using RAxML v.8.2.12 [60] and MrBayes v.3.2.7a [61], respectively. Rapid bootstrapping with 1000 replicates was executed for ML analysis with the GTR + GAMMA + I model chosen by ModelTest v.3.8 [62]. The BI analysis was run for one million generations, sampling the trees every 100 generations, and used four Markov Chain Monte Carlo (MCMC) chains. When the mean standard deviation of split frequencies was below 0.01, the runs were terminated. The burn-in summary of the top 25% of samples was performed using the “sumt” and “sump” commands to obtain posterior possibilities.
3. Results
The substantial proliferation of white and transparent dictyostelids colonizing the cap surface of one Morchella ascoma from a forest habitat was found in Chongqing, with the colonized area observed to be blackened and decayed (Figure 1). After a thorough inspection of the surrounding area, only one ascoma with dictyostelids growing on the surface was identified. The specimen of Morchella was identified to be Morchella sp. Mel-21 based on multi-gene phylogenetic analyses. Based on morphological observations, the slime molds were first considered to belong to the genus Dictyostelium and were further inferred to be D. implicatum by molecular phylogenetic analysis.
Figure 1.
Slime molds colonizing the ascoma of Morchella in the field. (A) Distant view; (B,C) close-up view. Slime molds indicated by white arrows.
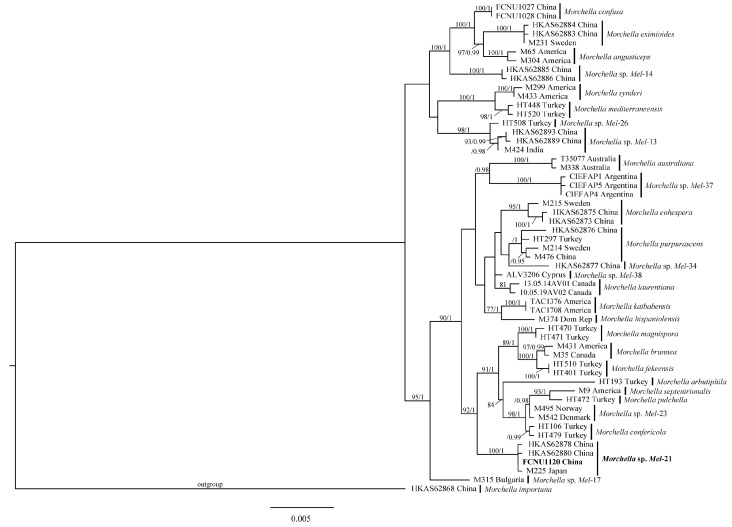
3.1. Molecular Phylogenetic Analysis of the Morchella Specimen
In this study, four sequences of the Morchella specimen were obtained through PCR amplification targeting the ITS, EF1-a, RPB1, and RPB2 genes with accession numbers PP658423, PP695543, PP693901, and PP693900. The alignments of sequences, which included those newly generated in this study and the 54 retrieved sequences from GenBank (Table 3) for ITS, EF1-a, RPB1, and RPB2 datasets, respectively, were 646, 777, 692, and 680 bp. The final aligned multi-gene sequence matrix contained 28 species and a total of 55 sequences with 3287 bp. The phylogenetic trees were inferred from the combined four-gene dataset based on ML and BI analyses. No significant topological differences were detected between the two analyses, and the ML phylogenetic tree is shown in Figure 2. The phylogenetic analyses strongly supported the studied specimen being Morchella sp. Mel-21 (Figure 2) since it clustered together with HKAS62878 and HKAS62880 from China and M225 from Japan with high support (100%/1); these were previously identified as Morchella sp. Mel-21 [51,63]. Therefore, based on molecular phylogenetic analyses, the species identity of the Morchella specimen used in this study was recognized as Morchella sp. Mel-21.
Figure 2.
The phylogenetic tree of 28 Morchella species inferred from ML analyses based on the concatenated dataset (ITS, EF1-a, RPB1, and RPB2). Bootstrap values over 75% and Bayesian posterior probabilities over 0.95 shown on the branches. The new specimen of Morchella used in this study indicated in bold.
3.2. Morphological Observation of Dictyostelids
Dictyostelium sp.
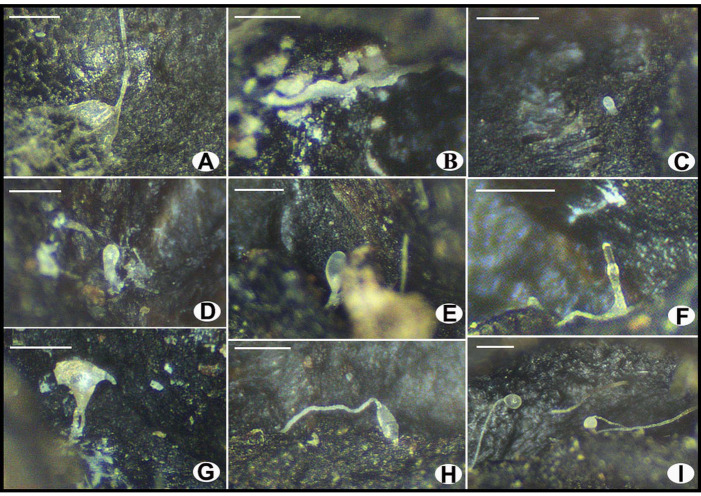
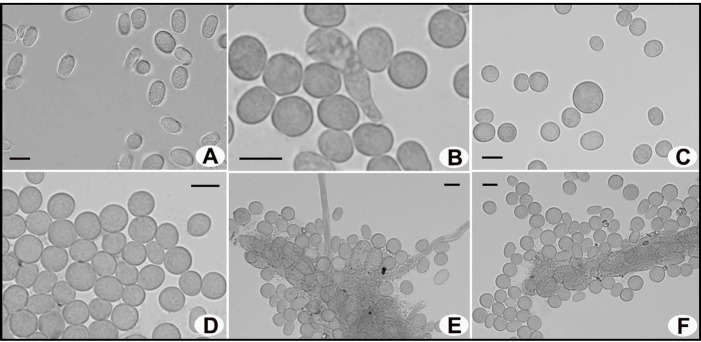
Cell aggregations (Figure 3A) with ample radiate streams. Pseudoplasmodia (Figure 3B) often migrating without sorophore formation. Mexican-hat-like protrusion (Figure 3C–E) and the sorocarp formation period with mastoid structure (Figure 3F–H) observed. Sorocarps (Figure 3I) solitary and unbranched, some erect while others prostrate. Sori white or milk-white, globose. Spores (Figure 4A) hyaline, elliptical, mostly 5.77–7.97 × 3.63–4.85 μm, without polar granules. Spore germination observed (Figure 4B). Microcyst (Figure 4C,D) globose. Sorophores generally stout, tapering from bases to tips, consisting of several tiers of cells, bases (Figure 4E) clavate, tips (Figure 4F) acuminate.
Figure 3.
Morphological characteristics of Dictyostelium sp. investigated in this study under a stereo microscope. (A) Cell aggregation; (B) pseudoplasmodium; (C–E) mexican-hat-like protrusion; (F–H) the sorocarp formation period with mastoid structure; (I) sorocarps. Scale bars = 200 μm.
Figure 4.
Microscopic morphological characteristics of Dictyostelium sp. observed under a light microscope. (A) Spores; (B) spore germination; (C,D) microcysts; (E) base of sorophores; (F) tip of sorophores. Scale bars = 5 μm.
Specimens examined. H1054. Isolated from the surface of one wild ascoma of Morchella in 2023 from Chongqing, China.
Known distribution. China, America, Germany, Korea, Japan, India, Pakistan, Ukraine, Thailand.
Commentary. The morphological observation was performed after the samples were dried, and the length and diameter of the fresh dictyostelids’ sorocarps and sori could not be measured. Consequently, the dictyostelids were initially identified as belonging to Dictyostelium sp. based solely on morphological features.
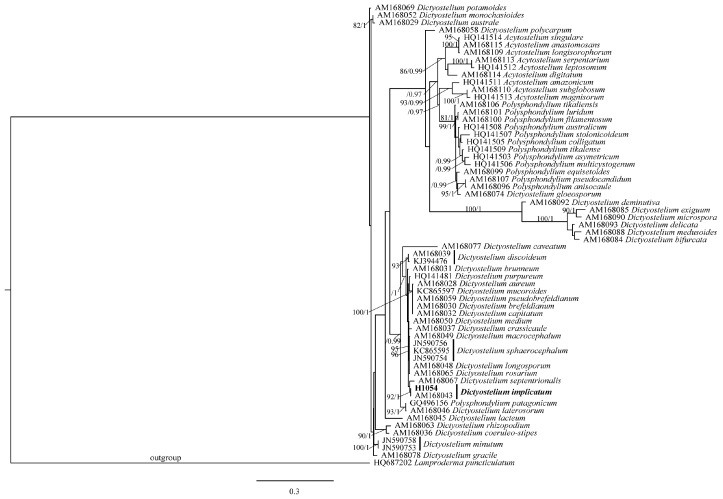
3.3. Molecular Phylogenetic Analysis of Dictyostelium Implicatum Specimens
The newly generated 18S rRNA sequence in this study was 531 bp with accession number PP658424 and aligned with the 61 sequences retrieved from GenBank (Table 4). The final alignment matrix contained 1259 bp with 62 sequences and a total of 57 species. The phylogenetic trees were obtained based on the ML and BI analyses, and the ML tree was presented in Figure 5. The dictyostelids in this study clustered together with AM168043 from Japan with high support (92%/1), which was previously identified as Dictyostelium implicatum [64]. Therefore, based on molecular phylogenetic analysis, dictyostelids that colonized the cap surface of Morchella ascoma found in this study were identified as D. implicatum.
Figure 5.
The phylogenetic tree of 57 species of dictyostelids inferred from ML analyses based on 18S rRNA. Bootstrap values over 75% and Bayesian posterior probabilities over 0.95 reported on the branches. The new collection of Dictyostelium used in this study indicated in bold.
4. Discussion
The large-scale commercial cultivation of morels has become a part of an emerging industry for edible fungi in China and globally, showcasing their significant economic and scientific value [6,7]. The colonization of bacteria and fungi is one of the key factors affecting the artificial cultivation of morels and causing serious economic losses [6,21,22,23,24,25]. Increasing numbers of bacteria and fungi, such as Ps. longispora [17,18,26,27], F. incarnatum-equiseti [16], C. solani [21], Pu. lilacinum [10], T. atroviride [8], Ps. chlororaphis subsp. aureofaciens and B. subtilis [15], A. alternata [13], Pe. raperi [14], and so on, have been discovered to colonize Morchella species and harm their fruiting bodies. Investigating the species diversity of these colonizing organisms is the premise for further revealing their colonization mechanisms and is also crucial for drawing the attention of planters and researchers to them during morel cultivation and in the field.
Dictyostelids, well known as dictyostelid cellular slime molds that feed on bacteria and other microbes [50], have never been reported to act as pathogens of any organisms before [36,37,38,39]. Though tiny and difficult to find in nature with the naked eye [65], dictyostelids have been documented worldwide [46], such as in China, America, Germany, Korea, Japan, India, Pakistan, Ukraine, Thailand, etc. [45,46,47,66,67,68,69,70,71,72,73,74,75,76,77]. In China, they have been previously reported in Beijing, Jilin, Shanxi, Heilongjiang, Liaoning, Hunan, Henan, Xizang, Yunnan, Sichuan, Guizhou, Hainan, Guangxi, Guangdong, Taiwan, and so on [46,47,76,77,78,79,80,81,82,83]. Based on the phylogenetic analysis of ITS, 18S rRNA, 5.8S rRNA, α-tubulin, and β-tubulin genes [64,84,85,86,87], dictyostelids have been reported to include 11 genera [88], of which Dictyostelium, Polysphondylium, and Acytostelium are the most common [89].
In this study, we found that dictyostelids had colonized the surface of one wild ascoma of Morchella, with the tissue being black and rotten. The wild ascoma was identified as Morchella sp. Mel-21 through molecular phylogenetic studies. Interestingly, this species was previously reported to be successfully cultivated in China, albeit with low and unstable yields [90,91]. Though the length and size of fresh sorocarps and sori are crucial for dictyostelids species identification [78], due to the availability of only dried specimens for the microscopic morphological observation of dictyostelids in this study, we were unable to obtain data on their length and size. Consequently, these dictyostelids were initially identified as belonging to the genus Dictyostelium based on morphology. Further molecular phylogenetic analysis was conducted to uncover the species identity of the dictyostelids using the 18S rRNA and ITS genes, which are widely accepted for species identification in the genus Dictyostelium [89,92,93,94]. However, only a clear 18S rRNA sequence was obtained with clean peaks, while the ITS sequences were always messy after multiple attempts and were then discarded. Based on the phylogenetic tree of the 18S rRNA dataset, the dictyostelids in this study were identified to be D. implicatum, with support values being 92%/1, slightly lower than 97%/1, probably due to the newly generated sequence (531 bp) being much shorter than the referenced ones (mainly around 670 bp), chosen according to An and Li [89], but no better sequences of 18S rRNA could be obtained from the dictyostelids after multiple attempts. Notably, to the best of our knowledge, the dictyostelids found in this study are reported for the first time from Chongqing, located in southwestern China, further broadening their distribution record in China.
Considering the lack of previous reports of dictyostelids acting as pathogens of any organisms [36,37,38,39], with the aim of conducting inoculation experiments to determine whether dictyostelids could be pathogens of Morchella based on our discovery, we tried to isolate the dictyostelids and hoped to reveal their colonization mechanisms. However, despite multiple attempts across different laboratories, we were unable to successfully isolate them. Given that abundant microbial communities have been reported on the ascomata of Morchella [3,4], based on the available literature and our data, we currently hypothesize that the colonization mechanism of dictyostelids discovered in this study is likely consuming the microorganisms present on the surface of the ascoma, and the activity of these microorganisms may, in turn, contribute indirectly to the blackening and decay of the Morchella ascoma. Subsequent studies will be conducted to continue trying to resolve this problem and provide a more comprehensive understanding.
This study represents the first report of dictyostelids (D. implicatum) colonizing the fruiting body of Morchella and introduces novel research advancements for exploring the life history and potential ecological significance of dictyostelids. While organism colonization has previously only been documented in cultivated morels, this study observes it for the first time on wild ascomata. The new finding of dictyostelids colonizing Morchella sp. Mel-21 could serve as a valuable reference and attract attention for artificial cultivation in the future.
Acknowledgments
The authors would like to thank Xue-Jiao Chen (Chongqing Normal University) for assisting with sample collection in the field, as well as Jia Ling, Qin Qin, and Si-Yue Wang (Chongqing Normal University) for their help during molecular and morphological experiments. Zhao-Juan Zhang (Jilin Agricultural University) is also appreciated for her experimental support. We also thank the reviewers for their constructive comments and suggestions.
Author Contributions
Conceptualization, X.-H.D.; methodology, W.-S.H., P.L. and X.-H.D.; validation, W.-S.H. and X.-H.D.; formal analysis, W.-S.H.; investigation, W.-S.H., L.-L.J., X.-Y.Z. and W.W.; resources, L.-L.J.; data curation, W.-S.H.; writing—original draft, W.-S.H.; writing—review and editing, W.-S.H., P.L. and X.-H.D.; visualization, W.-S.H. and X.-H.D.; supervision, X.-H.D.; project administration, X.-H.D.; funding acquisition, X.-H.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Newly generated sequences used in the study were uploaded to GenBank with accession numbers PP658423, PP695543, PP693901, PP693900, and PP658424.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 32270023), the Natural Science Foundation of Chongqing (grant numbers CSTB2022NSCQ-LZX0035, cstc2021jcyj-msxmX0425), the Scientific and Technological Research Program of Chongqing Municipal Education Commission (grant numbers KJQN202200562, KJQN202300503), and the Chongqing Germplasm Bank of Edible Fungi Program (grant number WSWZZ2020001).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Du X.H., Zhao Q., Yang Z.L. A review on research advances, issues, and perspectives of morels. Mycology. 2015;6:78–85. doi: 10.1080/21501203.2015.1016561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian J.F., Shang Y., Xiao Z.N. Research progress on nutrient composition, function and processing of morels. Sci. Technol. Food Ind. 2023;45:419–428. [Google Scholar]
- 3.Li Y.T., Chen H.Y., Zhang X. Cultivation, nutritional value, bioactive compounds of morels, and their health benefits: A systematic review. Front. Nutr. 2023;10:1159029. doi: 10.3389/fnut.2023.1159029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang K., Li L., Yang Z., Chen H., Qin Y., Brennan C. Variable characteristics of microbial communities and volatile organic compounds during post-harvest storage of wild morel mushrooms. Postharvest Biol. Technol. 2023;203:112401. doi: 10.1016/j.postharvbio.2023.112401. [DOI] [Google Scholar]
- 5.Liu W., Chen L.F., Cai Y.L., Zhang Q.Q., Bian Y.B. Opposite polarity monospore genome de novo sequencing and comparative analysis reveal the possible heterothallic life cycle of Morchella importuna. Int. J. Mol. Sci. 2018;19:2525. doi: 10.3390/ijms19092525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y.Y., Tang J., Wang Y., He X.L., Tan H., Yu Y., Chen Y., Peng W.H. Large scale commercial cultivation of morels: Current state and perspectives. Appl. Microbiol. Biot. 2022;106:4401–4412. doi: 10.1007/s00253-022-12012-y. [DOI] [PubMed] [Google Scholar]
- 7.Du X.H., Yang Z.L. Mating systems in true morels (Morchella) Microbiol. Mol. Biol. Rev. 2021;85:e0022020. doi: 10.1128/MMBR.00220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y.W., Wu H.H., Wang S., Yu Q., Tian D.S., Xu X. First report of Trichoderma atroviride causing rot of Morchella sextelata in Anhui Province, China. Crop Prot. 2023;168:106206. doi: 10.1016/j.cropro.2023.106206. [DOI] [Google Scholar]
- 9.Gupta S., Saxena S. Endophytes: Saviour of apples from post-harvest fungal pathogens. Biol. Control. 2023;182:105234. doi: 10.1016/j.biocontrol.2023.105234. [DOI] [Google Scholar]
- 10.Masaphy S. First report on Purpureocillium lilacinum infection of indoor-cultivated morel primordia. Agriculture. 2022;12:695. doi: 10.3390/agriculture12050695. [DOI] [Google Scholar]
- 11.Shi X.F., Liu D., He X.H., Liu W., Yu F.Q. Epidemic identification of fungal diseases in Morchella cultivation across China. J. Fungi. 2022;8:1107. doi: 10.3390/jof8101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X.X., Peng J.Y., Sun L., Bonito G., Guo Y.X., Li Y., Fu Y.P. Genome sequencing of Paecilomyces Penicillatus provides insights into its phylogenetic placement and mycoparasitism mechanisms on morel mushrooms. Pathogens. 2020;9:834. doi: 10.3390/pathogens9100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y.F., Fu M.H., Li W.L., Luo Y., Zhang Y.T., Sun W.L., Zou J. First report on apothecium deformity of Morchella importuna caused by Alternaria alternata in China. Plant Dis. 2024;108:1398. doi: 10.1094/PDIS-10-23-2122-PDN. [DOI] [PubMed] [Google Scholar]
- 14.Xu X., Yang C.B., Wang S., Jiao C.Y., Sun J.Z., Fan X.Y., Wang X.J., Xiang W.S., Zhao J.W. Penicillium raperi causes rot disease on Morchella sextelata in Heilongjiang Province, China. Crop Prot. 2024;175:106479. doi: 10.1016/j.cropro.2023.106479. [DOI] [Google Scholar]
- 15.Zhu X.T., Ma K.L., Sun M.Y., Zhang J.M., Liu L.J., Niu S.Q. Isolation and identification of pathogens of Morchella sextelata bacterial disease. Front. Microbiol. 2023;14:1231353. doi: 10.3389/fmicb.2023.1231353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M.P., Chen K., Wang G.Z., Bian Y.B. First report of stipe rot disease on Morchella importuna caused by Fusarium incarnatum–F. equiseti species complex in China. Plant Dis. 2016;100:2530. doi: 10.1094/PDIS-05-16-0633-PDN. [DOI] [Google Scholar]
- 17.He P.X., Li C.C., Cai Y.L., Ya Z., Bian Y.B., Liu W. First report of pileus rot disease on cultivated Morchella importuna caused by Diploöspora longispora in China. J. Gen. Plant Pathol. 2018;84:65–69. doi: 10.1007/s10327-017-0754-3. [DOI] [Google Scholar]
- 18.Hyde K.D., Maharachchikumbura SS N., Hongsanan S., Samarakoon M.C., Lücking R., Pem D., Harishchandra D., Jeewon R., Zhao R.L., Xu J.C., et al. The ranking of fungi: A tribute to David L. Hawksworth on his 70th birthday. Fungal Divers. 2017;84:1–23. doi: 10.1007/s13225-017-0383-3. [DOI] [Google Scholar]
- 19.Liu J.M., Feng J.C., Wu Z.T., Yang X.Z. Study on pathogen identification and biological morel of Morchella white mold disease in Hexi area. North. Hortic. 2023:116–123. [Google Scholar]
- 20.Tu S., Zhang Y., Chen X., Song L., Lv B., Chen Y. First report of Aspergillus niger causing rot of Morchella sextelata in China. Plant Dis. 2024;108:804. doi: 10.1094/PDIS-09-23-1889-PDN. [DOI] [PubMed] [Google Scholar]
- 21.Yu F.M., Jayawardena R.S., Luangharn T., Zeng X.Y., Li C.J.Y., Bao S.X., Ba H., Zhou D.Q., Tang S.M., Hyde K.D., et al. Species diversity of fungal pathogens on cultivated mushrooms: A case study on morels (Morchella, Pezizales) Fungal Divers. 2024;125:157–220. doi: 10.1007/s13225-023-00531-6. [DOI] [Google Scholar]
- 22.Liu W., Cai Y.L., He P.X., Ma X.L., Bian Y.B. Occurrence and control of pests and diseases in field cultivation of Morchella mushrooms. Acta Edulis Fungi. 2019;26:128–134. [Google Scholar]
- 23.Lv B., Yu S., Chen Y., Yu H., Mo Q. First report of Lecanicillium aphanocladii causing rot of Morchella sextelata in China. Plant Dis. 2022;106:3202. doi: 10.1094/PDIS-12-21-2656-PDN. [DOI] [PubMed] [Google Scholar]
- 24.Peng B., Bian Y.B., Gong Y.H., Xiao Y. Diseases of Morchella and their comprehensive prevention and control techniques. Edible Med. Mushrooms. 2024;32:129–135. [Google Scholar]
- 25.Zhao Q., Lü M.L., Li L., Huang W., Zhang Y.F., Hao Z. Temptation and trap of morel industry in China. J. Fungal Res. 2021;19:1232–1237. [Google Scholar]
- 26.Sun J.Z., Yu S., Lu Y.Z., Liu H.W., Liu X.Z. Proposal of a new family Pseudodiploösporeaceae fam. nov. (Hypocreales) based on phylogeny of Diploöspora longispora and Paecilomyces penicillatus. Mycology. 2022;14:60–73. doi: 10.1080/21501203.2022.2143919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q.Z., Ma H.S., Zhang Y., Dong C.H. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018;38:259–271. doi: 10.1080/07388551.2017.1333082. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z.H., Cong Y.L., Sossah F.L., Lu Y.Z., Kang J.C., Li Y. Characterization and genome analysis of Cladobotryum mycophilum, the causal agent of cobweb disease of Morchella sextelata in China. J. Fungi. 2023;9:411. doi: 10.3390/jof9040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X.S., Wang J., Wang Y.P., Li X.Y., Wang Y.Z., Zhu X.Q., Zhou L., Zhao M., Zhang N., Xie J.Y. The high-yield operating procedures of morels cultivation in southwest China. Edible Med. Mushrooms. 2020;28:454–460. [Google Scholar]
- 30.Li S., Dou W.J., Peng X.Y., Wang Q., Li Y. Research progress of Myxomycetes resources in China. J. Fungal Res. 2023;21:93–102. [Google Scholar]
- 31.Adl S.M., Leander B.S., Simpson AG B., Archibald J.M., Anderson O.R., Bass D., Bowser S.S., Brugerolle G., Farmer M.A., Karpov S., et al. Diversity, nomenclature, and taxonomy of protists. Syst. Biol. 2007;56:684–689. doi: 10.1080/10635150701494127. [DOI] [PubMed] [Google Scholar]
- 32.Cavalier-Smith T. Protist phylogeny and the high-level classification of Protozoa. Eur. J. Protistol. 2003;39:338–348. doi: 10.1078/0932-4739-00002. [DOI] [Google Scholar]
- 33.Cavalier-Smith T., Chao E.E.Y. Phylogeny and megasystematics of phagotrophic heterokonts (kingdom Chromista) J. Mol. Evol. 2006;62:388–420. doi: 10.1007/s00239-004-0353-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen S.L. The taxonomic history of Myxomycetes (Myxogastrea) Mycosystema. 2023;42:38–49. [Google Scholar]
- 35.Ruggiero M.A., Gordon D.P., Orrell T.M., Bailly N., Bourgoin T., Brusca R.C., Cavalier-Smith T., Guiry M.D., Kirk P.M. A higher level classification of all living organisms. PLoS ONE. 2015;10:e0119248. doi: 10.1371/journal.pone.0119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard F.L., Currie M.E. Parasitism of myxomycete plasmodia on fungous mycelia. J. Arnold Arbor. 1932;13:438–447. doi: 10.5962/p.185269. [DOI] [Google Scholar]
- 37.Michalczyk-Wetula D., Jakubowska M., Felska M., Skarżyński D., Mąkol J., Płonka P.M. Tyrophagus putrescentiae (Sarcoptiformes: Acaridae) in the in vitro cultures of slime molds (Mycetozoa): Accident, contamination, or interaction? Exp. Appl. Acarol. 2021;84:445–458. doi: 10.1007/s10493-021-00608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephenson S.L., Feest A. Ecology of soil eumycetozoans. Acta Protozool. 2012;51:201–208. [Google Scholar]
- 39.Zhang Z.J., Zhai C., Li Y., Stephenson S.L., Liu P. Slime molds (Myxomycetes) causing a “disease” in crop plants and cultivated mushrooms. Front. Plant Sci. 2024;15:1411231. doi: 10.3389/fpls.2024.1411231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cubeta M.A., Cody B., Williams P.H. First report of Plasmodiophora brassicae on cabbage in eastern North Carolina. Plant Dis. 1998;82:129. doi: 10.1094/PDIS.1998.82.1.129D. [DOI] [PubMed] [Google Scholar]
- 41.Ledingham G.A. Studies on Polymyxa graminis, n. gen.; n. sp.; a plasmodiophoraceous root parasite of wheat. Can. J. Res. 1939;17:38–51. doi: 10.1139/cjr39c-005. [DOI] [Google Scholar]
- 42.Lee J.H., Kim D.R., Kwak Y.S. First report of Stemonitis splendens Rostaf causing bark decay of oak logs used for shiitake cultivation in Korea. Mycobiology. 2014;42:279–281. doi: 10.5941/MYCO.2014.42.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y.S., Zhang B., Jiang S.C., Hsiang T., Li Y., Wang X.L. First report of Physarella oblonga on Lentinula edodes in China. Plant Dis. 2017;101:2146. doi: 10.1094/PDIS-02-17-0214-PDN. [DOI] [Google Scholar]
- 44.Zhang B., Li S., Li H.M., Li Y. First report of a new myxogastria (Stemonaria longa) causing rot disease on shiitake logs (Lentinula edodes) in China. Plant Dis. 2017;102:1032. doi: 10.1094/PDIS-05-17-0719-PDN. [DOI] [Google Scholar]
- 45.Liu P., Zou Y., Hou J., Stephenson S.L., Li Y. Dictyostelium purpureum var. pseudosessile, a new variant of dictyostelid from tropical China. BMC Evol. Biol. 2019;19:78. doi: 10.1186/s12862-019-1407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu P., Zhang S., Zou Y., Li Z., Stephenson S.L., Li Y. Distribution and ecology of dictyostelids in China. Fungal Biol. Rev. 2020;34:170–177. doi: 10.1016/j.fbr.2020.07.003. [DOI] [Google Scholar]
- 47.Zhang Z., Yang Y., Zhao J., Li Y., Stephenson S.L., Qiu J., Liu P. Environmental factors influencing the diversity and distribution of dictyostelid cellular slime molds in forest and farmland soils of western China. Microbio. Spectr. 2023;11:e0173223. doi: 10.1128/spectrum.01732-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou Y., Liu P. Research progress on ecology of dictyostelid cellular slime molds. Mycosystema. 2023;42:160–169. [Google Scholar]
- 49.Li Y., Wang Q., Li S. Development of myxomycetology. Mycosystema. 2021;40:261–269. [Google Scholar]
- 50.Zhou Y. Master’s Dissertation. Jilin Agricultural University; Jilin, China: May 1, 2019. Biodiversity of Dictyostelid Cellular Slime Molds in Different Habitat Gradients of Changbai Mountain. [Google Scholar]
- 51.Du X.H., Zhao Q., O’Donnell K., Rooney A.P., Yang Z.L. Multigene molecular phylogenetics reveals true morels (Morchella) are especially species-rich in China. Fungal Genet. Biol. 2012;49:455–469. doi: 10.1016/j.fgb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 52.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand H.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Volume 8. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 53.An Y., Li Y. Molecular phylogeny of dictyostelid cellular slime molds from China. J. Jilin Agric. Univ. 2019;41:294–302. [Google Scholar]
- 54.Kauserud H., Schumacher T. Outcrossing or inbreeding: DNA markers provide evidence for type of reproductive mode in Phellinus nigrolimitatus (Basidiomycota) Mycol. Res. 2001;105:676–683. doi: 10.1017/S0953756201004191. [DOI] [Google Scholar]
- 55.Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- 56.Gardes M., Bruns T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 57.Du X.H., Wu D.M., He G.Q., Wei W., Xu N., Li T.L. Six new species and two new records of Morchella in China using phylogenetic and morphological analyses. Mycologia. 2019;111:857–870. doi: 10.1080/00275514.2019.1640012. [DOI] [PubMed] [Google Scholar]
- 58.Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 60.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Posada D. ModelTest server: A web-based tool for the statistical selection of models of nucleotide substitution online. Nucleic Acids Res. 2006;34:W700–W703. doi: 10.1093/nar/gkl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Donnell K., Rooney A.P., Mills G.L., Kuo M., Weber N.S., Rehner S.A. Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic. Fungal Genet. Biol. 2011;48:252–265. doi: 10.1016/j.fgb.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Schaap P., Winckler T., Nelson M., Alvarez-Curto E., Elgie B., Hagiwara H., Cavender J., Milano-Curto A., Rozen D.E., Dingermann T., et al. Molecular phylogeny and evolution of morphology in the social amoebas. Science. 2006;314:661–663. doi: 10.1126/science.1130670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun J.Y. Master’s Dissertation. Jilin Agricultural University; Jilin, China: Jun 1, 2011. Studies on the Life Cycle of Important Taxa in Dictyosteliaceae. [Google Scholar]
- 66.Cavender J.C. Dictyostelium dimigraformum, Dictyostelium laterosorum and Acytostelium ellipticum: New Acrasieae from the American tropics. J. Gen. Microbiol. 1970;62:113–123. doi: 10.1099/00221287-62-1-113. [DOI] [Google Scholar]
- 67.Cavender J.C., Lakhanpal T.N. Distribution of dictyostelid cellular slime molds in forest soils of India. Mycologia. 1986;78:56–65. doi: 10.1080/00275514.1986.12025204. [DOI] [Google Scholar]
- 68.Cavender J.C., Cavender-Bares J., Hohl H.R. Ecological distribution of cellular slime molds in forest soils of Germany. Bot. Helv. 1995;105:199–219. [Google Scholar]
- 69.Cavender J.C., Vadell E.M., Perrigo A.L., Landolt J.C., Stephenson S.L., Liu P. Four new species of dictyostelids from soil systems in northern Thailand. J. Fungi. 2022;8:593. doi: 10.3390/jof8060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hagiwara H. The Taxonomic Study of Japanese Dictyostelid Cellular Slime Molds. National Science Museum Monographs; Tokyo, Japan: 1989. pp. 1–128. [Google Scholar]
- 71.Hagiwara H. Dictyostelid cellular some molds of Pakistan. I. Distribution and occurrence in soils of forests, cultivated fields and alpine pastures. In: Nakaike T., Malik S., editors. Cryptogamic Flora of Pakistan. Volume 1. Bulletin of the National Science Museum; Tokyo, Japan: 1992. pp. 87–98. [Google Scholar]
- 72.Liu P., Li Y. New species and new records of dictyostelids from Ukraine. Mycologia. 2011;103:641–645. doi: 10.3852/10-253. [DOI] [PubMed] [Google Scholar]
- 73.Liu P., Zou Y., Li S., Stephenson S.L., Wang Q., Li Y. Two new species of dictyostelid cellular slime molds in high-elevation habitats on the Qinghai-Tibet Plateau, China. Sci. Rep. 2019;9:5. doi: 10.1038/s41598-018-37896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu P., Zou Y., Li W., Li Y., Li X., Che S., Stephenson S.L. Dictyostelid cellular slime molds from Christmas Island, Indian Ocean. Msphere. 2019;4:10–1128. doi: 10.1128/mSphere.00133-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong J.S., Chang N.K. A new species of cellular slime molds from Korea, Dictyostelium flavidum sp. nov. J. Plant Biol. 1992;35:393–401. [Google Scholar]
- 76.Zhao M.J. Master’s Dissertation. Jilin Agricultural University; Jilin, China: May 1, 2014. Studies on Taxonomy of Dictyostelid Cellular Slime Molds of Eastern China. [Google Scholar]
- 77.Zou Y., Hou J., Guo S., Li C., Li Z., Stephenson S.L., Pavlov I.N., Liu P., Li Y. Diversity of dictyostelid cellular slime molds, including two species new to science, in forest soils of Changbai Mountain, China. Microbiol. Spectr. 2022;10:e0240222. doi: 10.1128/spectrum.02402-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He X.L. Master’s Dissertation. Jilin Agricultural University; Jilin, China: Jun 1, 2008. Preliminary Taxonomy Studies on Dictyosteliaceae in China. [Google Scholar]
- 79.Bai R.L. Preliminary study of Acrasiomycetes. Mycosystema. 1983;2:173–178. [Google Scholar]
- 80.Hagiwara H., Yeh Z.Y., Chien C.Y. Dictyostelium macrocephalum, a new dictyostelid cellular slime mold from Taiwan. Bull. Natl. Sci. Mus. Ser. B. 1985;11:103–108. [Google Scholar]
- 81.Li C. Master’s Dissertation. Jilin Agricultural University; Jilin, China: May 29, 2013. Studies on Taxonomy of Dictyostelid Cellular Slime Molds in Henan and Hunan Provinces. [Google Scholar]
- 82.Ren Y.Z. Master’s Dissertation. Jilin Agricultural University; Jilin, China: May 31, 2013. Studies on Distribution of Dictyostelid Cellular Slime Molds in the Tropic and Frigid Zone of China. [Google Scholar]
- 83.Yuan H.Y. Master’s Dissertation. Jilin Agricultural University; Jilin, China: May 28, 2013. Studies on Taxonomy of Dictyostelid Cellular Slime Molds in the Southwest of China. [Google Scholar]
- 84.McCarroll R., Olsen G.J., Stahl Y.D., Woese C.R., Sogin M.L. Nucleotide sequence of the Dictyostelium discoideum small-subunit ribosomal ribonucleic acid inferred from the gene sequence: Evolutionary implications. Biochemistry. 1983;22:5858–5868. doi: 10.1021/bi00294a027. [DOI] [Google Scholar]
- 85.Triviños-Lagos L., Ohmachi T., Albrightson C., Burns R.G., Ennis H.L., Chisholm R.L. The highly divergent α- and β-tubulins from Dictyostelium discoideum are encoded by single genes. J. Cell Sci. 1993;105:903–912. doi: 10.1242/jcs.105.4.903. [DOI] [PubMed] [Google Scholar]
- 86.Romeralo M., Spiegel F.W., Baldauf S.L. A fully resolved phylogeny of the social amoebas (Dictyostelia) based on combined SSU and ITS rDNA sequences. Protist. 2010;161:539–548. doi: 10.1016/j.protis.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Romeralo M., Cavender J.C., Landolt J.C., Stephenson S.L., Baldauf S.L. An expanded phylogeny of social amoebas (Dictyostelia) shows increasing diversity and new morphological patterns. BMC Evol. Biol. 2011;11:1–10. doi: 10.1186/1471-2148-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database. 2020;2020:baaa062. doi: 10.1093/database/baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.An Y. Ph.D. Dissertation. Jilin Agricultural University; Jilin, China: Dec 2, 2015. Studies on Multigene and Fatty-Acids Biomarker in Representative Genera Taxonomy of Dictyostelid Cellular Slime Molds. [Google Scholar]
- 90.Du X.H. Review on species resources, reproductive modes and genetic diversity of black morels. J. Fungal Res. 2019;17:240–251. [Google Scholar]
- 91.Cai Y.L., Ma X.L., Lu D.X., Zhang Y., Liu W. Phylogenetic analysis and domestication of wild morel Mel-21. Acta Edulis Fungi. 2020;27:23–29. [Google Scholar]
- 92.Romeralo M., Fiz-Palacios O., Lado C., Cavender J.C. A new concept for Dictyostelium sphaerocephalum based on morphology and phylogenetic analysis of nuclear ribosomal internal transcribed spacer region sequences. J. Can. Bot. 2007;85:104–110. doi: 10.1139/b06-147. [DOI] [Google Scholar]
- 93.Romeralo M.A., Escalante R., Sastre L., Lado C. Molecular systematics of dictyostelids: 5.8S ribosomal DNA and internal transcribed spacer region analyses. Eukaryot. Cell. 2007;6:110–116. doi: 10.1128/EC.00233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheikh S., Thulin M., Cavender J.C., Escalante R., Kawakami S.I., Lado C., Landolt J.C., Nanjundiah V., Queller D.C., Strassmann J.E., et al. A new classification of the dictyostelids. Protist. 2018;169:1–28. doi: 10.1016/j.protis.2017.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Newly generated sequences used in the study were uploaded to GenBank with accession numbers PP658423, PP695543, PP693901, PP693900, and PP658424.