Abstract
The high-yield expression of a neutralizing epitope from human immunodeficiency virus type 1 (HIV-1) on the surface of a plant virus and its immunogenicity are presented. The highly conserved ELDKWA epitope from glycoprotein (gp) 41 was expressed as an N-terminal translational fusion with the potato virus X (PVX) coat protein. The resulting chimeric virus particles (CVPs), purified and used to immunize mice intraperitoneally or intranasally, were able to elicit high levels of HIV-1-specific immunoglobulin G (IgG) and IgA antibodies. Furthermore, the human immune response to CVPs was studied with severe combined immunodeficient mice reconstituted with human peripheral blood lymphocytes (hu-PBL-SCID). hu-PBL-SCID mice immunized with CVP-pulsed autologous dendritic cells were able to mount a specific human primary antibody response against the gp41-derived epitope. Notably, sera from both normal and hu-PBL-SCID mice showed an anti-HIV-1-neutralizing activity. Thus, PVX-based CVPs carrying neutralizing epitopes can offer novel perspectives for the development of effective vaccines against HIV and, more generally, for the design of new vaccination strategies in humans.
The ideal requisites of any vaccine in inducing protective systemic and mucosal immunity include safety, efficacy, and low costs. Plants and plant viruses have recently been considered attractive systems for expressing and delivering foreign proteins or peptides as immunogens to be used for the development of new vaccination strategies (2, 18). The employment of plants for the production of therapeutic proteins offers several advantages such as absence of mammalian pathogens, cost effectiveness, large-scale production, and relative ease in expression and purification (13, 38). Plant virus coat proteins (CP) are particularly suitable carriers to present immunogenic peptides to the immune system. When properly fused at different positions on the capsid proteins, exogenous sequences are expressed in plants, originating recombinant viral CP able to self-assemble and generate chimeric virus particles (CVPs) displaying the foreign sequence on their outer surfaces. The “epitope-displaying” strategy using plant virus CP as carriers for both viral and bacterial antigens (14) has been successfully tested for experimental vaccines in animal models (7, 15, 20, 21, 36). The two systems most frequently used are tobacco mosaic virus and cowpea mosaic virus, whose structures have been determined to atomic resolution, allowing the design of fusion proteins carrying modifications expected to be located at the surface of the assembled virion. For rod-shaped viruses, such as potato virus X (PVX), although no crystallographic data are available, structural (3) and immunological (4) evidence reveals that the N terminus of the CP is exposed at the virion surface, enabling the decoration of the particle with a recombinant fusion peptide or protein. In fact, PVX has been used as a presentation system mainly for proteins such as green fluorescent protein (31), scFv antibody (34), and rotavirus major capsid protein (VP6) (25). Hence, if effective fusion strategies are devised, for instance, by introducing a sequence encoding the foot-and-mouth disease virus 2A catalytic peptide (30), there is no a priori constraint on the size of the inserted protein or peptide for the virion to be assembled and move. The high level of accumulation and ease of virus purification make PVX ideally suited for both small oligopeptide and protein fusions. Therefore we used PVX to produce CVPs displaying the immunogenic peptide.
The possibility to carry out a mucosal delivery of vaccine-expressing plants, potentially resulting also in the activation of the mucosa-associated immune system (6, 19), is particularly important for viruses transmitted mainly via mucosal surfaces such as human immunodeficiency virus type 1 (HIV-1) (17). A protective humoral immune response against HIV-1 requires antibodies able to properly bind to the virus envelope under physiological conditions. The vast majority of antibodies in seropositive individuals exhibit low affinity and weak neutralizing activity toward the native virus envelope (12, 27). Up to now, the only epitopes clearly identified as being well exposed are those recognized by neutralizing monoclonal antibodies (MAbs) 2F5, 2G12, and b12 (9). Among these, MAb 2F5 recognizes highly conserved linear epitope ELDKWAS (2F5e), located in the membrane-proximal part of the glycoprotein (gp) 41 ectodomain (23, 29). Therefore, we assayed the immunogenicity of PVX-derived CVPs displaying this epitope as an interesting candidate for the preparation of a vaccine against HIV-1.
Newly developed human vaccines are generally tested in normal mice before clinical experimentation, and concerns regarding the predictive value of studies with mouse models have been recently raised due to the marked differences in the regulation of the immune response between mice and humans (26). Therefore, it would be desirable, where possible, to extend the evaluation of candidate human vaccines from normal mice to animal models in which human primary immune responses can be studied. For this reason, we investigated the human immune response to PVX-derived CVPs displaying 2F5e in severe combined immunodeficient mice reconstituted with human peripheral blood lymphocytes (hu-PBL-SCID). This model exhibits unique features for studying in vivo human immune responses to pathogens (16, 32, 35).
In the present study, we provide evidence that (i) PVX-derived CVPs administered via different routes are able to elicit high levels of HIV-1-specific immunoglobulin G (IgG) and IgA antibodies in normal mice without adjuvants and (ii) hu-PBL-SCID mice immunized with CVP-pulsed autologous dendritic cells (DCs) are able to mount a specific human primary antibody response against the HIV-1-derived epitope. Remarkably, sera obtained from both normal and hu-PBL-SCID mice were endowed with anti-HIV-1-neutralizing activity.
MATERIALS AND METHODS
DNA construct.
The gp41 sequence ELDKWA was inserted into PVX CP as an N-terminal in-frame fusion. Briefly, the PVX-201 plasmid (5) (kind gift from D. Baulcombe, The Sainsbury Laboratory, Norwich, United Kingdom) was digested with restriction enzymes NheI and XhoI, and the excised fragment, containing most of the PVX CP-encoding sequence, was cloned into the corresponding sites of the pBluescript SK(+) (Stratagene) vector (pBS), previously modified in the polylinker to assemble restriction sites useful for the cloning, generating the pBS-CP plasmid. A double-stranded oligonucleotide encoding gp41 epitope ELDKWA, with SalI and NheI cohesive ends appended, was obtained by annealing two oligonucleotides: 5′-TCGACATGGAACTTGATAAGTGGGTTTCTG-3′ and 5′-CTAGCAGAAGCCCACTTATCAAGTTCCATG-3′. The double-stranded oligonucleotide was inserted between the SalI and NheI sites of the pBS-CP plasmid, yielding plasmid pBS-CP-H2F5. This plasmid was restricted with NheI and XhoI, and the excised fragment, coding for the modified PVX CP, was cloned into the PVX-201 plasmid, yielding plasmid pPVX-2F5E.
Plant infection and CVP purification.
Nicotiana benthamiana plants were infected with virus carrying plasmid pPVX-2F5E or wild-type (WT) PVX by abrading the surfaces of two leaves per plant with carborundum and inoculating each leaf with 20 μg of plasmid DNA. The inoculation was accomplished by gentle rubbing to spread the inoculum and further abrade the leaf surface. Upon systemic infection (10 to 12 days after inoculation), the correct expression of the foreign sequence was verified by reverse transcription-PCR (RT-PCR). Briefly, total RNA from infected tissues was extracted using an Rneasy plant minikit (Qiagen) and the RT-PCRs were performed using the GeneAmp RNA PCR kit (Perkin-Elmer). The cDNA strand was synthesized using oligo(dT)16, and PCR was performed with one primer mapping on the PVX genome (5′-CTGGGGAATCAATCACAGTGTTG-3′) and the other matching the 2F5e-encoding sequence (5′-CTAGCAGAAGCCCACTTATCAAGTTCCATG-3′). The presence of CVPs in the sap of infected plants was assayed by enzyme-linked immunosorbent assay (ELISA). Briefly, microtiter plates were coated by overnight incubation at 4°C with 100 μl of anti-PVX polyclonal antibodies diluted 1:2,000 in carbonate buffer. After being washed, the plates were blocked with 2% milk–0.1% gelatin for 2 h at 37°C. The infected plant tissue was harvested and homogenized in 1× phosphate-buffered saline (PBS). Extracts were then added (l00 μl/well), and plates were incubated at 37°C for 2 h. After the plates were washed, CVPs were detected by MAb 2F5 (8, 28, 29) (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program; catalog no. 1475) (1:150 dilution), followed by an anti-human Ig horseradish peroxidase-linked F(ab′)2 fragment (Amersham Pharmacia Biotech) diluted 1:5,000. The substrate was ABTS (2,2′-azinobis[3-ethylbenzthiazolinesulfonic acid]; KPL), and the colorimetric reaction was measured with an automated ELISA reader at 405 nm.
The engineered and WT virus particles were purified from symptomatic plant tissue 10 to 12 days after inoculum application, as described previously (1). Briefly, leaf tissue was ground and the sap was separated from cellular debris by centrifugation. Virus particles were purified on a cesium chloride density gradient, and the virus suspension was dialyzed against 1× PBS before immunization. The yield of CVPs was in the range of 4 to 6 mg for 10 g of fresh weight leaf tissue, and the corresponding amount of peptide was approximately 150 μg.
Mouse immunization
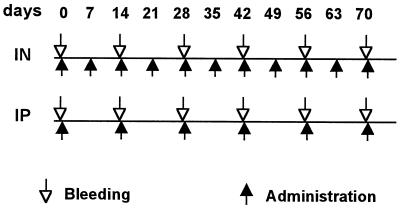
Four-week-old C57BL/10 female mice (six mice per group) were intranasally or intraperitoneally immunized with purified PVX-2F5E CVPs, as described in the schedule reported in Fig. 2. For both intranasal and intraperitoneal treatment, we included two control groups receiving WT PVX or PBS. A dose of 50 μg of PVX-2F5E (corresponding to 1.3 μg of 2F5e peptide) or 50 μg of WT PVX was administered per mouse. For each intranasal immunization, mice were anesthetized with an intraperitoneal injection of 50 mg of ketamine and 3 mg of xylazine/kg of body weight. Blood collection was performed by tail bleeding, and sera were stored at −20°C.
FIG. 2.
Schedule of mouse immunization. Three groups were treated intranasally (IN), while the others were treated by intraperitoneal injection (IP). Treatment groups were immunized with PVX-2F5E, WT PVX, or PBS. ↑, times of administration; ↓, bleeding times for serum collection.
Animal handling and maintenance were performed according to the interdisciplinary principles and guidelines for the use of animals in research, testing, and education prepared by the Ad Hoc Committee on Animal Research (The New York Academy of Sciences, New York, N.Y.).
ELISA of mouse and human Igs.
To detect anti-2F5e and anti-PVX antibodies, 96-well microtiter ELISA plates (Maxisorp; Nunc) were coated overnight at 4°C with 4 ng of a HIV-1 MN gp160-derived synthetic peptide (H66)/μl including the 2F5e sequence (QTQQEKNEQELLELDKWASL; the 2F5e sequence is underlined; NIH AIDS Research and Reference Reagent Program, catalog no. 2030) or 10 ng of WT PVX/μl, both diluted in carbonate buffer. The ability of MAb 2F5 to detect 2F5e in the H66 peptide was assessed by preliminary end point dilution ELISA experiments. Subsequently, MAb 2F5 diluted 1:150 was used in all ELISAs as a positive control. Coated plates were washed three times with 1× PBS–0.1% Tween 20 and once with 1× PBS. Blocking was performed with 2% milk–0.1% gelatin at 37°C for 2 h. After plates were washed (as described above), mouse sera (diluted 1:50 in 2% milk) were added in duplicate wells, and the plates incubated overnight at 4°C. Also after the plates were washed (as described above), secondary peroxidase-conjugated anti-mouse IgG (Amersham Pharmacia Biotech) or anti-mouse IgA (KPL) or anti-mouse IgG1 or IgG2a (ICN), diluted in 2% milk, as indicated by the manufacturer, was applied to each well, and plates were incubated for 1 h at 37°C. For MAb 2F5 detection, a peroxidase-conjugated anti-human total Ig (Cappel-Cooper Biomedical) was used. After incubation, plates were washed as described above and the chromogenic substrate (ABTS; KPL) was added. The colorimetric reaction was measured with an automated ELISA reader at 405 nm, and antibody levels were expressed as optical density values at 405 nm (OD405). ELISA experiments to evaluate human antibody responses in hu-PBL-SCID mice were performed according to the above-described procedure, except that peroxidase-conjugated anti-human total Ig (Cappel-Cooper Biomedical), diluted in 2% milk, as indicated by the manufacturer, was used as the secondary antibody.
For the end point titer evaluation, sera were serially diluted and titers were defined as the highest dilution at which the absorbance value was above the cutoff value, determined as the mean value of control sera (from PBS-injected mice) plus two standard deviations.
Extraction of fecal antibody.
Feces were collected 6 days after the last dose of immunogen (see Fig. 2) and dissolved by vortexing at 4°C for 15 min as a 10% (wt/vol) suspension in a mixture containing 100 mM NaCl, 10 mM Tris-HCl buffer, 1 mM CaCl2, 5% heat-inactivated fetal calf serum (Sigma), 0.05% Tween 20 (pH 7.4), and protease inhibitor cocktail (Complete; Roche). After being vortexed, samples were left to stand for 15 min, revortexed, and clarified by centrifugation. Supernatants were diluted 1/10 for the ELISA test. ELISAs were performed according to the above-described procedure.
DC preparation
Peripheral blood mononuclear cells were obtained from the blood of healthy donors by standard Ficoll-Paque density gradient centrifugation (Seromed). Monocytes were then isolated by subsequent Percoll density gradient centrifugation (32) and cultured in lipopolysaccharide-free flasks (Costar) at the concentration of 2 × 106 cells/ml in RPMI 1640 (GIBCO BRL) supplemented with 10% fetal calf serum, 500 U of granulocyte-macrophage colony-stimulating factor (R&D Systems)/ml, and 500 U of type I consensus interferon (specific activity, 109 U/mg of protein; Yamanouchi)/ml at 37°C in 5% CO2. After 3 days, nonadherent cells were collected and characterized for DC differentiation markers by fluorescence-activated cell sorter analysis as previously described (32). For pulsing, DCs (107) were incubated with 500 μg of PVX-2F5E or WT PVX (100 μg/ml) for 2 h at 37°C. After extensive washing with culture medium, DCs were used to immunize SCID mice previously reconstituted with autologous monocyte-depleted PBL (2 × 106 cells/mouse).
hu-PBL-SCID mouse model
Four-week-old CB17 scid/scid female mice (Charles River) (four mice per group), housed under specific-pathogen-free conditions, were intraperitoneally injected with 30 × 106 monocyte-depleted human PBL obtained from the blood of healthy donors and resuspended in 0.5 ml of RPMI 1640. Three and 10 days after reconstitution, mice were intraperitoneally injected with 2 × 106 human autologous DCs, previously pulsed with PVX-2F5E or with WT PVX (DCs injected 10 days after mice reconstitution were obtained from autologous peripheral blood mononuclear cells frozen at the time of preparation). On days 10, 17, and 25, blood samples were collected and anti-H66 and anti-PVX antibody levels in the sera were checked by ELISA.
Neutralization assay.
Neutralizing activity was tested by measuring the ability of sera to inhibit virus-induced syncytium formation in a standardized virus-cell system. The method is adapted from that described by Nara et al. (24). In such a test, a single infectious unit of virus infects a single cell, leading to a distinct response, which is the formation of a multinucleated giant cell. The addition of an antibody with neutralizing activity can prevent this event, and a quantitative measure of the residual infectivity can be determined. In our assay, C8166 target cells (a human T-lymphotropic virus type 1-transformed human umbilical cord CD4+ lymphoblastoid cell line) and the T-tropic HIV-1 IIIB virus prototype were used. Briefly, mouse sera, inactivated at 56°C for 30 min, were diluted twofold in medium and distributed in 96-well microtiter plates; there were four replicas (50 μl) per dilution. An equal volume of virus (100 50% tissue culture infective doses was then added, and the virus-serum mixture was incubated for 2 h at room temperature. Then 5 × 104 cells were added to each well in a final volume of 200 μl, and the plate was further incubated at 37°C in 5% CO2. After 5 days, syncytia were counted in each well under a microscope with the aid of a gridder ocular micrometer, and the mean percentage of neutralization was estimated by the formula 1 − Vn/V0, where Vn is the mean number of syncytia in antibody-treated cultures and V0 is the mean number of syncytia in virus control cultures (24).
RESULTS
Gene engineering and CVP production
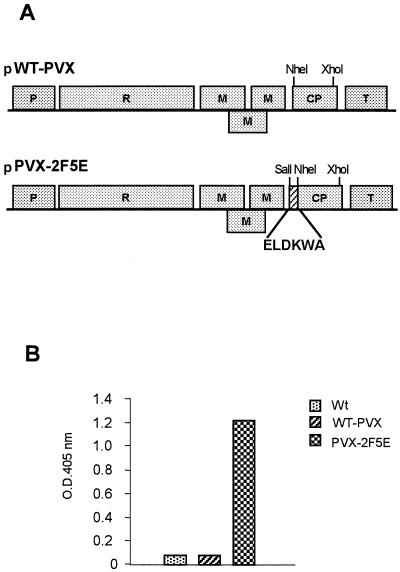
We modified the PVX CP-encoding gene by linking the sequence encoding HIV-1 gp41-derived 2F5e, as described in Materials and Methods (Fig. 1A). The plasmid containing the full-length cDNA of PVX with the modified CP sequence (pPVX-2F5E) was used to infect N. benthamiana plants. Twelve days later, leaves from plants showing systemic infection were collected, and the expression of the foreign sequence was verified by RT-PCR (data not shown). The correct display of the HIV-1 epitope on the outer surface of CVPs was assessed by ELISA using human MAb 2F5 (Fig. 1B). Further experiments demonstrated that the modified CP was highly stable and retained its capability to form CVPs after three cycles of reinfection (data not shown).
FIG. 1.
Genome structure of PVX-derived vectors and detection of CVPs by ELISA. (A) Full-length WT PVX cDNA was inserted between constitutive promoter 35S (P) derived from cauliflower mosaic virus and the transcription terminator (T) from the nopaline synthase gene, important for the regulation of the viral genome expression upon plant infection with plasmid DNA. pPVX-2F5E is the plasmid carrying the virus genome engineered to express the 2F5 epitope fused to CP. The amino acid sequence of the epitope is shown. R, viral replicase; M, movement protein. (B) Detection of 2F5e on the surface of the virus carrying PVX-2F5E by using MAb 2F5 as evaluated by ELISA. Histograms represent the adsorbance values of samples from plants infected with WT PVX or PVX-2F5E or from an uninfected control (Wt) in a representative experiment.
Immunogenicity of CVPs in mice.
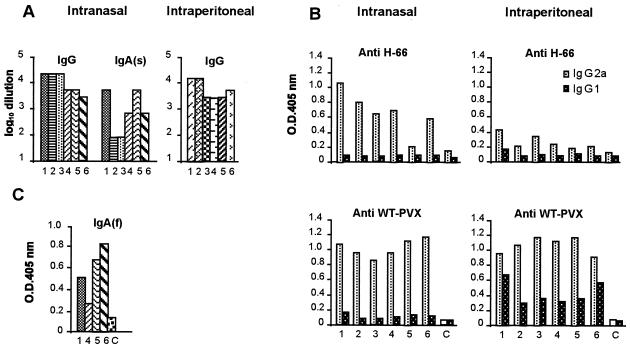
The immune response of mice intranasally or intraperitoneally immunized with purified CVPs (PVX-2F5E) or with WT PVX or PBS as controls was studied according to the schedule reported in Fig. 2. Sera from both intranasally and intraperitoneally PVX-2F5E-immunized mice showed high levels of IgG specific for the H66 peptide (Fig. 3A and C), while no reactivity was found in the sera of control animals (Fig. 3B and D). These results demonstrate that the gp41-derived sequence displayed on the surface of PVX is immunogenic in mice. The kinetics of the response shows that antibody levels rise after the fourth immunization (maximum peak on day 42) and after the second immunization (maximum peak on day 56) in intranasally and intraperitoneally treated mice, respectively (Fig. 3A and C). Two out of six intranasally immunized mice were unresponsive on day 56, while broad, prolonged antibody levels were detected in the others. Anti-H66 IgG titers, calculated as end point dilution, ranged from 2,000 (mouse 6) to more than 30,000 (mice 1, 2, and 3) (Fig. 4A) for the intranasally immunized group and from 2,000 (mice 3, 4, and 5) to 15,000 (mice 1 and 2) for the intraperitoneally immunized animals. It is noteworthy that mice immunized via the mucosal route showed the presence of anti-H66 IgA in the serum (Fig. 3E and 4A) and in fecal extracts (Fig. 4C), while no H66-specific IgA was detectable in fecal samples from intraperitoneally immunized mice (not shown).
FIG. 3.
Kinetics of serum antibody response in mice. Sera from mice immunized intranasally (IN) or intraperitoneally (IP) with PVX-2F5E or WT PVX were evaluated by ELISA. Shown are IgG (A to D) and IgA (E and F) antibody responses specific for the H66 peptide. Each line represents the kinetics of the response of a single mouse. Antibody levels are expressed as OD405 values.
FIG. 4.
Determination of antibody titers and isotypes. (A) Histograms visualizing the serum IgG and IgA end point titers evaluated by ELISA on days 42 and 70 in mice immunized intranasally or intraperitoneally with PVX-2F5E. The titers were determined as described in Materials and Methods. (B) Isotype determination of IgG evaluated by ELISA on day 42 in mice immunized with PVX-2F5E. Shown are the levels of IgG1 or IgG2a specific for both H66 and WT PVX. Each bar represents individual mouse responses. Antibody levels are expressed as OD405 values. (C) Presence of IgA(f) in feces evaluated by ELISA on day 76 in mice immunized intranasally with PVX-2F5E. C, mean absorbance value plus two standard deviations for control samples derived from the six mice immunized with PBS.
The isotyping of the anti-H66 or anti-WT PVX IgG response to PVX-2F5E or WT PVX showed that the IgG2a subclass was dominant in mice immunized via both the mucosal and the systemic routes with PVX-2F5E (Fig. 4B).
Human antibody response in hu-PBL-SCID mouse model.
To specifically address the issue of whether CVPs had the ability to induce a human DC-driven antibody response in vivo, we immunized hu-PBL-SCID mice with human autologous monocyte-derived DCs (32) pulsed with PVX-2F5E or WT PVX and mouse sera were analyzed for the presence of antigen-specific human antibodies at different time points. In several independent experiments, sera from chimeric mice immunized with PVX-2F5E-pulsed DCs showed significant levels of Igs specific for the H66 peptide, while no anti-2F5e reactivity was detected in the sera of mice injected with WT PVX-pulsed DCs or in that from PBS-injected controls (Fig. 5). Both DC-injected groups raised a human antibody response to WT PVX (Fig. 5).
FIG. 5.
Detection of anti-H66 and anti-WT PVX human Igs in sera of hu-PBL-SCID mice. hu-PBL-SCID mice (four mice per group) were intraperitoneally injected on days 3 and 10 with autologous DCs pulsed with PVX-2F5E, WT PVX, or PBS alone. Histograms represent the mean OD405 values of four samples ± standard deviations. Data of one representative experiment are shown.
Neutralization assay.
The HIV-1-neutralizing activity of sera obtained from both normal and hu-PBL-SCID mice was evaluated by a syncytium inhibition assay. As shown in Fig. 6, sera from mice intranasally immunized with PVX-2F5E showed a consistent capability to inhibit syncytium formation, compared to controls (WT PVX). Sera from hu-PBL-SCID mice immunized with PVX-2F5E-pulsed DCs also showed neutralizing activity (Fig. 6).
FIG. 6.
In vitro neutralization assay of HIV-1. Neutralizing activities of sera collected at day 42 from mice immunized intranasally with PVX-2F5E (M1, M4) or WT PVX (M7) and at day 17 from hu-PBL-SCID mice immunized with PVX-2F5E-pulsed (H4) or WT PVX-pulsed (H6) DCs were determined by syncytium inhibition assay. Percentages of neutralization, evaluated as described in Materials and Methods, are plotted against serum dilution. Each line shows the neutralizing activity of a representative serum from one mouse in each group. Similar results were obtained using serum from the other mice in the same group.
DISCUSSION
In this study, we have demonstrated the ability of plant CVPs displaying a highly conserved HIV-1 gp41-derived epitope to induce a neutralizing antibody response in mice. Furthermore, we have shown that these CVPs are able to induce a human primary antibody response in hu-PBL-SCID mice immunized with DCs pulsed with CVPs. Notably, both mouse and human antibodies show the ability to inhibit HIV-1 syncytium formation in vitro. Studies on the immunogenicity of B-cell epitope 2F5e, expressed as a fusion product with different protein carriers (11, 22, 37), performed with mice provide contrasting results and support the concept that the molecular context of peptide expression is crucial. Here, the efficiency of PVX carrying an HIV-1 epitope for eliciting a human protective response against HIV-1 is demonstrated for the first time, supporting the feasibility of using this type of vector in the realization of new anti-HIV-1 subunit vaccines.
A number of studies have recently documented the ability of CVPs displaying foreign peptides from several pathogens and delivered by different routes to induce protective immunity in animal models (6, 7, 15, 20, 21, 36). Notably, in the present study we have shown that intranasal delivery of CVPs, inducing higher antigen-specific antibody titers, evokes a more efficient immune response than delivery through the systemic route. Moreover, high levels of specific IgA were observed in intranasally immunized mice, not only in the serum but also in feces, indicating the presence of specific Igs in the mucosal districts. Thus the immune response evoked by the intranasal delivery of CVPs was quantitatively and qualitatively different from that elicited by systemic administration, implying activation of a mucosal response, which is considered to be crucial for protecting against pathogens, such as HIV-1, transmitted via the mucosal route. It should also be emphasized that these results were obtained without the use of adjuvant for immunization. Mucosal administration of antigens in the absence of adjuvants may result in immunity or tolerance depending on several factors, such as the type of antigen, dose, and cytokine milieu produced following antigen exposure (10). Interestingly, we have observed a strong immune response following repeated intranasal immunizations, suggesting that CVPs act as adjuvants, which are generally capable of breaking tolerance, shaping the response toward immunity. Typically, the breaking of tolerance is associated with a Th-1-type of immune response (10). Interestingly, the antibody responses observed in mice immunized intranasally with CVPs were predominantly of the IgG2a isotype, suggesting a Th1-type immune response. The polarization of the response to this T-helper-cell type is considered a reliable correlate of immune protection in viral infections, including HIV-1 (33). Thus the immune response induced by CVPs exhibited a Th profile potentially consistent with protection against HIV infection. Moreover, the antibody responses evoked in mice consistently showed neutralizing activity against HIV-1 in vitro.
Altogether, the results shown in this paper represent an important link between plant biotechnology and vaccine research. In fact, we have demonstrated that the 2F5 neutralizing epitope, generally giving controversial results when used as an immunogen fused to other carrier molecules (11, 22, 37), becomes highly immunogenic when linked to the CP of plant virus particles. This observation confirms the notion that the mode of presentation of an epitope on a heterologous carrier can dramatically affect its immunological properties. Furthermore, PVX-2F5E exhibits a powerful immunogenic potential, which includes the generation of a neutralizing response, not only in mice but also in a human context. Finally, human DCs pulsed with PVX-2F5E can trigger in vitro proliferation of autologous PBLs, suggesting that PVX-derived CVPs are able to activate cellular responses. Therefore, it would be interesting to investigate whether plant-derived CVPs carrying HIV-1 cytotoxic T-lymphocyte epitopes could be used, in combination with those displaying B-cell epitopes, to broaden the immune correlates of protection against HIV-1.
The CVP strategy described herein can offer new insights for the development of a highly effective and advantageous vaccine against HIV and, more generally, for the design of new vaccination strategies in humans. In this perspective, along with the evaluation of the effectiveness of these plant-derived vectors as carriers of molecules for vaccination purposes, safety issues regarding the possible side effects induced by the anti-CP immune response need to be addressed.
ACKNOWLEDGMENTS
We thank G. Scala and S. Baschieri for helpful suggestions and critical review of the manuscript and M. Piscitelli at the Animal House for advice and control in immunization experiments, performed in accordance with the recommendations of the ENEA Bioethics Committee. The following reagents were obtained through the AIDS Research and Reference Program, Division of AIDS, NIAID, NIH: the HIV-1 gp41 monoclonal antibody (2F5; from H. Katinger) and HIV MN66.
This work was supported by a grant from the Istituto Superiore di Sanità-Programma Nazionale sull'AIDS-Progetto: “Patogenesi, Immunità e Vaccino per l'AIDS” to E.B. (grant no. 40B.11).
REFERENCES
- 1.AbouHaidar M G, Xu H, Hefferon K L. Potexvirus isolation and RNA extraction. Methods Mol Biol. 1998;81:131–143. doi: 10.1385/0-89603-385-6:131. [DOI] [PubMed] [Google Scholar]
- 2.Arntzen C J. High-tech herbal medicine: plant-based vaccines. Nat Biotechnol. 1997;15:221–222. doi: 10.1038/nbt0397-221. [DOI] [PubMed] [Google Scholar]
- 3.Baratova L A, Grebenshchikov N I, Dobrov E N, Gedrovich A V, Kashirin I A, Shishkov A V, Efimov A V, Jarvekulg L, Radavsky Y L, Saarma M. The organization of potato virus X coat proteins in virus particles studied by tritium planigraphy and model building. Virology. 1992;188:175–180. doi: 10.1016/0042-6822(92)90747-d. [DOI] [PubMed] [Google Scholar]
- 4.Baratova L A, Grebenshchikov N I, Shishkov A V, Kashirin I A, Radavsky J L, Jarvekulg L, Saarma M. The topography of the surface of potato virus X: tritium planigraphy and immunological analysis. J Gen Virol. 1992;73:229–235. doi: 10.1099/0022-1317-73-2-229. [DOI] [PubMed] [Google Scholar]
- 5.Baulcombe D C, Chapman S, Santa Cruz S. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 1995;7:1045–1053. doi: 10.1046/j.1365-313x.1995.07061045.x. [DOI] [PubMed] [Google Scholar]
- 6.Brennan F R, Bellaby T, Helliwell S M, Jones T D, Kamstrup S, Dalsgaard K, Flock J I, Hamilton W D. Chimeric plant virus particles administered nasally or orally induce systemic and mucosal immune responses in mice. J Virol. 1999;73:930–938. doi: 10.1128/jvi.73.2.930-938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan F R, Gilleland L B, Staczek J, Bendig M M, Hamilton W D, Gilleland H E. A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology. 1999;145:2061–2067. doi: 10.1099/13500872-145-8-2061. [DOI] [PubMed] [Google Scholar]
- 8.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 9.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Anjuere F, McGhee J R, George-Chandy A, Holmgren J, Kieny M P, Fujiyashi K, Mestecky J F, Pierrefite-Carle V, Rask C, Sun J B. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev. 1999;170:197–222. doi: 10.1111/j.1600-065X.1999.tb01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckhart L, Raffelsberger W, Ferko B, Klima A, Purtscher M, Katinger H, Ruker F. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J Gen Virol. 1996;77:2001–2008. doi: 10.1099/0022-1317-77-9-2001. [DOI] [PubMed] [Google Scholar]
- 12.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000;18:1151–1155. doi: 10.1038/81132. [DOI] [PubMed] [Google Scholar]
- 14.Johnson J, Lin T, Lomonossoff G. Presentation of heterologous peptides on plant viruses: genetics, structure and function. Annu Rev Phytopathol. 1997;35:67–86. doi: 10.1146/annurev.phyto.35.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Koo M, Bendahmane M, Lettieri G A, Paoletti A D, Lane T E, Fitchen J H, Buchmeier M J, Beachy R N. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc Natl Acad Sci USA. 1999;96:7774–7779. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krensky A M. SCID mouse models: more than furry flasks. Nat Biotechnol. 1997;15:720–721. doi: 10.1038/nbt0897-720. [DOI] [PubMed] [Google Scholar]
- 17.Lehner T, Bergmeier L, Wang Y, Tao L, Mitchell E. A rationale basis for mucosal vaccination against HIV infection. Immunol Rev. 1999;170:183–196. doi: 10.1111/j.1600-065x.1999.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 18.Lomonossoff G P, Johnson J E. Use of macromolecular assemblies as expression systems for peptides and synthetic vaccines. Curr Opin Struct Biol. 1996;6:176–182. doi: 10.1016/S0959-440X(96)80072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason H S, Haq T A, Clements J D, Arntzen C J. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16:1336–1343. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 20.McLain L, Durrani Z, Wisniewski L A, Porta C, Lomonossoff G P, Dimmock N J. Stimulation of neutralizing antibodies to human immunodeficiency virus type 1 in three strains of mice immunized with a 22 amino acid peptide of gp41 expressed on the surface of a plant virus. Vaccine. 1996;14:799–810. doi: 10.1016/0264-410x(95)00229-t. [DOI] [PubMed] [Google Scholar]
- 21.Modelska A, Dietzschold B, Sleysh N, Fu Z F, Steplewski K, Hooper D C, Koprowski H, Yusibov V. Immunization against rabies with plant-derived antigen. Proc Natl Acad Sci USA. 1998;95:2481–2485. doi: 10.1073/pnas.95.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nara P L, Hatch W C, Dunlop N M, Robey W G, Arthur L O, Gonda M A, Fischinger P J. Simple, rapid, quantitative syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987;3:283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien G J, Bryant C J, Voogd C, Greenberg H B, Gardner R C, Bellamy A R. Rotavirus VP6 expressed by PVX vectors in Nicotiana benthamiana coats PVX rods and also assembles into viruslike particles. Virology. 2000;270:444–453. doi: 10.1006/viro.2000.0314. [DOI] [PubMed] [Google Scholar]
- 26.O'Shea J J, Visconti R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat Immunol. 2000;1:17–19. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]
- 27.Parren P W, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13:S137–S162. [PubMed] [Google Scholar]
- 28.Purtscher M, Trkola A, Grassauer A, Schulz P M, Klima A, Dopper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, Tauer C, Berger R, Barrett N, Jungbauer A, et al. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 30.Ryan M D, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994;13:928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santa Cruz S, Chapman S, Roberts A G, Roberts I M, Prior D A, Oparka K J. Assembly and movement of a plant virus carrying a green fluorescent protein overcoat. Proc Natl Acad Sci USA. 1996;93:6286–6290. doi: 10.1073/pnas.93.13.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santini S M, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type 1 interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shearer G M, Clerici M. Cytokine profiles in HIV type 1 disease and protection. AIDS Res Hum Retroviruses. 1998;14:S149–S152. [PubMed] [Google Scholar]
- 34.Smolenska L, Roberts I M, Learmonth D, Porter A J, Harris W J, Wilson T M, Santa Cruz S. Production of a functional single chain antibody attached to the surface of a plant virus. FEBS Lett. 1998;441:379–382. doi: 10.1016/s0014-5793(98)01586-5. [DOI] [PubMed] [Google Scholar]
- 35.Tary-Lehmann M, Saxon A, Lehmann P V. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529–533. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 36.Turpen T H, Reinl S J, Charoenvit Y, Hoffman S L, Fallarme V, Grill L K. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Bio/Technology. 1995;13:53–57. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y, Zhao Y, Lu Y, Chen Y H. Epitope-vaccine induces high levels of ELDKWA-epitope-specific neutralizing antibody. Immunol Investig. 2000;29:41–50. doi: 10.3109/08820130009105143. [DOI] [PubMed] [Google Scholar]
- 38.Yusibov V, Koprowski H. Plants as vectors for biomedical products. J Med Food. 1998;1:5–12. [Google Scholar]