Abstract
The survival of CD8+/CD44hi memory phenotype T cells depends on an IL-15 activity on nonlymphoid cells. Here, we report that IL-15 and its receptor were induced on dendritic cells (DCs) by a combination of IFN-γ and NF-κB relA inducers. IL-15 conferred in an autocrine loop resistance to apoptosis that accompanied the maturation process in DCs in vitro. As an apparent result in vivo, mice deficient in IL-15 or its receptor harbor few DCs. Injecting DCs into IL-15-/- mice was associated with the appearance of CD8+/CD44hi T cells that depended on IL-15 expression but also correlated with the longevity of the DCs. These findings support the hypothesis that DCs mediate the effect of IL-15 on CD8+/CD44hi memory phenotype T cells.
Keywords: apoptosis, cytokine, antigen-presenting cells, memory phenotype CD8 T cells
Anormal immune response to invading pathogens depends on the balanced proliferation and death of participating cells. The total number of lymphocytes in an organism is tightly controlled by cytokines, among other mechanisms. In this respect, IL-15 is crucial for the maintenance of CD8+/CD44hi memory phenotype T cells, natural killer (NK) cells, and CD8+ memory T cells (1-4).
IL-15 message has been found in a variety of cell types (5-7). However, IL-15 protein is secreted at low levels, so it is not detectable in the serum at picogram quantities under physiological conditions. The receptor for IL-15 is a heterotrimeric complex with two subunits (γc and IL-2/15Rβ) being components of receptors for other cytokines (3). Expression of the private IL-15Rα chain remains somewhat controversial. Some reports describe the chain's expression in lymphocytic cells that are affected by the cytokine (8-10). The highest expression levels are found in monocytic cells, including dendritic cells (DCs).
Transfer experiments revealed that the expression of the IL-15 receptor on nonlymphoid cells is necessary for the survival and homeostatic proliferation of NK and CD8+/CD44hi memory phenotype T cells. Koka et al. (11) described a rapid disappearance of WT NK cells if injected into IL-15Rα-deficient animals (11), whereas Burkett et al. (12) reported similar observations for CD8+/CD44hi T cells. These results point to at least one cell type that mediates the activity of IL-15. One explanation for both the failure to detect IL-15 in the serum and the necessity for IL-15Rα expression on nonlymphoid cells to support lymphocytes was proposed by previous studies where we showed that the simultaneous expression of IL-15 and IL-15Rα on one cell type is able to cause proliferation of T lymphocytes devoid in IL-15Rα expression (13). However, the mediating cell type has not been identified.
A number of reports (3, 14-16) describe an in vitro effect of IL-15 that inhibits apoptosis in various cell types, including fibroblasts and T lymphocytes. The relevance of these data remains to be shown because fibroblasts display no apparent defect in mice deficient for IL-15 or its receptor. Also, IL-15Rα-/- lymphocytes show no aberrations if transferred into IL-15Rα-competent mice.
Here, we describe an autocrine antiapoptotic effect of IL-15 in mature DCs and identify DCs as a candidate cell type that mediates the effect of IL-15 on CD8+/CD44hi memory phenotype T cells.
Materials and Methods
Reagents and Antibodies. The cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, IL-15, and IFN-γ were purchased from Peprotech (Rocky Hill, NJ), and Light was from R & D Systems. LPS, polyI:C, and CpG were from Sigma, CD40 ligand was from Alexis (San Diego), and propidium iodide and RNaseA were from Roche Diagnostics. The following antibodies used for FACS analyses were provided by Pharmingen: αCD8, αCD11c, αCD14, αCD25, αCD44, αCD62L, αCD83, αCD86, αCD122, αCD127, αCD132, αNK1.1, αThy1.1, αThy1.2, and αMHC II. Antibodies against human IL-15 and murine IL-15Rα were from R & D Systems. mAbs against human IL-15Rα and murine CD40 have been described (13). SC-12378, SC-671, and SC-668 (Santa Cruz Biotechnology) were used for immunoblotting human IL-15Rα, murine IL-2/15Rβ, and murine γc, respectively. For IL-15 binding, cells were washed twice in PBS, incubated with 1 nM human IL-15 (30 min, 4°C), washed, and stained with αIL-15.
Mice. C57BL/6 mice were purchased from the Frederick Cancer Research Facility. C57BL/6-IL-15-/- and C57BL/6-p53-/- mice were provided by Taconic Farms. IL-15Rα-/-, C57BL/6-IL-15Rβ-/-, and Thy1.1 congenic mice were from The Jackson Laboratory. IL-15Rα-/- mice were used after six backcross generations onto C57BL/6. Mice transgenic for human IL-15 have been described (15) and were backcrossed onto IL-15-/- mice.
Cell Culture. Human DCs were derived from elutriated monocytes (Blood Bank, National Cancer Institute) and used after 6 days of culture in the presence of 10% human serum and 25 ng/ml human GM-CSF and IL-4. Murine DCs were derived from bone marrow and cultured in 10% FBS and 20 ng/ml murine GM-CSF and 1 nM human IL-15 if indicated. Activation of DCs was done in the presence of cytokines by stimulation with 60 ng/ml LPS, 10 μg/ml polyI:C, 5 μg/ml CpG, 1 μg/ml CD40 ligand, 5 μg/ml αCD40 mAb, 20 ng/ml Light, and 20 ng/ml human or murine IFN-γ. The percentage of immature DCs was monitored by staining murine cells with αCD11c and human cells with αCD83 and αCD14. Maturation was analyzed 24 h after stimulation. The amount of hypodiploid DNA was determined 4 days after treatment unless otherwise indicated. Cells were washed in PBS, stained against CD11c, fixed in 2% formaldehyde (30 min, 4°C), permeabilized in 70% ethanol (15 min, 4°C), exposed to 25 μg/ml RNaseA (30 min, 37°C), and stained with 50 μg/ml propidium iodide.
Retroviral Infection. The sequence for murine Bcl-2 was amplified by PCR, sequenced, and inserted into a retroviral vector up-stream of an internal ribosome entry site-driven GFP (kindly provided by G. P. Nolan, Stanford University, Stanford, CA). This vector or the empty vector were cotransfected with a plasmid encoding ecotropic gag, env, and pol into 293HEK cells by using Lipofectamine 2000 (Invitrogen). Retrovirus supernatants were collected 36 h later and filtered, and DCs were spin-infected at day 3 of culture. Two days after infection, DCs were collected, washed, and sorted by GFP expression. Cells were grown in culture for an additional 24 h in the presence of 20 ng/ml murine IFN-γ and 60 ng/ml LPS and injected into mice. For in vitro analyses, DCs were grown for an additional 4 days with or without LPS/IFN-γ.
Manipulation of Mice. Mice were injected i.p. with 106 DCs that had been infected with the retroviral Bcl-2 or the mock constructs. Seventy microliters of tail blood was removed 24 h before injection and at days 3 and 6 after injection. Blood samples were separated over Ficoll gradients and analyzed by FACS. Blood, spleen, and lymph node samples were also analyzed from untreated WT, IL-15Rα-/-, IL-15-/-, and IL-15-/-.hIL-15Tg mice as well as 3 days after the i.p. injection of 5 μg of LPS and 1 μg of murine IFN-γ or after the injection of 50 μg of αCD122 (TMβ1, Pharmingen).
Immunoprecipitation and Western Blot. To detect IL-15Rα in DCs, cell lysates were prepared from human DCs by lysis in 150 mM NaCl, 1% Triton X-100, 25 mM Hepes (pH 7.3), and 10% glycerol, supplemented with protease inhibitors (Roche Diagnostics). Cell lysates were immunoprecipitated by using αIL-15Rα covalently bound to agarose beads (Pierce), size-fractionated by SDS/PAGE, and immunoblotted. For analysis of IL-2/15Rβ (CD122) and γc (CD132) expression, equal numbers of DCs and activated T cells were lysed in 4% SDS, 100 mM Tris (pH 6.8), and 20% glycerol, sonicated, and subjected to SDS/PAGE.
Results
Expression of IL-15 and Its Receptor in DCs. Although IL-15 is necessary for the existence of CD8+/CD44hi T cells, its activity is mediated by nonlymphoid cells. We hypothesized that these intermediary cell types are DCs that are intimately connected with the fate of lymphocytes, and we analyzed whether IL-15 affects DCs. It appears likely that the highest effect of a cytokine on a given cell correlates with the expression of its receptor. Therefore, we analyzed the expression levels of IL-15Rα in DCs.
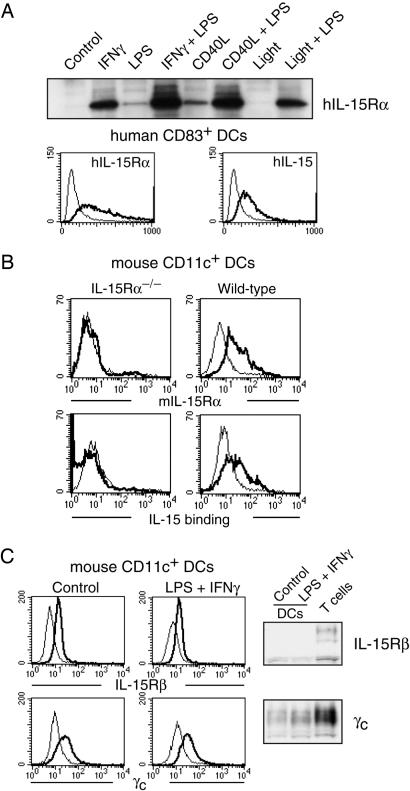
The promoters of both IL-15 and IL-15Rα were shown to contain binding sites for IFN-responsive factors and NF-κB (17-21). To analyze IL-15Rα expression we used three different methods: cytometry, IL-15 binding, and immunoprecipitation/immunoblot. We derived DCs from both murine bone marrow and human blood. In both cases, treatment with IFN-γ led to the expression of IL-15Rα as did treatment with NF-κB relA inducers such as CD40 ligand, LPS, CpG, and polyI:C (Fig. 1 A and B and Fig. 7, which is published as supporting information on the PNAS web site). The expression was highest if both stimuli were combined. In contrast, treatment of DCs with Light that induces NF-κB relB by p100 processing (data not shown) did not influence IL-15Rα expression (Fig. 1 A). Treatment of DCs with IFN-γ and relA inducers also caused the expression of the cytokine IL-15 itself (Fig. 1 A). Further analyses revealed that the remaining two subunits of the IL-15 receptor, IL-2/15Rβ and γc, were constitutively expressed at low levels in DCs (Fig. 1C).
Fig. 1.
Expression of IL-15 and its receptor in DCs. (A) Human blood-derived DCs were grown for 6 days in GM-CSF/IL-4 and stimulated for 24 h as indicated. (Upper) Immunoprecipitation/immunoblot analysis reveals that IL-15Rα was most effectively induced by a combination of 20 ng/ml IFN-γ and 60 ng/ml LPS or 1 μg/ml CD40 ligand. (Lower) FACS analyses showed that human DCs identified by CD83 induced the surface expression of both IL-15Rα and IL-15 in response to LPS/IFN-γ treatment (bold lines). Light lines indicate untreated DCs. (B) Murine DCs were derived from the bone marrow of IL-15Rα-/- (Left) and WT mice (Right) and grown for 6 days in GM-CSF. LPS/IFN-γ treatment induced the expression of IL-15Rα and the binding of human IL-15 on WT DCs (CD11c+) only. (C) Murine DCs express both IL-2/15Rβ and γc at low levels that did not appear to be affected by LPS/IFN-γ treatment as analyzed by FACS (light lines represent isotype controls) and Western blotting.
Treatment with IFN-γ/LPS is known to induce maturation in DCs. We followed this process by staining the maturation markers CD86 and MHC II 24 h after treatment (see Fig. 8, which is published as supporting information on the PNAS web site). Maturation of DCs was observed but appeared to be independent of IL-15 because no difference was detected among WT, IL-15-/-, IL-15Rα-/-, and IL-2/15Rβ-/- cells. Taken together, treatment with IFN-γ/relA inducers leads to the expression of IL-15 and its receptor on DCs.
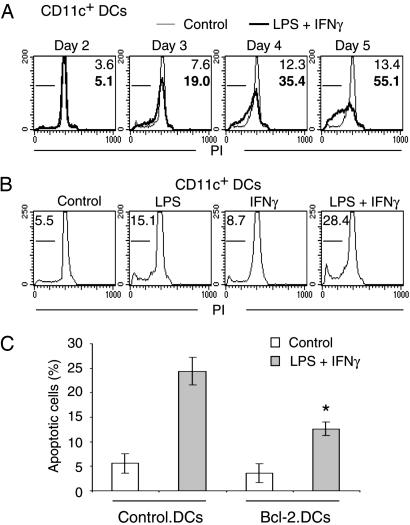
Maturation Is Accompanied by Apoptosis in DCs. The maturation of DCs is necessary for the activation of naïve T lymphocytes and is regulated by cytokines and the interaction with pathogens. To prevent excessive immune responses, the removal of mature DCs should also be regulated. Programmed cell death is one mechanism to assure the noninflammatory disposal of unnecessary cells. Therefore, we studied apoptosis in DCs by staining their DNA with propidium iodide. Treatment with IFN-γ/LPS induced a slow appearance of hypodiploid DNA as a marker of apoptosis in DCs (Fig. 2A). As was observed for the expression of IL-15 and IL-15Rα, the combination was most effective in inducing apoptosis (Fig. 2B).
Fig. 2.
Induction of apoptosis in DCs. Murine DCs were derived as described in Fig. 1, stimulated as indicated, and stained with propidium iodide (PI). (A) Treatment of DCs with 60 ng/ml LPS and 20 ng/ml IFN-γ (bold lines) caused the appearance of hypodiploid DNA as a marker of apoptosis that was not observed under control conditions (light lines). (B) Treatment of DCs with LPS or IFN-γ alone caused less apoptotic death than the combination. (C) Overexpression of Bcl-2 protects LPS/IFN-γ-treated DCs from apoptotic death. The asterisk indicates that the difference between Bcl-2-expressing and control DCs proved significant as determined by t test (P < 0.01, n = 4).
Most apoptotic cell deaths involve mitochondrial pathways. Bcl-2 is an antiapoptotic factor that inhibits this part of the apoptotic cascade. We introduced Bcl-2 via retroviral infection of murine DCs. Overexpression of Bcl-2 indeed inhibited the rate of IFN-γ/LPS-induced apoptosis but had little effect on untreated DCs (Fig. 2C). We also observed that p53 does not appear to be involved in maturation-induced cell death because WT and p53-/- cells had identical rates of cell death in response to IFN-γ/LPS (data not shown). In summary, treatment with IFN-γ/LPS causes DCs to die of apoptosis, a phenomenon that can be inhibited by overexpression of Bcl-2.
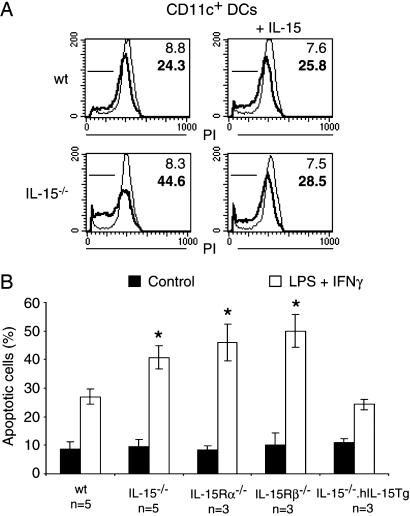
Autocrine Effect of IL-15 on Apoptosis. IL-15 has been shown to inhibit apoptosis in vitro (14, 16). To investigate whether IL-15 affects apoptosis of DCs we derived cells from mice with genetic deletions of IL-15 and its receptor. Comparisons with WT cells revealed that IL-15-/- DCs had increased rates of programmed cell death if stimulated by IFN-γ/LPS (Fig. 3). The presence of 1 nM IL-15 in the tissue culture medium suppressed apoptosis of IL-15-/- DCs to levels of WT cells but had no effect on WT DCs themselves (Fig. 3A). Similarly, a transgenic expression of IL-15 corrected the proapoptotic effect of a genetic IL-15 deletion (Fig. 3B).
Fig. 3.
Endogenous IL-15 decreases the apoptotic death of DCs in vitro.(A) Murine DCs were derived as described and were either left untreated (light lines and numbers) or stimulated with 60 ng/ml LPS and 20 ng/ml IFN-γ for 4 days (bold lines and numbers). (Left) DNA stains revealed an increased amount of hypodiploid DNA as a late marker of apoptosis if cells had been derived from IL-15-/- mice. (Right) The presence of 1 nM IL-15 in the tissue culture medium reduced apoptotic death in IL-15-/- cells. (B) Average numbers of apoptotic DCs with (open bars) or without (filled bars) LPS/IFN-γ treatment. DCs with deficiencies in IL-15, IL-15Rα, or IL-2/15Rβ showed increased rates of apoptosis after LPS/IFN-γ treatment. The transgenic expression of IL-15 reversed the proapoptotic effect of IL-15 deficiency. *, significant differences when compared with WT samples as determined by t test (P < 0.01).
We also investigated a role for the IL-15 receptor in determining the rate of apoptosis in DCs. Like the cytokine, cells with deficiencies in the receptor α and β chains displayed increased rates of apoptotic cell death in response to IFN-γ/LPS (Fig. 3B). We conclude that the expression of IL-15 confers an autocrine resistance to apoptosis on mature DCs via IL-15Rα/β.
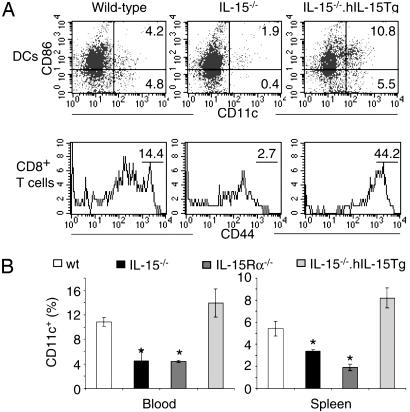
Reduction of DCs in IL-15-/- and IL-15Rα-/- Mice. To investigate whether the increased fragility of IL-15-deficient DCs that we observed in vitro is reflected in vivo we analyzed the frequencies of cells in blood and spleen. In comparison with results found in WT mice, the total number of DCs as detected by a CD11c stain was reduced in both organs in IL-15-/- and IL-15Rα-/- mice (Fig. 4). This reduction was observed in both the immature and mature (CD86+) DC population. The defect in the number of DCs in IL-15-/- mice could be corrected by transgenic expression of IL-15 (Fig. 4) or an injection of human IL-15 (data not shown).
Fig. 4.
IL-15 is necessary for DCs in vivo. Samples were taken from WT, IL-15-/-, IL-15Rα-/-, and IL-15-/-.hIL-15tg mice and analyzed by FACS. (A) (Upper) Blood samples from IL-15-/- mice contained fewer CD11c+ DCs with a reduction in both the mature (CD86+) and immature compartments. A transgenic expression of human IL-15 on the IL-15-/- background increased the number of DCs. (Lower) The number of DCs correlated with the percentage of circulating CD3+/CD8+/CD44hi T cells. (B) Average numbers of CD11c+ revealed that a lack of IL-15 or IL-15Rα was associated with a stronger reduction of DCs in blood (Left) when compared with spleen (Right) samples. *, significant differences when compared with WT mice based on t test (P < 0.01, n = 5 for each bar).
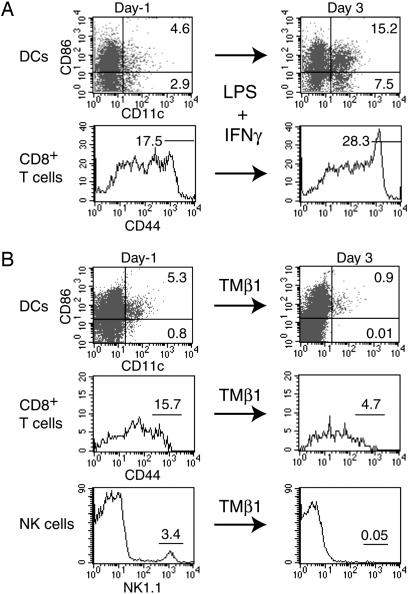
A simultaneous stain for CD8/CD44 revealed a correlation between the number of DCs and the number of CD8+ memory phenotype T cells (Fig. 4A). In addition, an i.p. injection of 5 μg of LPS and 1 μg of IFN-γ induced an increase in both DCs and CD8+/CD44hi T cells (Fig. 5A). Conversely, injecting 50 μg of TMβ1, an αCD122 (IL-2/15Rβ) mAb that blocks IL-15 signaling, reduced the population sizes of circulating DCs, CD8+/CD44hi T cells, and NK cells (Fig. 5B). The number of CD8+/CD44lo T lymphocytes remained nearly constant after TMβ1 injection (data not shown), indicating that the antibody treatment did not delete all CD122-expressing cells. We conclude that the requirement of IL-15 for the presence of CD8+/CD44hi T cells correlates with the number of DCs.
Fig. 5.
Induced changes in the population size of CD3+/CD8+/CD44hi T cells correlate with the number of DCs. (A) Treatment of WT mice with 5 μg of LPS and 1 μg of IFN-γ leads to an increase of immature DCs (CD11c+/CD86-), mature DCs (CD11c+/CD86+), and CD8+/CD44hi T cells in blood samples 3 days after injection. (B) An injection of 50 μg of TMβ1, an αCD122 (IL-2/15Rβ) antibody that blocks IL-15 signaling, reduced the number of both DCs (CD11c+) and CD3+/CD8+/CD44hi T cells in blood samples. In addition, the antibody injection strongly reduced the number of NK1.1-positive cells. Similar, but less dramatic, changes were observed for all three cell types in spleen and lymph node samples (data not shown).
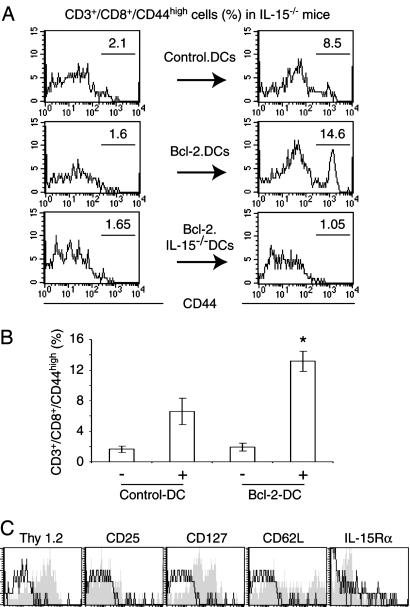
Induction of CD8+/CD44hi Cells by DCs. We wanted to establish causality between the lack of DCs and CD8+/CD44hi T cells in IL-15-deficient mice. If a reduced survival of mature DCs is responsible for a lack of CD8+ memory phenotype T cells, increasing the lifespan of DCs should increase the number of CD8+/CD44hi T cells. Therefore, we generated long-lived DCs by overexpression of Bcl-2 (1-4, 22, 23). These and control cells were sorted by FACS based on a coexpressed GFP, matured for 24 h with IFN-γ/LPS in vitro, and injected into the peritoneum of IL-15-/- mice. The percentage of circulating CD44hi among CD3/CD8+ cells ranged from 10% to 20% in untreated WT mice but was <4% in IL-15-/- animals. The injection of mock-infected DCs led to a transient increase of CD8+/CD44hi T cells in IL-15-/- mice 3 days after treatment (Fig. 6 A and B). Injecting Bcl-2-expressing DCs caused a more robust appearance of CD8+/CD44hi T cells, reaching numbers that were similar to WT mice. In contrast, no increase in the population size of CD8+/CD44hi T cells was observed 3 days after injecting either control or Bcl-2-expressing IL-15-/- DCs. This finding demonstrates a strict IL-15 dependency of the CD8+/CD44hi T cell-inducing effect of DCs because the expression of Bcl-2 increased the survival of both WT and IL-15-/- DCs (see Fig. 9, which is published as supporting information on the PNAS web site). The CD8+/CD44hi T cells that we observed at day 3 after injecting WT DCs originated from host tissues because these cells were positive for the congenic marker Thy1.2, whereas injected DCs were from Thy1.1-bearing mice (Fig. 6C). An analysis of the Bcl-2-DC-induced CD8+ memory phenotype population for additional memory T cell surface markers revealed them to be negative for CD25 (IL-2Rα) and IL-15Rα, positive for CD127 (IL-7Rα), and partially positive for CD62L (Fig. 6C). Taken together, these data indicate that the longevity of mature DCs correlates with the percentage of CD8+/CD44hi T cells among circulating lymphocytes.
Fig. 6.
Longevity of DCs affects the number of CD8+/CD44hi T cells in vivo. Murine DCs were derived from bone marrow of Thy1.1-congenic mice, infected with empty or Bcl-2-encoding retroviruses, sorted, matured in 60 ng/ml LPS and 20 ng/ml IFN-γ for 24 h, and injected into the peritoneum of IL-15-/- mice. Blood samples were analyzed 1 day before (Left) and 3 days after (Right) injection. (A) Whereas mock-infected DCs increased the number of CD8+/CD44hi T cells, a stronger increase was observed if Bcl-2-expressing DCs were injected. In contrast, the injection of Bcl-2-expressing IL-15-/- DCs did not affect the number of CD8+/CD44hi T cells. (B) Average numbers of CD8+/CD44hi T cells that were derived from three mice showed a significant effect of the expression of Bcl-2 in WT DCs as determined by t test (P < 0.01). (C) The CD8+/CD44hi T cells originated form host tissues because these cells expressed the congenic marker Thy1.2 of host cells but not Thy1.1 of the injected cells. Further FACS analysis revealed that the Bcl-2-DC-induced CD8+/CD44hi T cells were negative for CD25 (IL-2Rα) and IL-15Rα and positive for CD127 (IL-7Rα). These cells also included a subpopulation that expressed CD62L. Specific antibody staining (gray areas) was compared with isotype controls (thin lines).
Discussion
The cytokine IL-15 is crucial for the existence of a number of lymphocytes that include NK, NKT, γδT, and CD8+CD44hi T cells (1-4). Here, we report that IL-15 is also necessary for DCs. We observed a reduced percentage of DCs among blood leukocytes in mice with genetic deletions in IL-15 or its high-affinity receptor IL-15Rα. Both the injection of IL-15 and its transgenic expression were able to at least partially restore the number of DCs.
Our in vitro data suggest that one cause of the disappearance of DCs is an increased proclivity to apoptotic cell death during maturation. Similar to the in vivo effect, external addition of IL-15 restored the resistance to apoptosis of IL-15-/- DCs in vitro. No benefit was observed if IL-15 was administered to IL-15-proficient cells. The increased fragility of IL-15-/- DCs may not be the only factor that contributes to the disappearance of DCs in IL-15-/- mice because we observed a decrease of both mature and immature DCs. Other mechanisms may include effects of IL-15 on proliferation and migration. However, no effect of IL-15 on the proliferation of immature DCs was observed in vitro (data not shown). In addition, analyzing tissue samples from blood, spleen (Fig. 4), and lymph nodes (data not shown), we found no actual increases in the number of DCs in IL-15-/- mice when compared with WT mice, which does not support an effect of IL-15 on DC migration. These data do not exclude the possibility of increased numbers of DCs in other peripheral tissues such as skin of IL-15-/- mice.
The mechanisms that mediate the antiapoptotic effect of endogenous IL-15 on mature DCs are currently unknown. It has been shown that Bcl-2 is rapidly down-regulated upon the induction of DC maturation (24). In addition, CD40 ligand, RANK ligand, TNF-α, IL-12, IL-15, LPS, and CpG were shown to inhibit apoptosis in DCs via up-regulations of Bcl-2, Bcl-xL, and cellular inhibitor of apoptosis proteins (25-28). We have investigated WT and IL-15-/- DCs in vitro in response to LPS/IFN-γ and found no differences in the expression of Bcl-2, Bcl-xL, Mcl-1, Bid, Bim, Bax, Bak, IAP-1, IAP-2, XIAP, or caspase-3 (data not shown). Clearly more experiments are necessary to investigate antiapoptotic pathways that underlie the response of DCs to endogenous IL-15.
Several of our results indicate that DCs are the primary target for IL-15, which, in turn, mediate the known effect of the cytokine on the number of CD8+CD44hi T cells. First, the expression of IL-15Rα could clearly be shown on mature DCs by using FACS analysis, IL-15 binding, and immunoprecipitation/immunoblot as well as by comparing WT and IL-15Rα-/- cells in murine samples (29, 30). In contrast, the same analysis failed to detect the expression of IL-15Rα on either human or murine, naïve or activated T cells. Our data stand in conflict with other reports that show the expression of IL-15Rα at all stages of T cell development past the naïve stage (8). The reason for this discrepancy remains to be determined. Second, an injection of mature DCs led to a generation of CD8+CD44hi T cells in IL-15-/- hosts. This generation depended on the longevity of injected DCs because a statistical difference in the generation of CD8+CD44hi T cells was observed after the injection of control as compared with long-lived, Bcl-2-expressing DCs. Additional surface marker stains also revealed increases of NK cells in IL-15-/- mice in response to the injection of DCs (data not shown). However, those increases were less dramatic in amplitude when compared with the effect on CD8+ memory phenotype cells.
The necessity of IL-15 for the generation and maintenance of true CD8+ memory has been shown in three different model systems (12, 31, 32). In these studies, IL-15 did not affect the population size of antigen-specific CD8+ T cells after their initial expansion in response to immunization. Depending on the immunogen used, however, differences in the number of antigen-specific CD8+ cells appeared starting at ≈2 weeks postimmunization in IL-15- and IL-15Rα-deficient mice. Our experiments do not directly address the function of IL-15 in the generation of true CD8+ memory T cells. However, the involvement of the longevity of DCs in this function appears to be an attractive hypothesis. Several publications showed that the acquisition of homeostatic growth that is characteristic for true CD8+ memory T cells requires an extended period (8, 33). It appears conceivable that this process includes a teaching process and that DCs via their intimate contact with T cells fulfill this function. In this respect, the death of DCs early during an immune response caused by the lack of the antiapoptotic activity of IL-15 would reduce the size of the eventual true CD8+ memory population.
One mechanism that DCs potentially could use to affect memory phenotype CD8+CD44hi T cells is transpresentation of IL-15. We previously described an in vitro effect in which IL-15 and its high-affinity receptor IL-15Rα are bound on the surface of monocytic cells and induce T cells to proliferate even though these T cells lack the expression of the complete IL-15 receptor (13). We argued that IL-15Rα expression on DCs complements the expression of IL-2/15Rβ and γc on T cells. The data presented here further support an effect of IL-15 via transpresentation by IL-15Rα because Bcl-2-expressing IL-15-/- DCs were unable to support CD8+CD44hi T cells despite a similar antiapoptotic effect of Bcl-2 in both WT and IL-15-deficient DCs. In addition to this T cell-proliferative effect, our data reveal an involvement of IL-15 in the survival of DCs. Therefore, IL-15 appears to fulfill two crucial functions at the DC-CD8+ T lymphocyte synapse that include a prolongation of the life of DCs and the induction of T cell proliferation.
In summary, IL-15 mediates the survival of mature DCs. This DC longevity, in turn, affects the fate of CD8+CD44hi T cells.
Supplementary Material
Acknowledgments
We thank Dr. A. Singer for critically reading the manuscript and making helpful suggestions and Dr. S. Sharrow for cell sortings.
Author contributions: S.P.D., T.A.W., and J.R.M. designed research; S.P.D. and J.R.M. performed research; and J.R.M. wrote the paper.
Abbreviations: NK, natural killer; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor.
References
- 1.Sprent, J. (2003) Microbes Infect. 5, 227-231. [DOI] [PubMed] [Google Scholar]
- 2.Schluns, K. S. & Lefrancois, L. (2003) Nat. Rev. Immunol. 3, 269-279. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann, T. A., Dubois, S. & Tagaya, Y. (2001) Immunity 14, 105-110. [PubMed] [Google Scholar]
- 4.Lodolce, J., Burkett, P., Koka, R., Boone, D., Chien, M., Chan, F., Madonia, M., Chai, S. & Ma, A. (2002) Mol. Immunol. 39, 537-544. [DOI] [PubMed] [Google Scholar]
- 5.Grabstein, K. H., Eisenman, J., Shanebeck, K., Rauch, C., Srinivasan, S., Fung, V., Beers, C., Richardson, J., Schoenborn, M. A. & Ahdieh, M. (1994) Science 264, 965-968. [DOI] [PubMed] [Google Scholar]
- 6.Musso, T., Calosso, L., Zucca, M., Millesimo, M., Ravarino, D., Giovarelli, M., Malavasi, F., Ponzi, A. N., Paus, R. & Bulfone-Paus, S. (1999) Blood 93, 3531-3539. [PubMed] [Google Scholar]
- 7.Neely, G. G., Robbins, S. M., Amankwah, E. K., Epelman, S., Wong, H., Spurrell, J. C., Jandu, K. K., Zhu, W., Fogg, D. K., Brown, C. B. & Mody, C. H. (2001) J. Immunol. 167, 5011-5017. [DOI] [PubMed] [Google Scholar]
- 8.Wherry, E. J. & Ahmed, R. (2004) J. Virol. 78, 5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giri, J. G., Kumaki, S., Ahdieh, M., Friend, D. J., Loomis, A., Shanebeck, K., DuBose, R., Cosman, D., Park, L. S. & Anderson, D. M. (1995) EMBO J. 14, 3654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh, S., Perera, L. P., Burke, D. S., Waldmann, T. A. & Berzofsky, J. A. (2004) Proc. Natl. Acad. Sci. USA 101, 15154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koka, R., Burkett, P. R., Chien, M., Chai, S., Chan, F., Lodolce, J. P., Boone, D. L. & Ma, A. (2003) J. Exp. Med. 197, 977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkett, P. R., Koka, R., Chien, M., Chai, S., Chan, F., Ma, A. & Boone, D. L. (2003) Proc. Natl. Acad. Sci. USA 100, 4724-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. (2002) Immunity 17, 537-547. [DOI] [PubMed] [Google Scholar]
- 14.Bulfone-Paus, S., Ungureanu, D., Pohl, T., Lindner, G., Paus, R., Ruckert, R., Krause, H. & Kunzendorf, U. (1997) Nat. Med. 3, 1124-1128. [DOI] [PubMed] [Google Scholar]
- 15.Marks-Konczalik, J., Dubois, S., Losi, J. M., Sabzevari, H., Yamada, N., Feigenbaum, L., Waldmann, T. A. & Tagaya, Y. (2000) Proc. Natl. Acad. Sci. USA 97, 11445-11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulfone-Pau, S. S., Bulanova, E., Pohl, T., Budagian, V., Durkop, H., Ruckert, R., Kunzendorf, U., Paus, R. & Krause, H. (1999) FASEB J. 13, 1575-1585. [DOI] [PubMed] [Google Scholar]
- 17.Mariner, J. M., Lantz, V., Waldmann, T. A. & Azimi, N. (2001) J. Immunol. 166, 2602-2609. [DOI] [PubMed] [Google Scholar]
- 18.Mariner, J. M., Mamane, Y., Hiscott, J., Waldmann, T. A. & Azimi, N. (2002) J. Immunol. 168, 5667-5674. [DOI] [PubMed] [Google Scholar]
- 19.Azimi, N., Brown, K., Bamford, R. N., Tagaya, Y., Siebenlist, U. & Waldmann, T. A. (1998) Proc. Natl. Acad. Sci. USA 95, 2452-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azimi, N., Shiramizu, K. M., Tagaya, Y., Mariner, J. & Waldmann, T. A. (2000) J. Virol. 74, 7338-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogasawara, K., Hida, S., Azimi, N., Tagaya, Y., Sato, T., Yokochi-Fukuda, T., Waldmann, T. A., Taniguchi, T. & Taki, S. (1998) Nature 391, 700-703. [DOI] [PubMed] [Google Scholar]
- 22.Nopora, A. & Brocker, T. (2002) J. Immunol. 169, 3006-3014. [DOI] [PubMed] [Google Scholar]
- 23.Hou, W. S. & Van Parijs, L. (2004) Nat. Immunol. 5, 583-589. [DOI] [PubMed] [Google Scholar]
- 24.Granucci, F., Vizzardelli, C., Pavelka, N., Feau, S., Persico, M., Virzi, E., Rescigno, M., Moro, G. & Ricciardi-Castagnoli, P. (2001) Nat. Immunol. 2, 882-888. [DOI] [PubMed] [Google Scholar]
- 25.Bjorck, P., Banchereau, J. & Flores-Romo, L. (1997) Int. Immunol. 9, 365-372. [DOI] [PubMed] [Google Scholar]
- 26.Lundqvist, A., Nagata, T., Kiessling, R. & Pisa, P. (2002) Cancer Immunol. Immunother. 51, 139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, Y., Lee, S. W. & Sung, Y. C. (2002) J. Immunol. 168, 5-8. [DOI] [PubMed] [Google Scholar]
- 28.Pirtskhalaishvili, G., Shurin, G. V., Esche, C., Cai, Q., Salup, R. R., Bykovskaia, S. N., Lotze, M. T. & Shurin, M. R. (2000) Br. J. Cancer 83, 506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferlazzo, G., Pack, M., Thomas, D., Paludan, C., Schmid, D., Strowig, T., Bougras, G., Muller, W. A., Moretta, L. & Munz, C. (2004) Proc. Natl. Acad. Sci. USA 101, 16606-16611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koka, R., Burkett, P., Chien, M., Chai, S., Boone, D. L. & Ma, A. (2004) J. Immunol. 173, 3594-3598. [DOI] [PubMed] [Google Scholar]
- 31.Becker, T. C., Wherry, E. J., Boone, D., Murali-Krishna, K., Antia, R., Ma, A. & Ahmed, R. (2002) J. Exp. Med. 195, 1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schluns, K. S., Williams, K., Ma, A., Zheng, X. X. & Lefrancois, L. (2002) J. Immunol. 168, 4827-4831. [DOI] [PubMed] [Google Scholar]
- 33.Selin, L. K. & Welsh, R. M. (2004) Immunity 20, 5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.