Abstract
We previously demonstrated that recombinant plant virus particles containing a chimeric peptide representing two rabies virus epitopes stimulate virus neutralizing antibody synthesis in immunized mice. We show here that mice immunized intraperitoneally or orally (by gastric intubation or by feeding on virus-infected spinach leaves) with engineered plant virus particles containing rabies antigen mount a local and systemic immune response. After the third dose of antigen, given intraperitoneally, 40% of the mice were protected against challenge infection with a lethal dose of rabies virus. Oral administration of the antigen stimulated serum IgG and IgA synthesis and ameliorated the clinical signs caused by intranasal infection with an attenuated rabies virus strain.
Increasing knowledge of the molecular biology of plant viruses has raised the possibility of using these viruses as antigen expression systems (1–9). Unlike conventional recombinant vaccines, which are largely derived from live-recombinant, live-attenuated, or killed pathogens (10–14), plant viruses are generally recognized to be nonpathogenic in humans and other animals. Thus, interest in plant viruses as a system for the expression and delivery of antigens is growing. Tobacco mosaic virus (TMV), cowpea mosaic virus, and tomato bushy stunt virus have been successfully used to express antigenic determinants from different human or animal pathogens (1–9). We have adapted alfalfa mosaic virus (AIMV) coat protein (CP) to express foreign peptides and have demonstrated that mice immunized with such engineered virus produce antibodies specific for the target antigen (7). Importantly, immunization with recombinant AIMV particles containing antigenic determinants of rabies virus or HIV did not require coadministration of adjuvant for an effective immune response (7). Effective immunization with chimeric rabies peptide alone, however, require coadministration of adjuvant (15).
Vaccines that are administered parenterally can usually prevent systemic spread of invasive pathogens, but do not prevent infection of mucosal tissues (16–24), which is the primary gateway for many pathogens of humans and animals. There has been considerable progress in the development of heterologous expression systems (25–29) for the oral administration of antigens that stimulate common mucosal–immune systems. In the late 1980s, plants became part of the vaccine development process as an effective, inexpensive, and safe production and delivery system for vaccines. Antigens produced in plants can be used to generate neutralizing or protective antibodies in animal models (30–33). Nevertheless, adjuvant appears to be a requirement for an effective immune response against those antigens (30–33). On the other hand, presentation of antigen as a constituent of bacteria or virus particles significantly enhances the immune response to the antigen (34, 35). We have demonstrated that vaccine antigens can be expressed in an immunogenic form using engineered plant virus (7). This offers us the ability to not only produce high levels of virus economically, but also to examine whether virus administered as a constituent of untreated plant tissue will be more immunogenic via the oral route than the purified virus alone. Therefore, in this study, we immunized mice parenterally or orally with recombinant AIMV particles (purified or nonpurified in virus-infected spinach leaves) containing a chimeric peptide of rabies virus previously shown to protect mice immunized intramuscularly (i.m.) against lethal challenge with rabies virus (15). Our data demonstrate a similar protection, as well as the generation of a protective antibody response after the oral administration of virus particles containing this peptide.
MATERIALS AND METHODS
In Vitro Transcription.
In vitro transcripts of recombinant TMV were synthesized using T7 RNA polymerase (Promega) and purified plasmid DNA, according to the manufacturer’s guidelines. Transcripts were capped using the RNA cap structure analog m7G(5)ppp(5)G (Biolabs, Beverly, MA).
Plant Infection and Virus Isolation.
The leaves of Nicotiana benthamiana and Spinacia oleracea plants were inoculated with in vitro synthesized RNA products of recombinant TMV strains as described (7). The virus was isolated from N. benthamiana plants 12–14 days after the inoculum was applied (4). Leaf tissue was ground and the sap separated from cell debris by centrifugation. Virus particles were selectively precipitated using 5% polyethylene glycol. The leaves of S. oleracea were not processed prior to mice feeding.
Mice.
Eight-week-old female Swiss–Webster mice (Taconic Farms) were used in these experiments. Mice were housed in a temperature and light-cycle controlled room at the Thomas Jefferson University Animal Facility.
Viruses and Cell Culture.
CVS-11, CVS-24, and CVS-F3 strain rabies viruses were propagated in baby hamster kidney cells (BHK)-21 (36). The BHK-21 cell line used for rabies virus neutralization assay was maintained as described (36).
Immunization of Mice with Engineered Plant Virus.
Mice (10 in each group) were immunized as follows: (i) Intraperitoneally (i.p.) with three doses (50 μg per injection) of recombinant virus particles at 2-week intervals (7). Control mice received a mixture of wild-type AIMV plus 30BRz (7); (ii) Orally, by gastric intubation, with four doses of purified recombinant virus particles (250 μg per dose) at 2-week intervals. Control mice received a mixture of wild-type AIMV plus 30BRz (A/TMV); (iii) Orally, by feeding on spinach leaves containing recombinant virus particles (1 g per dose) for 7 consecutive days in addition to their normal food, followed by 7 days of normal diet. This feeding cycle was repeated four times. Control mice were fed noninfected spinach leaves. In all groups, serum samples and fecal pellets were collected 2 days before each immunization.
ELISA.
Mouse sera and supernatants from fecal pellets were analyzed for the presence of antigen-specific antibody by ELISA as described (7), using 96-well plates coated with 100 μl per well of inactivated ERA strain rabies virus (5 μg/ml) or with AIMV CP (5 μg per ml) overnight at 24°C. Peroxidase-conjugated goat anti-mouse IgG (γ-chain-specific) and IgA (α-chain-specific) were used as a secondary antibody.
Neutralization Assay and Challenge.
Sera from mice inoculated with recombinant AIMV particles consisting of CPDrg24, a fusion of AIMV CP and rabies virus peptide Drg24 (7) or control virus (A/TMV) were heat inactivated at 56°C for 30 min and incubated with CVS-11 strain rabies virus. Pretreated virus was used to infect BHK indicator cells (36). The neutralizing activity of rabies virus-specific serum antibodies was determined using a rapid fluorescent focus-forming inhibition test as described (36).
Fourteen days after the last immunization, mice immunized i.p. with purified virus particles were challenged i.m. with a lethal dose (10 IMLD50) of CVS-24 strain rabies virus and monitored for the appearance of clinical signs of disease. Mice immunized orally via gastric intubation or spinach leaf feeding were challenged intranasally (i.n.) under anesthesia with 10 μl PBS containing 105 focus-forming units of CVS-F3 strain rabies virus. Mice were examined for the appearance of clinical signs of disease and weighed daily.
Statistics.
Standard deviation (SD) and Statistical significance of differences (P) were calculated. The differences were considered significant at α = 0.05.
RESULTS AND DISCUSSION
Intraperitoneal Immunization of Mice with A/TMV Containing Rabies Peptide CPDrg24 Protects Against Lethal Rabies Virus Dose.
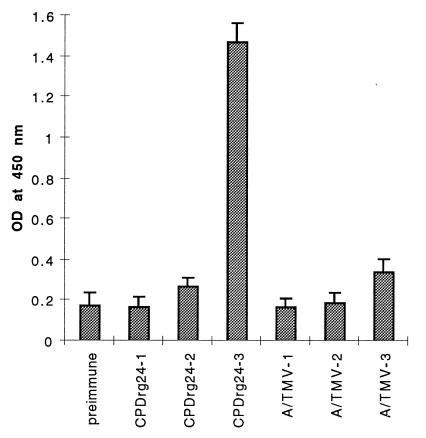
Analysis of serum samples collected from individual mice 12 days after each of three i.p. immunizations with CPDrg24 (Fig. 1) revealed no rabies virus-specific antibodies after the first immunization, only a slight difference in titers between sera of control, and CPDrg24-immunized mice after the second immunization, and a significant response following the third administration. ELISA titers of rabies-specific antibodies in sera of CPDrg24-immunized mice were 3-fold higher than background levels seen with preimmune and control sera A/TMV. Serum samples collected from CPDrg24-immunized mice after the third immunization also showed neutralizing activity against CVS-11 strain rabies virus, with a mean virus neutralizing titer of 165 (SD ± 11.7, Table 1). Control sera from mice immunized with A/TMV or from nonimmunized mice showed no neutralizing activity (Table 1). Upon i.m. challenge with a lethal dose (10 IMLD50) of CVS-24 strain rabies virus at 14 days after the third immunization, all control mice (nonimmune and mice immunized with A/TMV) developed the clinical signs of rabies and succumbed to infection between days 6 and 7 after challenge. All of the CPDrg24-immunized mice remained alive at days 6 to 7. Forty percent of CPDrg24-immunized mice survived and never developed the clinical signs of rabies. Sixty percent of CPDrg24-immunized mice died between days 11 and 15 after challenge, significantly later than the sham-immunized mice. Possibly, the serum antibody levels in these mice were not high enough to protect them against the challenge virus. These results support the notion that plant viruses can be developed as a system for the production and delivery of vaccine antigens.
Figure 1.
Rabies virus-specific serum antibody (IgG) response of mice immunized i.p. with CPDrg24. Serum antibody responses were measured by ELISA on plates coated with ERA strain rabies virus. Bars represent mean values obtained using preimmune and sera after each inoculation of antigen. A serum dilution of 1:160 was used. CPDrg24–1, -2, and -3 indicate the serum antibody response observed after the first, second, and third immunizations, respectively. A/TMV-1, -2, and -3 represent rabies-specific serum antibody responses from control-immunized mice (AIMV plus TMV).
Table 1.
Neutralization titers of sera from mice immunized i.p. with CPDrg24 and challenge infection of these mice with CVS-24 strain rabies virus
| Groups of mice | Neutralization titers (mean titer) | Challenge with CVS-24 strain rabies virus
|
|||
|---|---|---|---|---|---|
| Mortality days after challenge
|
Survival | ||||
| 6–7 | 10–11 | 15–16 | |||
| Mice immunized with CPDrg24 | 165 ± 11.7 | 0/10 | 5/10 | 6/10 | 4/10 |
| Mice immunized with A/TMV | 0 | 10/10 | 0 | 0 | 0 |
| Nonimmune mice | 0 | 10/10 | 0 | 0 | 0 |
Oral Administration of Vaccine Antigens.
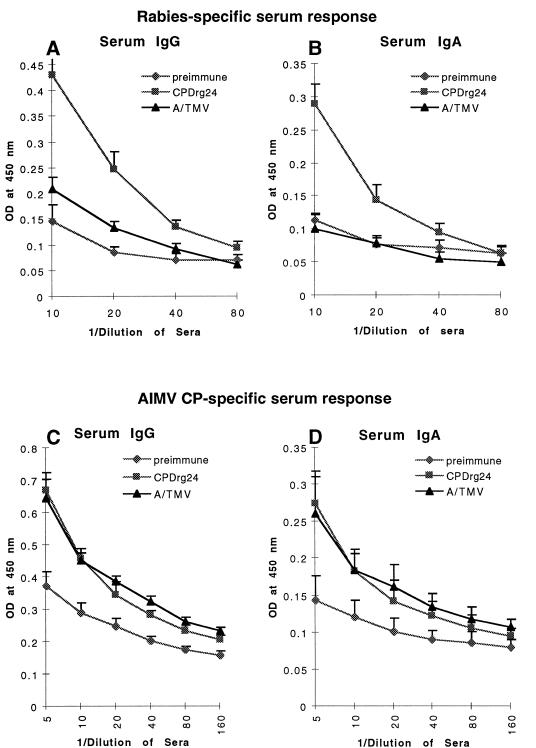
Plant viral CP can self-assemble into particles displaying antigenic peptides, generating a structure that may be valuable for oral or i.n. immunization to stimulate mucosal and systemic immunity. Indeed, it was recently shown that immune responses to polypeptides were significantly enhanced upon their delivery as particulate vaccines (32, 33). To determine whether the plant virus particles containing antigen will survive in the gastrointestinal tract and stimulate mucosal and systemic immunity, mice were immunized by gastric intubation four times with recombinant AIMV particles consisting of CPDrg24. Serum samples obtained after the fourth immunization were assessed by ELISA for the presence of IgG and IgA specific for both rabies virus and carrier protein (AIMV CP). The rabies-specific serum IgG and IgA responses of mice immunized orally with CPDrg24 were 2-fold higher (P = 0.015) than background levels seen with preimmune and control sera (Fig. 2 A and B). The serum IgG and IgA responses specific for AIMV CP were similar both in mice immunized with CPDrg24 and control mice that received A/TMV (Fig. 2 C and D), whereas the titers of both IgG and IgA in preimmune sera were 1.8-fold (P = 0.01) lower. Together these results suggest that the rabies virus epitope was effectively displayed on the surface of engineered AIMV particles. Moreover, the results of gastric intubation of CPDrg24 suggest that the virus particles survived in the gastrointestinal tract and stimulated specific immune responses.
Figure 2.
Rabies- and AIMV-specific serum antibody (IgG and IgA) response of mice immunized with CPDrg24 orally by gastric intubation. Rabies-specific and AIMV-specific serum antibody responses were measured by ELISA on plates coated with a synthetic peptide resembling the linear epitope (G5–24) of rabies virus glycoprotein and on plates coated with the AIMV CP, respectively. Data represent averages obtained using preimmune and sera after the last (fourth) administration of antigen. (A and B) Serum IgG and IgA response specific for the rabies, respectively; (C and D) Serum IgG and IgA response specific for the carrier molecule AIMV CP.
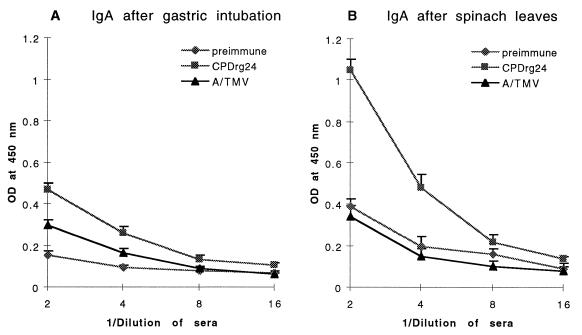
Mucosal rabies-specific responses were compared in mice receiving AIMV CPDrg24 by gastric intubation and mice that were fed spinach leaf tissue containing CPDrg24 through infection with chimeric TMV (7). Gastric intubation of CPDrg24 elicited a rabies-specific IgA response that was 1.6-fold higher (P = 0.014) than background from supernatants of control (A/TMV) fecal pellets (Fig. 3A). In contrast to gastric intubation, the level of rabies-specific IgA detected in supernatants of fecal pellets from mice fed CPDrg24-infected spinach leaves was 2.8-fold higher (P = 0.01) than the background seen with control fecal pellets (A/TMV; Fig. 3B). Mice consumed an estimated 25 μg of antigen per dose by feeding on spinach leaves, which is 10-fold less than the amount of antigen administered by gastric intubation (250 μg per dose). The higher levels of immune response generated by the leaf-feeding approach as compared with gastric intubation raises the possibility that the plant cells enhanced the delivery of virus particles to the sites of immune responses. However, we do not exclude the possibility that the low dose of antigen (25 μg) given to mice during spinach feeding may have stimulated elevated IgA synthesis. An important advantage of producing vaccine antigens in plants is the possible use of plants as a delivery vehicle for oral immunizations. In contrast to a high level of rabies-specific IgA, observed in fecal pellets of mice fed CPDrg24-containing spinach leaves, the serum IgG and IgA responses of these mice were weak (results not shown).
Figure 3.
Rabies virus-specific IgA response in supernatants of fecal pellets from mice immunized with CPDrg24 orally by (A) gastric intubation or (B) by feeding on fresh spinach leaves. Rabies-specific antibody responses were measured by ELISA on plates coated with synthetic peptide resembling the linear epitope of rabies virus glycoprotein. Data represent mean values obtained using preimmune and supernatants of fecal pellets after the last (fourth) administration of the antigen.
Protection of Orally Immunized Animals Against CVS-F3 Challenge.
Fourteen days after the last oral immunization (by gastric intubation or by feeding), mice were infected i.n. with attenuated CVS-F3 strain rabies virus. All infected mice from both groups showed signs of disease (rough fur) and in average 23% loss of body weight (Fig. 4). However, CPDrg24-immunized mice began to gain body weight 2 days earlier than the control mice. By day 20, immunized mice regained 90–95% (P = 0.015) of their original weight in contrast to 79–81% of control (Fig. 4). These results suggest further that oral immunization of mice with virus particles, especially as a part of food, generates local and systemic immune responses against rabies virus. It should be noted that generation of the observed immune responses did not require use of adjuvants. These data support the potential of plants as oral delivery vehicles and plant-produced antigens as vaccine material.
Figure 4.
Weight loss in mice immunized orally with CPDrg24 and challenged with CVS-F3 strain rabies virus. Data represent mean percent daily weight loss in mice (10 per group) immunized with CPDrg24 or control (A/TMV). Percent daily weight loss of mice immunized by (A) gastric intubation and (B) by feeding on spinach leaves.
Acknowledgments
We thank Shannon Cox for technical help. This work was funded by a grant from the Commonwealth of Pennsylvania.
ABBREVIATIONS
- AIMV
alfalfa mosiac virus
- TMV
tobacco mosiac virus
- CP
coat protein
- A/TMV
AIMV plus 30BRz
References
- 1.Hamamoto H, Sugiyama Y, Nakagawa N, Hashida E, Matsunaga Y, Takemoto S, Watanabe Y, Okada Y. Bio/Technology. 1993;11:930–932. doi: 10.1038/nbt0893-930. [DOI] [PubMed] [Google Scholar]
- 2.Usha R, Rohll J B, Spall V E, Shanks M, Maule A J, Johnson J E, Lomonossoff G P. Virology. 1993;197:366–374. doi: 10.1006/viro.1993.1598. [DOI] [PubMed] [Google Scholar]
- 3.Porta C, Spall V E, Loveland J, Johnson J E, Barker P J, Lomonossoff G P. Virology. 1994;202:949–955. doi: 10.1006/viro.1994.1417. [DOI] [PubMed] [Google Scholar]
- 4.Fitchen J, Beachy R N, Hein M B. Vaccine. 1995;13:1051–1057. doi: 10.1016/0264-410x(95)00075-c. [DOI] [PubMed] [Google Scholar]
- 5.McLain L, Porta C, Lomonossoff G P, Durrani Z, Dimmock N J. AIDS Res Hum Retroviruses. 1995;11:327–334. doi: 10.1089/aid.1995.11.327. [DOI] [PubMed] [Google Scholar]
- 6.Turpen T H, Reini S J, Charoenvit Y, Hoffman S L, Fallarme V, Grill L K. Bio/Technology. 1995;13:53–57. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- 7.Yusibov V, Modelska A, Steplewski K, Agadjanyan M, Weiner D, Hooper C, Koprowski H. Proc Natl Acad Sci USA. 1997;94:5784–5788. doi: 10.1073/pnas.94.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joelson T, Akerblom L, Oxelfelt P, Strandberg B, Tomenius K, Morris T. J Gen Virol. 1997;78:1213–1217. doi: 10.1099/0022-1317-78-6-1213. [DOI] [PubMed] [Google Scholar]
- 9.Dalsgard K, Uttenthal A, Jones T. Bio/Technology. 1997;15:248–252. [Google Scholar]
- 10.Sabin A, Boulger L. J Biol Stand. 1973;1:115. [Google Scholar]
- 11.Ada G. Semin Virol. 1990;1:3. [Google Scholar]
- 12.Blancou J, Kieny M, Lathe R, Lecocq J, Pastoret P, Soulebot J, Desmettre P. Nature (London) 1986;322:373. doi: 10.1038/322373a0. [DOI] [PubMed] [Google Scholar]
- 13.Rupprecht C, Wiktor T, Johnston D, Hamir A, Dietzschold B, Wunner W, Glicman L, Koprowski H. Proc Natl Acad Sci USA. 1986;83:7949–7950. doi: 10.1073/pnas.83.20.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Z, Rupprecht C, Dietzschold B, Saikumar P, Niu H, Babka I, Wunner W, Koprowski H. Vaccine. 1993;11:925–927. doi: 10.1016/0264-410x(93)90379-c. [DOI] [PubMed] [Google Scholar]
- 15.Dietzschold B, Gore M, Marchadier D, Niu H-S, Bunschoten H M, Otvos L, Wunner W H, Ertl H C J, Osterhaus A D M E, Koprowski H. J Virol. 1990;64:3804–3809. doi: 10.1128/jvi.64.8.3804-3809.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce N, Koster F. J Immunol. 1980;124:307–311. [PubMed] [Google Scholar]
- 17.Mestecky J. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 18.Kanesaki T, Murphy B, Collins P, Ogra P. J Virol. 1991;65:657–663. doi: 10.1128/jvi.65.2.657-663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGhee J, Mestecky J, Dertzbaugh M, Eldridge J, Hirasawa M, Kiyono H. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 20.Oien N, Brideau R, Walsh E, Wathen M. Vaccine. 1994;12:731–735. doi: 10.1016/0264-410x(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 21.Wu H-Y, Nahm M, Guo Y, Russel M, Briles D. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 22.Aldovini A, Young R. Nature (London) 1991;351:479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- 23.Fairweather N, Lyness V, Maskell D. Infect Immunol. 1987;55:2541–2934. doi: 10.1128/iai.55.11.2541-2545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairweather N, Chatfield S, Charles I, Roberts M, Lipscombe M, Li L, Strugnell D, Comerford S, Tite J, Dougan G. Res Microbiol. 1990;141:769–773. doi: 10.1016/0923-2508(90)90109-4. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood J, Willis A E, Perham R N. J Mol Biol. 1991;220:821–827. doi: 10.1016/0022-2836(91)90354-9. [DOI] [PubMed] [Google Scholar]
- 26.Mastico R A, Talbot S J, Stockley P G. J Gen Virol. 1993;74:541–548. doi: 10.1099/0022-1317-74-4-541. [DOI] [PubMed] [Google Scholar]
- 27.Burke K L, Dunn G, Ferguson M, Minor P D, Almond J W. Nature (London) 1988;332:81–82. doi: 10.1038/332081a0. [DOI] [PubMed] [Google Scholar]
- 28.Dedieu J F, Ronco J, van der Werf S, Hogle J M, Henin Y, Girard M. J Virol. 1992;66:3161–3167. doi: 10.1128/jvi.66.5.3161-3167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold G F, Resnick D A, Li Y, Zhang A, Smith A D, Geisler S C, Jacobo-Molina A, Lee W, Webster R G, Arnold E. Virology. 1994;198:703–708. doi: 10.1006/viro.1994.1082. [DOI] [PubMed] [Google Scholar]
- 30.Mason H S, Lam D M-K, Arntzen C J. Proc Natl Acad Sci USA. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haq T A, Mason H, Clements J D, Arntzen C J. Science. 1995;268:714–716. doi: 10.1126/science.7732379. [DOI] [PubMed] [Google Scholar]
- 32.McGarvey P B, Hammond J, Dienelt M M, Hooper D C, Fu Z F, Dietzschold B, Koprowski H, Michaels F H. Bio/Technology. 1995;13:1484–1487. doi: 10.1038/nbt1295-1484. [DOI] [PubMed] [Google Scholar]
- 33.Mason H S, Ball J M, Shi J J, Jiang X, Estes M K, Arntzen C J. Proc Natl Acad Sci USA. 1996;93:5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox D, Taubman M. Int Arch Allergy Appl Immunol. 1984;73:394. doi: 10.1159/000233602. [DOI] [PubMed] [Google Scholar]
- 35.Wold A, Dahlgren U, Hanson L, Mattsby-Baltzer I, Mionverdt T. Infect Immunol. 1989;57:2666–2672. doi: 10.1128/iai.57.9.2666-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiktor T J, Macfarlan R I, Foggin C M, Koprowski H. Dev Biol. 1984;57:199–211. [PubMed] [Google Scholar]