Abstract
The function of the human T-cell leukemia virus (HTLV) Rex phosphoprotein is to increase the level of the viral structural and enzymatic gene products expressed from the incompletely spliced viral RNAs containing the Rex-responsive element. The phosphorylation of HTLV type 2 Rex (Rex-2), predominantly on serine residues, correlates with an altered conformation, as detected by a gel mobility shift, and is required for specific binding to its viral RNA target sequence. Thus, the phosphorylation state of Rex in the infected cell may be a switch that determines whether the virus exists in a latent or a productive state. A mutational analysis of Rex-2 that focused on serine and threonine residues was performed to identify regions or domains within Rex-2 important for function, with a specific emphasis on identifying Rex-2 phosphorylation mutants. We identified mutations near the carboxy terminus that disrupted a novel region or domain and abrogated Rex-2 function. Mutant M17 (with S151A and S153A mutations) displayed reduced phosphorylation that correlated with reduced function. Replacement of both serine residues 151 and 153 with phosphomimetic aspartic acid restored Rex-2 function and locked Rex-2 in a phosphorylated active conformation. A mutant containing threonine residues at positions 151 and 153 displayed a phenotype indistinguishable from that of wild-type Rex. Furthermore, this same mutant showed increased threonine phosphorylation and decreased serine phosphorylation, providing conclusive evidence that one or both of these residues are phosphorylated in vivo. Our results provide the first direct evidence that the phosphorylation of Rex-2 is important for function. Further understanding of HTLV Rex phosphorylation will provide insight into the regulatory control of HTLV replication and ultimately the pathobiology of HTLV.
Human T-cell leukemia virus (HTLV) types 1 (HTLV-1) and 2 (HTLV-2) are complex retroviruses that have been causally associated with leukemia and neurological disorders in humans (21). In addition to structural and enzymatic genes gag, pol, and env, HTLV encodes two trans-acting regulatory gene products, Tax and Rex, both of which are essential for viral replication (15, 17, 24). Tax acts to increase the rate of transcription from the viral long terminal repeat (LTR). In addition, Tax modulates the transcription of various cellular genes involved in growth and differentiation and disrupts cell cycle control and DNA repair processes (3, 4, 38, 45, 47). These pleiotropic effects of Tax on cellular processes are likely important in the ability of HTLV to mediate oncogenesis. Consistent with this hypothesis, Tax was recently shown to be essential for cellular transformation of primary human T lymphocytes in cultures (39, 41).
Rex, the other trans-acting regulatory protein, specifically binds unspliced and singly spliced viral RNAs, resulting in RNA stabilization and their selective trafficking to the cytoplasm (6, 30). These RNAs encode viral structural and enzymatic gene products. Rex function is mediated by a cis-acting RNA sequence, termed the Rex-responsive element (RxRE), located in the R region of the viral LTR (6, 9, 13, 20, 27, 35). Several functional domains of Rex have been identified to date. A highly basic amino acid sequence at the amino terminus of HTLV-1 Rex (Rex-1) and HTLV-2 Rex (Rex-2) is required for RNA binding and subcellular targeting to the nucleus or nucleolus (25, 34, 43). Both Rex-1 and Rex-2 have a leucine-rich activation domain that is required for interaction with cellular factors, including the human nucleoporin-like Rev/Rex activation domain binding protein (Rab) and human Rev interactive protein (hRIP) cofactors involved in nuclear export (18, 33). Recently, this activation domain was also shown to encompass a nuclear export signal (NES) and to be indispensable for Rex function (37). Mutational and chemical analyses of Rex-1 and the functionally analogous human immunodeficiency virus (HIV) type 1 (HIV-1) Rev implicated a third domain, responsible for multimerization of these proteins. Although the exact nature of multimerization is debatable, it has been suggested that multimerization is critical for Rex and HIV-1 Rev function (12, 26, 31, 36).
Two forms of Rex-2, p24rex and p26rex, are detected in HTLV-2-infected T cells (40, 42). Both species of Rex-2 have also been detected in cell lines transfected with rex expression plasmids; these include 729 human B cells, SF9 insect cells, COS cells, and JM4 human T cells (22–24, 40, 49). A previous study indicated that p24rex and p26rex share the same amino acid backbone and that they differ in the extent of serine phosphorylation (23). Thus, p26rex is the result of an altered conformation induced by the phosphorylation of a subset of serine residues. Similarly, Rex-1 is phosphorylated on serine, but phosphorylation does not result in significant altered gel mobility (1). Immunofluorescence studies have shown that p24rex is present only in the cytoplasm, whereas the phosphorylated form, p26rex, is predominantly localized in the nucleus and nucleolus (16). Biochemical studies have indicated that the phosphorylated form of Rex-2, p26rex, binds RxRE-containing RNA and thus is biologically active (24). Interestingly, Tax has been shown to be phosphorylated on a serine residue(s). One study suggested that the phosphorylation of either serine 300 or serine 301 is required for localization to nuclear bodies and Tax-mediated activation of gene expression (8). Another study identified serine-to-alanine mutations important for Tax transactivation function, but these serine residues were not phosphorylated (11). The precise role of phosphorylation in the regulation of either Rex or Tax function has not yet been determined.
In this study, a mutational analysis of Rex-2 targeting serine and threonine residues was performed to identify regions or domains within Rex-2 important for function, with a specific emphasis on identifying Rex-2 phosphorylation mutants. In addition to identifying mutations that disrupt the RNA binding-nuclear localization domain, the activation-effector-NES domain, and the multimerization domain, we identified a novel region or domain at the carboxy terminus that is important for Rex-2 function. One of the C-terminal mutants (with S151A and S153A mutations [S151A,S153A]) displayed a reduction in the p26 phosphorylated conformation of Rex that also correlated with reduced Rex function. Replacement of both serine residues 151 and 153 with aspartic acid locked Rex-2 in a phosphorylated active state and restored Rex-2 function. Two-dimensional (2D) phosphoamino acid analysis of a Rex-2 mutant containing threonine residues at positions 151 and 153 revealed decreased serine phosphorylation in conjunction with increased threonine phosphorylation, providing further evidence of in vivo phosphorylation of these residues. Our data provide the first direct evidence that the phosphorylation of critical residues at the carboxy terminus of Rex-2 is critical for function. Further understanding of HTLV Rex phosphorylation will provide insight into the regulatory control of HTLV replication and ultimately HTLV pathogenesis.
MATERIALS AND METHODS
Cells.
293 T cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented to contain 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine.
Plasmids.
Rex expression vector BC20.2, containing HTLV-2 tax/rex cDNA expressed from the cytomegalovirus (CMV) immediate-early gene promoter, has been described earlier (14, 23). Various rex mutants were generated by site-directed mutagenesis (with Quick Change; Stratagene) using BC20.2 as a template. Mutations were confirmed by dideoxy DNA sequencing. HIV-1 Tat expression vector pctat contains HIV-1 tat cDNA cloned downstream of the CMV promoter. Reporter pCgagRxRE-II (a kind gift from Vincenzo Ciminale, University of Padua, Padua, Italy) contains the HIV-1 LTR promoter and gag gene linked to a 445-bp fragment of HTLV-2 spanning the RxRE (nucleotides 316 to 760 of the R-U5 region) (16). A CMV-luciferase plasmid was used to control for transfection efficiency in each experiment (luciferase assay system; Promega).
Transfection and p24gag enzyme-linked immunosorbent assay.
Wild-type rex or various rex mutant expression plasmids were introduced into 293 T cells using the calcium phosphate transfection protocol. Briefly, 2 × 105 cells were transfected with 1 μg of pctat, 3 μg of pCgagRxRE-II, 1 μg of CMV-luciferase plasmid, and 5 μg of wild-type rex or various rex mutant expression plasmids or negative control. Cell lysates were made 48 h posttransfection using lysis buffer, containing 100 mM Tris (pH 7.6) and 0.5% Triton X-100. Luciferase activity for each sample was determined to control for transfection efficiency. HIV-1 p24gag levels in cell lysates were determined using a p24gag enzyme-linked immunosorbent assay (p24 HIV antigen assay kit; Beckman-Coulter). p24gag calibration curves were generated using HIV-1 p24 antigen standards as described by the kit manufacturer; the detection sensitivity was 1 pg/ml. All the experiments were performed in triplicate and normalized for transfection efficiency. Statistical significance relative to results for wild-type Rex was determined by the Student t test.
Metabolic labeling and immunoprecipitation.
Twenty-five micrograms of wild-type rex or various rex mutant expression plasmids or negative control was electroporated into 5 × 106 293 T cells (975 μF and 250 V). Cells were metabolically labeled 24 h posttransfection with [35S]methionine-[35S]cysteine (Trans-35S-label, 100 mCi/ml; Amersham) in methionine-cysteine-free RPMI 1640 supplemented with 20% dialyzed fetal calf serum. Cells were lysed in immunoprecipitation buffer (0.05 M Tris [pH 8.0], 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 0.15 M NaCl, 2 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 1 μg of pepstatin A/ml), and the lysates were clarified by centrifugation at 17,000 × g for 30 min at 4°C. The lysates were subsequently immunoprecipitated using anti-Rex antiserum (directed against C-terminal amino acid residues 139 to 170) for 16 h at 4°C. Protein A–Sepharose CL-4B (Sigma) was used to collect the immune complexes. Proteins were directly electrophoresed on an SDS–10% polyacrylamide gel; alternatively, for in vitro dephosphorylation experiments, prior to electrophoresis the immune complexes were incubated with 600 U of bacterial alkaline phosphatase (BAP) (GIBCO BRL) for 3 h at 65°C in 50 μl of BAP buffer, containing 50 mM NaCl, 10 mM Tris-Cl (pH 8.0), and 10 mM MgCl2. 35S-labeled proteins were visualized by autoradiography and quantified by PhosphorImager analysis (Molecular Dynamics).
2D phosphoamino acid analysis.
293 T cells (5 × 106) were electroporated with 25 μg of wild-type rex expression plasmid BC20.2 or rex mutant S151T,S153T at 975 μF and 250 V. At 24 h posttransfection, cells were metabolically labeled with 2 mCi of 32Pi/ml, and cell lysates were prepared as described above. p26rex phosphorylated in vivo was resolved on an SDS–10% polyacrylamide gel, transferred to an Immobilon-P membrane (Millipore), and detected by autoradiography. Samples were prepared and 2D phosphoamino acid analysis was performed as previously described. Briefly, membrane pieces containing phosphorylated p26rex were incubated in 0.5% polyvinylpyrrolidone in 100 mM acetic acid for 30 min at 37°C. The membrane was then washed three times in H2O and subjected to hydrolysis using 5.7 M HCl at 110°C for 60 min. The supernatant containing the hydrolyzed amino acids was centrifuged at 17,000 × g for 10 min, washed by repeated lyophilization, and subjected to 2D phosphoamino acid analysis using a thin-layer electrophoresis system (HTLE-7000; CBS Scientific, Del Mar, Calif.). The hydrolyzed amino acids were applied to thin-layer cellulose plates along with phosphorylated Ser (p-Ser), p-Thr, and a p-Tyr standards at 0.5 μg each per μl; electrophoresed at pH 1.9 for 25 min at 1.0 kV (∼13 mA) in the first dimension; and then subjected to electrophoresis in the second dimension at pH 3.5 for 20 min at 1.5 V (∼16 mA). Amino acid standards were visualized by staining with 0.25% ninhydrin in acetone, and the radiolabeled amino acids were visualized by direct autoradiography and quantified by PhosphorImager analysis.
RESULTS
Generation of Rex-2 mutants.
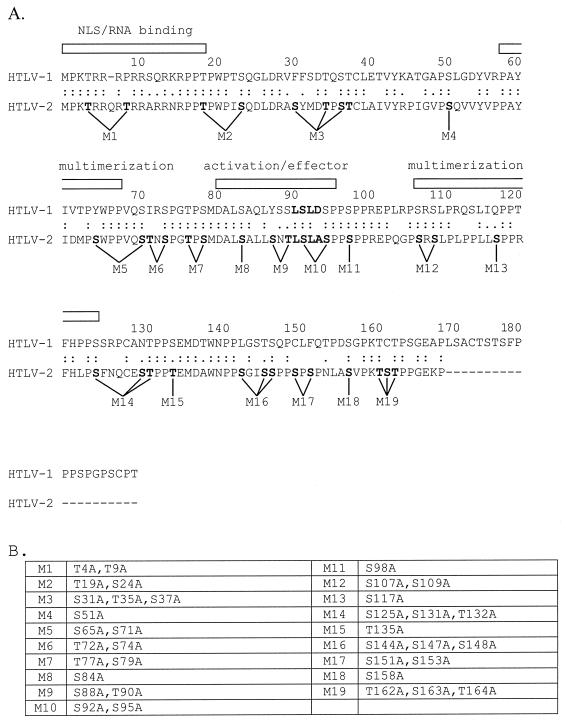
The phosphorylation of Rex-2 primarily on serine residues has been shown to be critical for functional RNA binding (23, 24). The 170-amino-acid Rex-2 protein contains 25 serine and 12 threonine residues that are potential phosphorylation sites. Our objective was to introduce mutations throughout the wild-type rex-2 expression plasmid, BC20.2, which would cause the substitution of alanine for serine or threonine residues. Based on previous reports and on alignment with Rex-1, several of our mutations were predicted to occur in the RNA binding region or nuclear localization domain (amino acids 1 to 19), activation-effector domain containing the overlapping NES (amino acids 81 to 94), and multimerization domain (amino acids 57 to 66 and 106 to 124). In all, 19 Rex-2 mutants were generated consisting of 6 single amino acid substitutions, 9 double amino acid substitutions, and 4 triple amino acid changes. Figure 1A shows the alignment of Rex-1 and Rex-2 and the previously identified domains. Figure 1B shows the predicted amino acid substitutions in our panel of mutants.
FIG. 1.
(A) Amino acid alignment of Rex-1 (top) and Rex-2 (bottom). Identities are indicated by double dots, and similarities are indicated by single dots. Rex-2 is 19 amino acid residues shorter than Rex-1. Rex-1 domains, including the nuclear localization (NLS)-RNA binding (25, 34, 43), multimerization, and activation-effector domains, are indicated (12, 25, 31, 34, 43). The locations of the Rex-2 mutants are indicated. (B) Summary of all mutations (M1 to M19).
Expression of Rex-2 mutants.
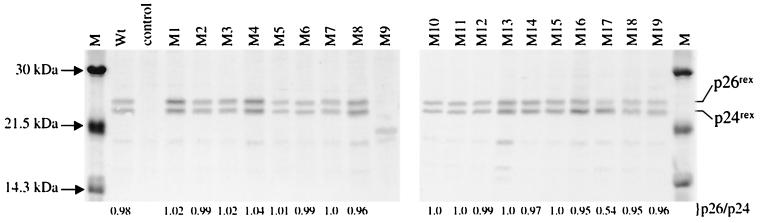
The stable expression of the various Rex-2 mutants from metabolically labeled 293 T cells was determined by radioimmunoprecipitation and SDS-polyacrylamide gel electrophoresis (PAGE) using anti-Rex-2 peptide antiserum. As expected, in cells transfected with our wild-type rex-2 expression construct, the p24rex form and the phosphorylated p26rex conformation were detected (Fig. 2). All of the Rex-2 mutants appeared to be stably expressed. With the exception of M9, all of the mutants displayed both forms of the Rex protein. To determine if there were significant differences in the levels of p26rex and p24rex, we quantitated their amounts over four independent experiments; the results are summarized as an average p26rex/p24rex ratio below each lane of Fig. 2. The majority of the mutants displayed ratios similar to that of wild-type Rex (a p26rex/p24rex ratio of nearly 1). Interestingly, a mutant with a mutation at the carboxy terminus, M17 (S151A,S153A), showed a significant reduction in the amount of p26rex in multiple experiments, with an average p26rex/p24rex ratio of 0.54 (Fig. 2, lane M17; see also Fig. 4 and 7B). These results suggest that residues 151 and/or 153 might be important for protein modification and/or conformation.
FIG. 2.
Detection of Rex-2 mutants in 293 T cells. 293 T cells were transfected with 25 μg of wild-type (Wt) or various rex mutant expression vectors or vector alone (control). At 24 h posttransfection, cells were metabolically labeled using [35S]methionine-[35S]cysteine; cell lysates were made as described in Materials and Methods. Lysates were immunoprecipitated using anti-Rex antiserum in the presence of protein A-Sepharose. Immunoprecipitated proteins were fractionated by SDS-PAGE and visualized by autoradiography. 14C low-molecular-mass markers are indicated on the left (lane M). The results indicate that all of the Rex mutants are efficiently expressed in 293 T cells. p26rex and p24rex were quantitated by PhosphorImager analysis over four independent experiments, and values were averaged. The p26rex/p24rex ratio is presented below each lane. (Note that M17 displays a significant reproducible reduction in the level of p26rex.)
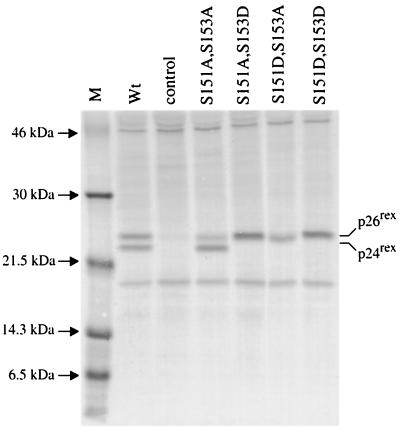
FIG. 4.
Detection of Rex-2 phosphorylation mutants in 293 T cells. Transfection, immunoprecipitation, and SDS-PAGE analysis were performed as described in the legend to Fig. 2. Wild-type (Wt) Rex, various Rex mutants, and the control are indicated. The data indicate that serines 151 and 153 are required for the phosphorylation-induced mobility shift from p24rex to p26rex and that replacement of either one or both serines 151 and 153 with aspartic acid locks the protein in a phosphorylated conformation. Lane M, markers.
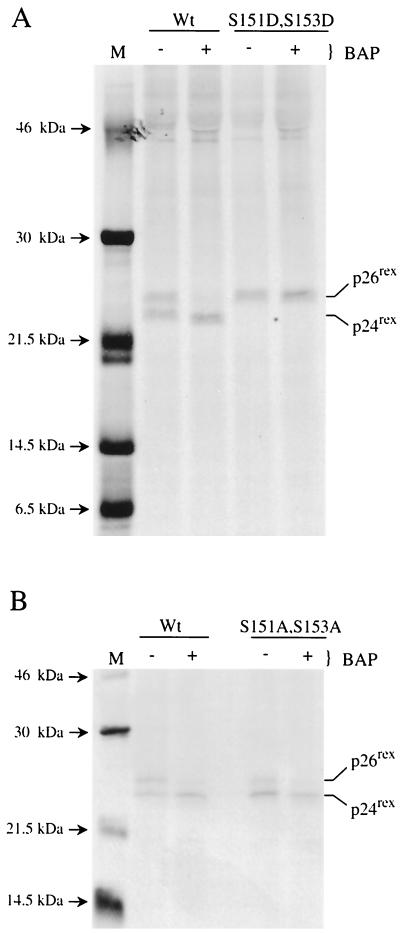
FIG. 7.
In vitro dephosphorylation of wild-type Rex-2, the S151D,S153D mutant, and mutant M17. (A) 293 T cells were transfected with 25 μg of wild-type (Wt) or rex mutant (S151D,S153D) expression vector. At 24 h posttransfection, cells were metabolically labeled using [35S]methionine-[35S]cysteine, and cell lysates were made as described in Materials and Methods. Transfected cell lysates were immunoprecipitated using anti-Rex-antiserum. Immune complexes were collected using protein A-Sepharose and incubated with 600 U of BAP for 3 h or mock treated, and the proteins were resolved by SDS-PAGE and visualized by autoradiography. 14C low-molecular-mass markers are indicated on the left (lane M). The results indicate that the phosphorylation-induced change in mobility on the SDS gel is due to the presence of aspartic acid residues at positions 151 and 153. (B) An experiment was performed as described for panel A to compare Wt Rex to mutant M17. The results indicate that the p26rex conformation of mutant M17 is the result of phosphorylation.
Rex-2 mutants segregate into four functional regions or domains.
Our series of Rex-2 mutants were tested for their ability to function in a quantitative bioassay using reporter plasmid pCgagRxRE-II. This plasmid contains the HIV-1 LTR and gag gene linked to the RxRE of HTLV-2 and the simian virus 40 polyadenylation signal or site (16). Efficient expression of Gag is dependent on Tat-mediated transcription and functional Rex binding to RxRE sequences. 293 T cells were cotransfected with pctat, pCgagRxRE-II, and wild-type or mutant rex expression vectors or negative control, and p24gag production was monitored using a Gag antigen capture assay. To test the sensitivity of this system, we performed a titration with increasing concentrations of rex expression plasmid, keeping the concentrations of pctat and pCgagRxRE II constant. We did not see any significant difference in p24gag values at concentrations between 1 and 5 μg of rex plasmid (data not shown). Therefore, all the subsequent experiments were performed with 5 μg of rex expression plasmid (saturating concentration of rex expression plasmid).
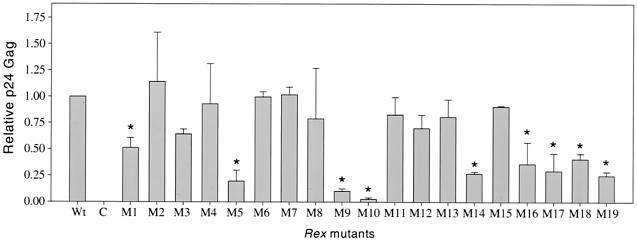
Our analysis revealed functionally deficient Rex-2 mutants clustering in four regions or domains (Fig. 3). Mutant M1, with mutations within the amino-terminal RNA binding-nuclear localization domain, showed p24gag levels 50% those in our reporter assay. Mutants M9 and M10, with mutations within the effector-activation domain (also containing the overlapping NES), showed drastic reductions in p24gag production compared to wild-type Rex-2. Previous studies have shown that the activation domain or effector domain is required for various protein-protein interactions and is indispensable for Rex-1 and Rex-2 function (18, 37). Mutants M5 and M14 exhibited significant reductions in p24gag levels compared to wild-type Rex-2. Amino acid alignment of Rex-1 and Rex-2 suggested that these mutations are within the multimerization domain identified for Rex-1. Previous work has suggested that multimerization is required for Rex-1 function (12, 48). Therefore, it would appear that a similar domain is present in Rex-2 and that multimerization is required for function.
FIG. 3.
Rex-2 functional assay. 293 T cells (2 × 105) were transfected with 1 μg of pctat, 3 μg of pCgagRxRE-II, 1 μg of luciferase, and 5 μg of wild-type (Wt) or various rex mutant expression vectors or vector alone (C). At 48 h posttransfection, cells were harvested and assayed for p24gag as described in Materials and Methods. Lysates were assayed for luciferase activity to control for transfection efficiency. The values, which represent relative p24gag levels for three independent experiments, are normalized to the value for Wt rex (set as 1). Error bars indicate standard deviations. Significance relative to the results for Wt Rex was determined by the Student t test; values that are statistically different are indicated by asterisks. The data suggest that functionally deficient Rex-2 mutants segregate into four groups.
M16, M17, M18, and M19, containing carboxy-terminal mutations, displayed approximately 25 to 40% the activity of wild-type Rex. The majority of functionally deficient mutants had p26rex/p24rex ratios similar to that of wild-type Rex, with the exception of M17. Although our results suggest a novel functional domain located at the carboxy terminus, we focused the remainder of our study on residues 151 and 153 because the reduction in M17 activity or function correlated with altered mobility or, more specifically, a reduction in the amount of phosphorylated p26rex.
Phosphomimetic substitutions at serine 151 and serine 153 lock Rex in a p26 functional state.
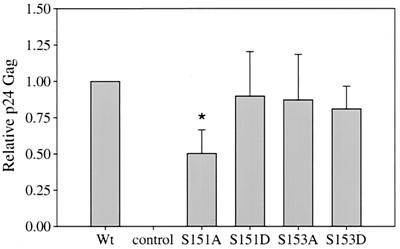
To test the hypothesis that serines 151 and 153 are residues that are phosphorylated and critical for function, the serine residues were mutated either individually or in combination to alanine and/or aspartic acid to mimic phosphoserine. We first investigated the expression and mobility of these mutants by SDS-PAGE. Interestingly, aspartic acid residues at positions 151 and/or 153 migrated as a single 26-kDa band (p26rex) with no detectable p24rex (Fig. 4). This result indicates that the replacement of serine at position 151 or 153 by aspartic acid appears to lock the protein in a phosphorylated state or conformation. We next tested the aspartic acid mutants in the Rex functional reporter assay. The results indicate that all three aspartic acid mutants are fully functional (Fig. 5). Together, these results further support the notion that the phosphorylated p26rex conformation is the active form of Rex and suggest that modification of residues 151 and/or 153 contributes to this conformation and activity.
FIG. 5.
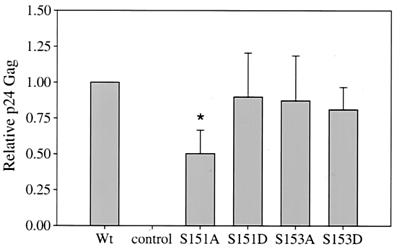
Rex-2 functional assay for phosphorylation mutants. The Rex functional assay was performed as described in the legend to Fig. 3. Wild-type (Wt) Rex, various Rex mutants, and the control are indicated. Error bars indicate standard deviations. Significance relative to the results for Wt Rex was determined by the Student t test; a value that is statistically different is indicated by an asterisk. The results suggest that serines 151 and 153 are necessary for function and that replacement of these sites with a phosphomimetic amino acid restores the activity to Wt levels.
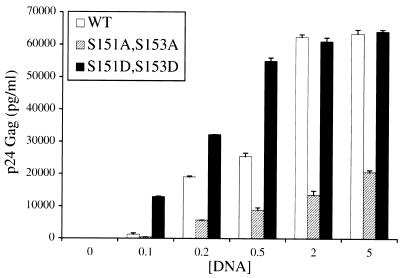
If the level of p26rex correlates with function, then one might expect that since the aspartic acid mutants with mutations at positions 151 and/or 153 are found exclusively in the phosphorylated p26rex active conformation, these mutants should show higher levels of functional activity than wild-type Rex. To test this hypothesis, the activities of wild-type Rex and the M17 (S151A,S153A) and S151D,S153D double mutants were assessed by use of the Gag reporter assay with increasing DNA concentrations of wild-type and rex mutant plasmids. Our results indicate that the activity of M17 (S151A,S153A) is approximately 25 to 30% that of wild-type Rex over a range of rex expression plasmid concentrations (0.1 to 5 μg), whereas S151D,S153D is significantly more active at the lowest concentration tested; this difference is gradually lost and disappears at higher concentrations (Fig. 6). Taken together, these results are consistent with the conclusion that the phosphorylation of serines 151 and/or 153 of Rex-2 results in a hyperphosphorylated and biologically functional Rex protein (p26rex).
FIG. 6.
The S151D,S153D mutant is more active than wild-type Rex. 293 T cells (2 × 105) were transfected with 1 μg of pctat, 3 μg of pCgagRxRE-II, 1 μg of luciferase, and increasing concentrations (0.1 to 5 μg) of wild-type (WT) or rex mutant (M17 [S151A,S153A] or S151D,S153D) expression vectors. At 48 h posttransfection, cells were harvested and assayed for p24gag as described in Materials and Methods. Lysates were assayed for luciferase activity to control for transfection efficiency. The values represent actual p24gag levels for three independent experiments. Error bars indicate standard deviations. The results indicate that at lower DNA concentrations, Rex mutant S151D,S153D (exclusively in the p26rex conformation) is significantly more active than WT Rex.
We next determined whether phosphorylation at some other site was responsible or required for maintaining the aspartic acid mutant with mutations at positions 151 and 153 exclusively in the p26rex conformation. The S151D,S153D mutant protein was subjected to BAP treatment in vitro. As previously reported (24), BAP treatment of wild-type Rex-2 resulted in the loss of p26rex and a concomitant increase in the amount of p24rex (Fig. 7A). However, BAP treatment had no effect on the mobility of the S151D,S153D p26rex mutant (Fig. 7A). These results provide further evidence that phosphomimetic aspartic acid at positions 151 and 153 locks the protein in a phosphorylated active conformation. Similar analysis of the M17 mutant (S151A,S153A) indicated that the reduced or remaining amount of p26rex was subject to loss following BAP treatment (Fig. 7B). These results indicate that the phosphorylation of an additional site(s) leads to the conformational change and further suggest that the phosphorylation of residues 151 and/or 153 likely stabilizes that conformation.
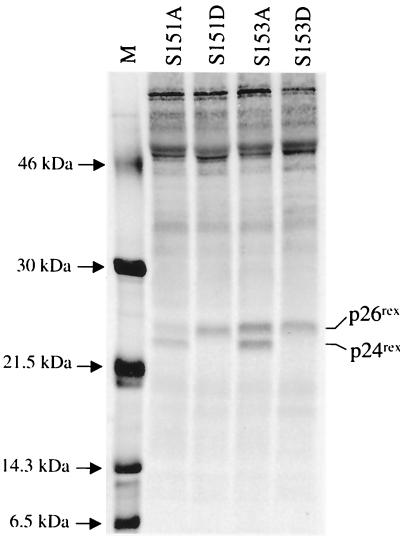
To investigate the individual contributions of serines 151 and 153 to the phosphorylation-induced conformational change and Rex-2 function, we made single amino acid changes at either position 151 or position153 by mutating the amino acid to either alanine or aspartic acid. SDS gel analysis of the S151A mutant shows a predominant p24rex form and a reduced p26rex form, similar to the results obtained with the alanine double mutant M17. The S153A mutant shows equal levels of p24rex and p26rex, similar to those seen with wild-type Rex (Fig. 8). These results suggest that serine 151 plays a greater role than serine 153 in the phosphorylation-induced mobility change on the SDS gel. Functional analyses of these mutants are consistent with the level of p26rex. S151A shows a significant reduction in activity, whereas S153A function is similar to that of wild-type Rex (Fig. 9). Interestingly, replacement of either serine 151 or serine 153 with aspartic acid is capable of locking the protein in a phosphorylated state, as measured by decreased mobility (Fig. 8). BAP treatment of both the S151D and the S153D mutants did not result in the loss of p26rex (data not shown). In addition, double mutants (S151A,S153D and S151D,S153A) are fully functional and migrate as a single p26rex species (Fig. 4 and 5). Taken together, these data indicate that aspartic acid at either or both residues 151 and 153 is sufficient to lock the protein in the p26rex conformation, consistent with a phosphorylated state. However, when tested alone, residue 151 appears to play a more significant role.
FIG. 8.
Detection of single phosphorylation mutants with mutations at residues 151 and 153 in 293 T cells. Transfection, immunoprecipitation, and SDS-PAGE analysis were performed as described in the legend to Fig. 1. The various Rex mutants analyzed are indicated. The data suggest that serine 151 is more important than serine 153 for the phosphorylation-induced mobility shift from p24rex to p26rex.
FIG. 9.
Rex-2 functional assay for single phosphorylation mutants with mutations at residues 151 and 153. The methods used are described in the legend to Fig. 3. Wild-type (Wt) Rex and the various Rex mutants analyzed are indicated. Error bars indicate standard deviations. Significance relative to the results for Wt Rex was determined by the Student t test; a value that is statistically different is indicated by an asterisk.The results suggest that serine 151 is more important than serine 153 in Rex function.
Serine 151 and serine 153 are critical phosphorylation sites.
We next directly evaluated whether residues 151 and 153 are truly phosphorylated in vivo. Earlier studies had shown that Rex-2 is phosphorylated predominantly on serine residues (23). Therefore, if these residues are phosphorylated, replacement of serines 151 and 153 with threonine should result in increased threonine phosphorylation in conjunction with decreased serine phosphorylation. SDS gel and functional analyses of the S151T,S153T mutant revealed that its mobility and functional activity are similar to those of wild-type Rex-2, suggesting that threonine at these residues can efficiently substitute for serine (data not shown).
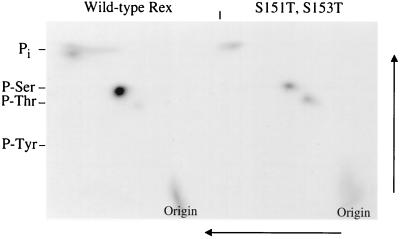
We next performed 2D phosphoamino acid analysis of the S151T,S153T mutant and wild-type Rex. We found a clear increase in threonine phosphorylation (Fig. 10). PhosphorImager analysis indicates that this increased threonine phosphorylation in the S151T,S153T mutant coincides with a 70% reduction in serine phosphorylation compared to the results for wild-type Rex. This finding provides direct evidence that residues 151 and/or 153 are phosphorylated in vivo. Interestingly, the S151T,S153T mutant still retained some serine phosphorylation, indicating that there is an additional serine(s) phosphorylated on Rex-2. This finding is consistent with the results of the BAP experiments shown in Fig. 7B, in which M17 still contained phosphorylated residues that contributed to the p24rex-to-p26rex conformational change. However, mutation of serine residues 151 and/or 153, either preventing phosphorylation or mimicking phosphoserine, has a direct effect on Rex-2 mobility or conformation and function.
FIG. 10.
2D phosphoamino acid analysis for the wild type and threonine mutants. 293 T cells were transfected with 25 μg of wild-type Rex or the Rex threonine mutant. At 24 h posttransfection, cells were metabolically labeled using 2 mCi of [32Pi], and cell lysates were made as described in Materials and Methods. Transfected cell lysates were immunoprecipitated using anti-Rex antiserum, and proteins were transferred to an Immobilon-P membrane. The band corresponding to p26rex was cut out of the membrane and digested with 5.7 M HCl. 2D phosphoamino acid analysis was done on thin-layer cellulose plates in the first dimension at pH 1.9 followed by the second dimension perpendicular to the first at pH 3.4 as indicated in Materials and Methods. The data indicate that residues 151 and/or 153 are phosphorylated in vivo.
DISCUSSION
It was previously demonstrated that phosphorylation of HTLV-2 Rex is required for efficient binding to viral target RNAs containing the RxRE (24). In this study, we subjected HTLV-2 Rex to mutational analysis to identify functional domains, with specific emphasis on identifying Rex-2 phosphorylation mutants. We identified four distinct groups of functionally deficient Rex-2 mutants. In addition to identifying mutants that disrupt the RNA binding-nuclear localization domain, the activation-effector domain (also containing the overlapping NES), and the multimerization domain, we identified a novel region or domain at the carboxy terminus that disrupts Rex-2 function. One carboxy-terminal mutant (S151A,S153A) displayed reduced phosphorylation that correlated with reduced function. Our further analysis indicated that serine residues 151 and/or 153 are phosphorylated in vivo and that this phosphorylation is likely critical to maintain Rex-2 in an active conformation required for efficient function.
It has been well documented that posttranslational modification of proteins by phosphorylation affects protein conformation and/or function. For example, a mobility change detected by SDS-PAGE analysis of the fos nuclear oncoprotein (7), polyomavirus large T antigen (10), and adenovirus E1A (44) results from phosphorylation on one or more serine residues. It has been hypothesized that this mobility shift is likely due to a change in protein structure which might be critical for protein function. Interestingly, a recent study investigating the signaling protein nitrogen regulatory protein C (NtrC) revealed that activation by phosphorylation involved stabilization of a preexisting condition or conformation. Nuclear magnetic resonance measurements indicated that phosphorylation shifted the equilibrium of NtrC far toward the active conformation (46).
A previous study indicated that p26rex is a phosphorylated form of p24rex and that phosphorylation on serine residues is critical for its ability to specifically bind RxRE-containing RNA (24). In addition, it has been shown that the phosphorylated form of Rex-2 is required to inhibit RNA splicing reactions in vitro (5). Thus, the p26rex phosphorylated form is the active form which would be required for HTLV to be in the productive phase of viral replication. In HTLV-infected cells, both phosphorylated and nonphosphorylated forms of Rex are present. We speculate that the concentration of phosphorylated Rex is dependent on environmental stimuli and the balance of highly regulated cellular kinase and phosphatase activities.
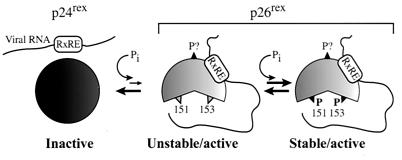
We propose a model for the role of phosphorylation and function of Rex-2 consistent with the current literature and our experimental data (Fig. 11). The initial Rex translation product, p24rex, found only in the cytoplasm, is phosphorylated at a currently unidentified serine(s), resulting in an active p26rex unstable intermediate. Several lines of evidence indicate that Rex is phosphorylated on at least one residue in addition to serines 151 and/or 153. First, tryptic digestion of in vivo 32Pi-labeled p26rex revealed multiple 32Pi-incorporating peptides (24). However, the functional relevance of these phosphorylation sites has not been elucidated. Second, in this report we show by 2D phosphoamino acid analysis that the threonine mutant (S151T,S153T) still contains significant levels of serine phosphorylation. Last, a reduced amount of p26rex is still detected in the S151A,S153A mutant and shows 30 to 40% the functional activity of wild-type Rex. We propose that the p26rex intermediate is unstable and is subject to dephosphorylation (returning to the p24rex conformation) or subsequent phosphorylation at serines 151 and/or 153, which is required for the optimal function of Rex-2. This further phosphorylation at residues 151 and/or 153 may stabilize the p26rex conformation, possibly rendering it more resistant to phosphatases and dephosphorylation. Alanine substitutions at positions 151 and/or 153 (reduced p26rex) likely result in the destabilization of p26rex, since it cannot be further phosphorylated and thus is more sensitive to dephosphorylation, pushing the equilibrium toward the inactive p24rex form. The replacement of serines 151 and/or 153 with phosphomimetic aspartic acid locks the protein in the p26rex conformation, consistent with an irreversible equilibrium shift toward the stable and active p26rex form. Thus far, we have not been able to identify another phosphorylation site(s) critical for function. However, one possible candidate is serine 88. Our results indicate that mutant M9 (S88A,T90A) is nonfunctional and migrates as a single unphosphorylated protein (Fig. 2, lane M9, and data not shown).
FIG. 11.
Model for Rex-2 phosphorylation and function. p24rex is the primary Rex-2 translation product that is initially phosphorylated on an as-yet-unidentified serine(s), resulting in an unstable, but functional p26rex intermediate. This intermediate can now be subsequently phosphorylated on serines 151 and/or 153, resulting in a functional, stable p26rex form.
There are several reports investigating the role of phosphorylation in the function of the HTLV Rex protein or the functionally analogous HIV Rev protein. Recently, Fouts et al. demonstrated that phosphorylation is required for recombinant HIV-1 Rev protein to enter rapidly into an efficient RNA binding state (19). The investigators also mapped the phosphorylation sites (Ser-54 and Ser-56) to be within the multimerization domain and showed that phosphorylation does not affect multimerization (19). Interestingly, this phosphorylation sequence is not conserved in the less pathogenic HIV-2. Studies of HTLV-1 Rex phosphorylation have revealed three phosphorylation sites, corresponding to Ser-70, Thr-174, and Ser-177 (1). Again, detailed functional studies of mutants to test the biological significance of phosphorylation and Rex function have not been performed. However, treatment of HTLV-1-infected T cells with protein kinase inhibitor H-7 results in a decreased level of Rex-regulated viral unspliced gag-pol mRNA, corresponding to reduced in vivo phosphorylation of Rex, suggesting a role for protein kinase C (2).
Amino acid sequence analysis of Rex-2 reveals that serine 151 falls in a predicted consensus site for phosphorylation by casein kinase 1, Sp/Tp-X2-3-S/T-X (28, 29). HIV-1 Rev has recently been shown to be phosphorylated on Ser-5 and Ser-8 both in vitro and in vivo by casein kinase 2, and this phosphorylation is important for its function (32). Thus, identification of cellular kinases phosphorylating Rex-2 will be critical to understanding the control of Rex activity in the HTLV life cycle and may identify distinct differences between HTLV-1 and HTLV-2.
In conclusion, our data provide the first direct evidence that phosphorylation is critical for the function of Rex-2. One implication of this finding is that HTLV-2 gene expression is regulated at the cellular level. A virus such as HTLV that encodes its own regulatory genes would be better able to adapt if it could respond to regulatory signals of the cells it infects. Therefore, the dependence of Rex function on phosphorylation provides an additional level of replication control and might better allow the virus to escape the immune pressures of the infected host.
ACKNOWLEDGMENTS
We thank Kathleen Boris-Lawrie and Michael Lairmore for critical comments. We also thank Tim Vojt for preparation of the figures.
This work was supported by a grant from the National Institutes of Health (CA 59581).
REFERENCES
- 1.Adachi Y, Copeland T D, Takahashi C, Nosaka T, Ahmed A, Oroszlan S, Hatanaka M. Phosphorylation of the Rex protein of human T-cell leukemia virus type I. J Biol Chem. 1992;267:21977–21981. [PubMed] [Google Scholar]
- 2.Adachi Y, Nosaka T, Hatanaka M. Protein kinase inhibitor H-7 blocks accumulation of unspliced mRNA of human T-cell leukemia virus type I (HTLV-II) Biochem Biophys Res Commun. 1990;169:469–475. doi: 10.1016/0006-291x(90)90355-q. [DOI] [PubMed] [Google Scholar]
- 3.Akagi T, Ono H, Shimotohno K. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene. 1996;12:1645–1652. [PubMed] [Google Scholar]
- 4.Armstrong A P, Franklin A A, Henbogaard M N, Giebler H A, Nyborg J K. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc Natl Acad Sci USA. 1993;90:7303–7307. doi: 10.1073/pnas.90.15.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker A L X, Ruland C T, Stephens D W, Black A C, Rosenblatt J D. Human T-cell leukemia virus type 2 Rex inhibits pre-mRNA splicing in vitro at an early stage of spliceosome formation. J Virol. 1996;70:5511–5518. doi: 10.1128/jvi.70.8.5511-5518.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballaun C, Farrington G R, Dobrovnik M, Rusche J, Hauber J, Bohnlein E. Functional analysis of human T-cell leukemia virus type I Rex-response element: direct RNA binding of Rex protein correlates with in vivo binding activity. J Virol. 1991;65:4408–4413. doi: 10.1128/jvi.65.8.4408-4413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber J R, Verma I M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987;7:2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bex F, Murphy K, Wattiez R, Burny A, Gaynor R B. Phosphorylation of the human T-cell leukemia virus type 1 transactivator Tax on adjacent serine residues is critical for Tax activation. J Virol. 1999;73:738–745. doi: 10.1128/jvi.73.1.738-745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black A, Chen I S, Arrigo S, Ruland C T, Allogiamento T, Chin E, Rosenblatt J D. Regulation of HTLV-II gene expression by Rex involves positive and negative cis-acting elements in the 5′ long terminal repeat. Virology. 1991;181:433–444. doi: 10.1016/0042-6822(91)90875-c. [DOI] [PubMed] [Google Scholar]
- 10.Bockus B J, Schaffhausen B. Phosphorylation of polyomavirus large T antigen: effects of viral mutations and cell growth state. J Virol. 1987;61:1147–1154. doi: 10.1128/jvi.61.4.1147-1154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehm A K, Stawhecher J A, Semmes O J, Jankowski P E, Lewis R, Hinrichs S H. Analysis of potential phophorylation sites in human T cell leukemia virus type 1 Tax. J Biomed Sci. 1999;6:206–212. doi: 10.1007/BF02255904. [DOI] [PubMed] [Google Scholar]
- 12.Bogerd H, Greene W C. Dominant negative mutants of human T-cell leukemia virus type I Rex and human immunodeficiency virus type 1 Rev fail to multimerize in vivo. J Virol. 1993;67:2496–2502. doi: 10.1128/jvi.67.5.2496-2502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogerd H P, Tiley L S, Cullen B R. Specific binding of the human T-cell leukemia virus type I Rex protein to a short RNA sequence located within the Rex-response element. J Virol. 1992;66:7572–7575. doi: 10.1128/jvi.66.12.7572-7575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cann A J, Koyanagi Y, Chen I S Y. High efficiency transfection of primary human lymphocytes and studies of gene expression. Oncogene. 1988;3:123–128. [Google Scholar]
- 15.Chen I S Y, Slamon D J, Rosenblatt J D, Shah N P, Quan S G, Wachsman W. The x gene is essential for HTLV replication. Science. 1985;229:54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- 16.Ciminale V, Zotti L, D'Agostino D M, Chieco-Bianchi L. Inhibition of human T-cell leukemia virus type 2 Rex function by truncated forms of Rex encoded in alternatively spliced mRNAs. J Virol. 1997;71:2810–2818. doi: 10.1128/jvi.71.4.2810-2818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen B R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992;56:375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elfgang C, Rosorius O, Hofer L, Jaksche H, Hauber J, Bevec D. Evidence for specific nucleocytoplasmic transport pathways used by leucine-rich nuclear export signals. Proc Natl Acad Sci USA. 1999;96:6229–6234. doi: 10.1073/pnas.96.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouts D E, True H L, Cengel K A, Celander D W. Site-specific phosphorylation of the human immunodeficiency virus type-1 Rev protein accelerates formation of an efficient RNA-binding conformation. Biochemistry. 1997;36:13256–13262. doi: 10.1021/bi971551d. [DOI] [PubMed] [Google Scholar]
- 20.Grassmann R, Berchtolds S, Aepinus C, Ballaun C, Bohnlein E, Fleckenstein B. In vitro binding of human T-cell leukemia virus Rex proteins to the Rex-response element of viral transcripts. J Virol. 1991;65:3721–3727. doi: 10.1128/jvi.65.7.3721-3727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green P L, Chen I S Y. Molecular features of the human T-cell leukemia virus: mechanisms of transformation and leukemogenicity. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1994. pp. 227–311. [Google Scholar]
- 22.Green P L, Xie Y, Chen I S Y. The internal methionine codons of the human T-cell leukemia virus type II rex gene are not required for p24rex production or virus replication and transformation. J Virol. 1990;64:4914–4921. doi: 10.1128/jvi.64.10.4914-4921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green P L, Xie Y, Chen I S Y. The Rex proteins of human T-cell leukemia virus type II differ by serine phosphorylation. J Virol. 1991;65:546–550. doi: 10.1128/jvi.65.1.546-550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green P L, Yip M T, Xie Y, Chen I S Y. Phosphorylation regulates RNA binding by the human T-cell leukemia virus Rex protein. J Virol. 1992;66:4325–4330. doi: 10.1128/jvi.66.7.4325-4330.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammes S R, Greene W C. Multiple arginine residues within the basic domain of HTLV-I Rex are required for specific RNA binding and function. Virology. 1993;193:41–49. doi: 10.1006/viro.1993.1101. [DOI] [PubMed] [Google Scholar]
- 26.Hataka Y, Umemoto T, Matsushita S, Shida H. Involvement of human CRM1 (exportin 1) in the export and multimerization of the Rex protein of human T-cell leukemia virus type 1. J Virol. 1998;72:6602–6607. doi: 10.1128/jvi.72.8.6602-6607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J H, Kaufman P A, Hanly S M, Rimsky L T, Greene W C. Rex transregulation of human T-cell leukemia virus type II gene expression. J Virol. 1991;65:405–414. doi: 10.1128/jvi.65.1.405-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreegipuu A, Blom N, Brunak S. PhosphoBase, a database of phosphorylation sites: release 2.0. Nucleic Acids Res. 1999;27:237–239. doi: 10.1093/nar/27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreegipuu A, Blom N, Brunak S, Jarv J. Statistical analysis of protein kinase specificity determinants. FEBS Lett. 1998;430:45–50. doi: 10.1016/s0014-5793(98)00503-1. [DOI] [PubMed] [Google Scholar]
- 30.Kusuhara K, Anderson M, Pettiford S M, Green P L. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J Virol. 1999;73:8112–8119. doi: 10.1128/jvi.73.10.8112-8119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madore S J, Tiley L S, Malim M H, Cullen B R. Sequence requirements for Rev multimerization in vivo. Virology. 1994;202:186–194. doi: 10.1006/viro.1994.1334. [DOI] [PubMed] [Google Scholar]
- 32.Marin O, Sarno S, Boschetti M, Pagano M A, Meggio F, Ciminale V, D'Agostino D M, Pinna L A. Unique features of HIV-1 Rev protein phosphorylation by protein kinase CK2 (‘casein kinase-2′) FEBS Lett. 2000;481:63–67. doi: 10.1016/s0014-5793(00)01971-2. [DOI] [PubMed] [Google Scholar]
- 33.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 34.Nosaka T, Siomi H, Adachi Y, Ishibashi M, Kubota S, Maki M, Hatanaka M. Nucleolar targeting signal of human T-cell leukemia virus type I Rex-encoded protein is essential for cytoplasmic accumulation of unspliced viral mRNA. Proc Natl Acad Sci USA. 1989;86:9798–9802. doi: 10.1073/pnas.86.24.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohta M, Nyunoya H, Tanaka H, Okamoto T, Akagi T, Shimotohno K. Identification of a cis-regulatory element involved in accumulation of human T-cell leukemia virus type II genomic mRNA. J Virol. 1988;62:4445–4451. doi: 10.1128/jvi.62.12.4445-4451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen H S, Cochrane A W, Dillon P J, Nalin C M, Rosen C A. Interaction of the human immunodeficiency virus type 1 Rev protein with a structured region in env mRNA is dependent on multimer formation mediated through a basic stretch of amino acids. Genes Dev. 1990;4:1357–1364. doi: 10.1101/gad.4.8.1357. [DOI] [PubMed] [Google Scholar]
- 37.Palmeri D, Malim M H. The human T-cell leukemia virus type 1 posttranscriptional trans-activator Rex contains a nuclear export signal. J Virol. 1996;70:6442–6445. doi: 10.1128/jvi.70.9.6442-6445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ressler S, Morris G F, Marriott S J. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J Virol. 1997;71:1181–1190. doi: 10.1128/jvi.71.2.1181-1190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robek M D, Ratner L. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73:4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenblatt J D, Cann A J, Slamon D J, Smalberg I S, Shah N P, Fujii J, Wachsman W, Chen I S Y. HTLV-II trans-activation is regulated by two overlapping nonstructural genes. Science. 1988;240:916–919. doi: 10.1126/science.2834826. [DOI] [PubMed] [Google Scholar]
- 41.Ross T M, Pettiford S M, Green P L. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J Virol. 1996;70:5194–5202. doi: 10.1128/jvi.70.8.5194-5202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shima H, Takano M, Shimotohno K, Miwa M. Identification of p26xb and p24xb of human T-cell leukemia virus type II. FEBS Lett. 1986;209:289–294. doi: 10.1016/0014-5793(86)81129-2. [DOI] [PubMed] [Google Scholar]
- 43.Siomi H, Shida H, Nam S H, Nosaka T, Maki M, Hatanaka M. Sequence requirements for nucleolar localization of human T-cell leukemia virus type I pX protein which regulates viral RNA processing. Cell. 1988;55:197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- 44.Smith C L, Debouck C, Rosenberg M, Culp J S. Phosphorylation of serine residue 89 of human adenovirus E1A proteins is responsible for their characteristic electrophoretic mobility shifts, and its mutation affects biological function. J Virol. 1989;63:1569–1577. doi: 10.1128/jvi.63.4.1569-1577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trejo S R, Fahl W E, Ratner L. c-sis/PDGF-B promoter transactivation by the Yax protein of human T-cell leukemia virus type 1. J Biol Chem. 1996;271:14584–14590. doi: 10.1074/jbc.271.24.14584. [DOI] [PubMed] [Google Scholar]
- 46.Volkman B F, Lipson D, Wemmer D E, Kern D. Two-state allosteric behavior in a single-domain signaling protein. Science. 2001;291:2429–2433. doi: 10.1126/science.291.5512.2429. [DOI] [PubMed] [Google Scholar]
- 47.Wano Y, Feinberg M, Hosking J B, Bogerd H, Greene W C. Stable expression of the HTLV-I Tax protein in human T-cells activates specific cellular genes involved in growth. Proc Natl Acad Sci USA. 1988;85:9733–9737. doi: 10.1073/pnas.85.24.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weichselbraun I, Berger J, Dobrovnik M, Bogerd H, Grassmann R, Greene W C, Hauber J, Bohnlein E. Dominant-negative mutants are clustered in a domain of the human T-cell leukemia virus type I Rex protein: implications for trans dominance. J Virol. 1992;66:4540–4545. doi: 10.1128/jvi.66.7.4540-4545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yip M T, Dynan W S, Green P L, Black A C, Arrigo S J, Torbati A, Heaphy S, Ruland C, Rosenblatt J D, Chen I S Y. Human T-cell leukemia virus (HTLV) type II Rex protein binds specifically to RNA sequences of the HTLV long terminal repeat but poorly to the human immunodeficiency virus type 1 Rev-responsive element. J Virol. 1991;65:2261–2272. doi: 10.1128/jvi.65.5.2261-2272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]