Abstract
Molecular and pharmacological studies in vitro suggest that protein kinase C (PKC) family members play important roles in intracellular signal transduction. Nevertheless, the in vivo roles of PKC are poorly understood. We show here that nPKC-epsilon/eta TTX-4 in the nematode Caenorhabditis elegans is required for the regulation of signal transduction in various sensory neurons for temperature, odor, taste, and high osmolality. Interestingly, the requirement for TTX-4 differs in different sensory neurons. In AFD thermosensory neurons, gain or loss of TTX-4 function inactivates or hyperactivates the neural activity, respectively, suggesting negative regulation of temperature sensation by TTX-4. In contrast, TTX-4 positively regulates the signal sensation of ASH nociceptive neurons. Moreover, in AWA and AWC olfactory neurons, TTX-4 plays a partially redundant role with another nPKC, TPA-1, to regulate olfactory signaling. These results suggest that C. elegans nPKCs regulate different sensory signaling in various sensory neurons. Thus, C. elegans provides an ideal model to reveal genetically novel components of nPKC-mediated molecular pathways in sensory signaling.
Keywords: C. elegans, DAG, nPKC, sensory signaling, thermotaxis
Introduction
Sensory neurons are mostly specialized to detect different environmental stimuli. For example, photoreceptor cells sense light exclusively and olfactory neurons sense odorants. In each sensory neuron, different molecules are involved in primary sensory signal transduction. In mammals, light is received by the G protein-coupled receptor rhodopsin, which in turn activates Gα transducin and phosphodiesterase (PDE), and leads to the closure of a cGMP-gated cation (CNG) channel (Ebrey and Koutalos, 2001). Activation of an odorant receptor by an odorant stimulates Golf protein, which increases the level of cAMP via activation of adenylyl cyclase, resulting in the opening of a cAMP-gated cation (CNG) channel (Pace et al, 1985; Firestein et al, 1991). Nociceptive signals directly activate TRP channels, TRPV1 and TRPV2 (Caterina et al, 1997, 1999). Given the numerous specific sensory neurons, we are led to ask how the activation or inactivation of the signaling molecules is regulated to respond properly to environmental stimuli. Molecular mechanisms for the highly tuned regulation of these signaling pathways still remain largely unclear.
The protein kinase C (PKC) family is a group of serine/threonine protein kinases that play important roles in intracellular signal transduction. The PKC family comprises at least 10 isozymes, which are divided into three subgroups based on their structures: conventional PKC (cPKC), novel PKC (nPKC), and atypical PKC (aPKC) (Nishizuka, 1992). cPKCs require Ca2+ and diacylglycerol (DAG) for activation, nPKCs require DAG but not Ca2+, and aPKCs require neither Ca2+ nor DAG. Extensive pharmacological analyses show that PKC members are involved in such cellular events as cellular proliferation and differentiation (Nishizuka, 1995), suggesting roles of PKCs in various intracellular signal transductions. In sensory neurons, PKCs mediate the sensitization of the heat response induced by the inflammatory peptide bradykinin in rat nociceptive neurons (Cesare and McNaughton, 1996), inhibit the phototransduction cascade in the photoreceptor of horseshoe crabs (Dabdoub and Payne, 1999), and are required for the response to synthetic sweeteners by mammalian taste cells (Varkevisser and Kinnamon, 2000). Nevertheless, the in vivo function of most members of the PKC family in sensory neurons has remained unknown.
The nematode Caenorhabditis elegans can sense various environmental stimuli mainly by means of sensory neurons in the head (amphid neurons). The sensory neurons for each specific stimulus have been identified by a series of laser ablation experiments. For example, temperature stimulus is sensed by thermosensory neurons AFD (Mori and Ohshima, 1995), attractive odorants are sensed by olfactory neurons AWA and AWC (Bargmann et al, 1993), and aversive high osmolality is sensed by nociceptive neurons ASH (Hart et al, 1995; Maricq et al, 1995). Genetic analyses of mutants that are defective in sensory behaviors to these stimuli have identified the several molecules in sensory signaling pathways: tax-4 and tax-2 encode the alpha and beta subunits of cyclic nucleotide-gated (CNG) channel, respectively, which are required for thermosensation in AFD neurons and olfaction in AWC neurons (Coburn and Bargmann, 1996; Komatsu et al, 1996, 1999), osm-9 and ocr-2 encode TRPV channel homologs, which are required for olfaction in AWA neurons and nociception in ASH neurons (Colbert et al, 1997; Tobin et al, 2002), odr-3 encodes Gα protein, which is required for olfaction in AWA and AWC neurons and nociception in ASH neurons (Roayaie et al, 1998), and odr-1 and daf-11 encode guanylyl cyclases, which are required for olfaction in AWC neurons (Birnby et al, 2000; L'Etoile and Bargmann, 2000). These molecules are homologs of the molecules that function in similar types of sensory neurons of other animals, implicating evolutionarily conserved roles for these molecular pathways in each type of sensory signaling.
In this study, we found that nPKC-epsilon/eta plays essential and different roles in many different types of sensory neurons of C. elegans. We isolated thermotaxis-defective mutants from a genetic screen. Of these, a novel mutant, ttx-4, showed a thermophilic phenotype in thermotaxis, and other behavioral abnormalities in osmotic avoidance and chemotaxis to odorants and NaCl. We revealed that ttx-4 encodes nPKC-epsilon/eta and is expressed and functions in many head sensory neurons responsible for the behaviors that are abnormal in the mutant. The loss-of-function (lf) mutation in TTX-4 caused hyperactivation of the AFD neurons, whereas the gain-of-function (gf) form led to their inactivation. In contrast, TTX-4 lf caused inactivation of the ASH neurons, whereas TTX-4 gf led to their hyperactivation. Further, pharmacological and genetic analyses showed that sensory signaling in AFD thermosensory neurons is regulated exclusively by TTX-4, which likely functions in the downstream of diacylglycerol kinase (DGK)-1 and in the upstream of TAX-4 CNG channel, while sensory signalings in AWA and AWC olfactory neurons are regulated by TTX-4 and nPKC-delta/theta TPA-1. Our results suggest that TTX-4 nPKC-epsilon/eta plays diverse roles in various sensory neurons to regulate positively or negatively different cell signaling pathways.
Results
ttx-4 mutants show defects in thermotaxis and other sensory behaviors
C. elegans senses temperature mainly by a pair of head sensory neurons AFD, and the sensation of temperature is observed as a characteristic thermotaxis behavior (Hedgecock and Russell, 1975; Mori and Ohshima, 1995; Mori, 1999). In the thermotaxis behavior, wild-type animals migrate to their previously cultivated temperature on a radial temperature gradient from 17 to 25°C (Hedgecock and Russell, 1975; Mori and Ohshima, 1995); For example, the wild-type animals migrate to 20°C and stay there isothermally on a radial temperature gradient after cultivation at 20°C with sufficient food (Figure 1A and B). To elucidate the molecular mechanism of thermosensation, a genetic screen was performed for mutants defective in thermotaxis (ttx). Two mutants, nj1 and nj3, defined a novel gene ttx-4. Another mutant, ttx-4(nj4), was isolated in a different screen (A Mohri, M Koike, and I Mori, unpublished). All three ttx-4 alleles were genetically recessive (data not shown). The ttx-4 mutants showed a thermophilic phenotype, in which the animals always migrate to a temperature higher than the cultivation temperature (Figure 1A and B, and Supplementary data). The ttx-4(nj1) and ttx-4(nj3) mutants were severely defective and the ttx-4(nj4) mutants were partially defective.
Figure 1.
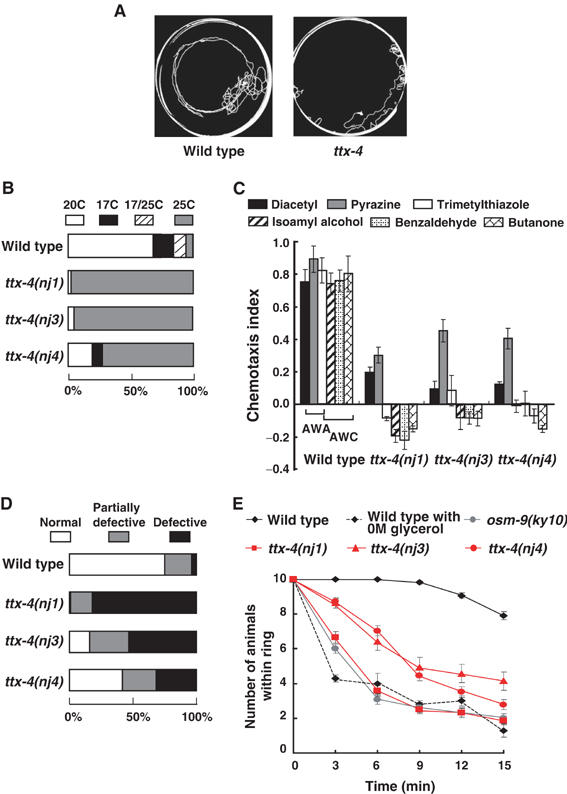
Phenotypes of ttx-4. (A) Tracks of wild-type and ttx-4 mutant animals showing thermotaxis behaviors on a radial temperature gradient ranging from 17°C (center) to 25°C (periphery). Animals were grown at 20°C. (B) The spectra of thermotaxis phenotypes of wild type and ttx-4 mutants. The phenotypic categories are described in Materials and methods. For each genotype, 100–160 animals were individually assayed on thermotaxis assay plates. (C) Chemotaxis of wild type and ttx-4 mutants for odorants. The concentration of odorants is described in Materials and methods. For each assay, 50–100 animals were analyzed. The bars represent the means of three independent assays with an error bar showing s.e.m. (D) Chemotaxis to NaCl. For each genotype, 100–170 animals were individually assayed. The phenotypic categories are described in Materials and methods. (E) Time-course assay for osmotic avoidance against 8 M glycerol. For each assay, 10 animals were placed inside of a high osmotic strength ring made from the given concentrations of 8 M glycerol solution, and the numbers of animals remaining within the ring were scored after 3, 6, 9, 12, and 15 min. ‘Wild type with 0 M glycerol' shows the animals' free movement without the osmotic barrier. The bars represent the means of more than three independent assays with an error bar showing s.e.m.
All ttx-4 mutants also showed severe defects in chemotactic responses to odorants sensed by AWA or AWC olfactory neurons, except that they were capable of partially responding to pyrazine sensed by AWA neurons (Figure 1C). In addition, these mutants were defective in chemotaxis to the water-soluble compound NaCl sensed mainly by ASE gustatory neurons (Figure 1D and Supplementary data). The ttx-4(nj1) mutants were severely defective in chemotaxis to NaCl, and ttx-4(nj3) and ttx-4(nj4) were partially defective. Furthermore, ttx-4 mutants showed a defect in osmotic avoidance behavior mediated by ASH nociceptive neurons (Figure 1E). A time-course assay was performed to observe how animals respond to osmotic strength that is decreasing over time probably due to diffusion. The ttx-4(nj1) mutants showed a defect as severe as that of the osm-9 mutants (Figure 1E). osm-9 encodes a TRPV channel homolog, and its null mutation completely abolishes the function of ASH neurons (Colbert et al, 1997). ttx-4(nj3) and ttx-4(nj4) mutants were less defective than ttx-4(nj1) mutants in osmotic avoidance behavior (Figure 1E).
ttx-4 gene encodes an nPKC-epsilon/eta ortholog
The ttx-4 gene was identified by a series of genetic mapping and subsequent rescue experiments with cloned genomic fragments (Figure 2A). Behavioral defects in the ttx-4 mutants were rescued by introduction of the cosmid C38F2 as well as the cloned genomic fragment containing two predicted genes, F57F5.5 and F10C2.3. The cloned genomic fragment without F10C2.3 also rescued the defects in ttx-4 mutants, indicating that F57F5.5 is the ttx-4 gene.
Figure 2.
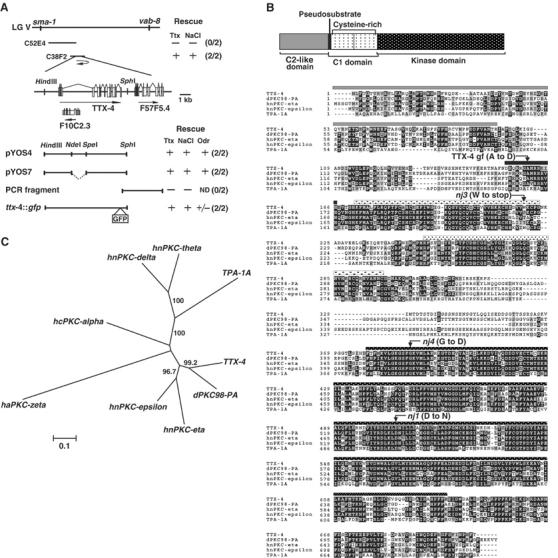
Genetic and molecular analyses of ttx-4. (A) Genetic map position of the ttx-4 gene on chromosome V and results of rescue. The ttx-4(nj1) gene was mapped onto the region between sma-1 and vab-8, which is covered by about 10 cosmids. The numbers in parentheses indicate the fraction of rescued lines. For each genotype, 100–170 animals were individually assayed for thermotaxis or for chemotaxis to NaCl. Chemotaxis to odorants was evaluated based on more than three independent assays for each transgenic line. (B) Structure and sequence of TTX-4. Upper panel: Predicted structure of TTX-4. Lower panel: Alignment of TTX-4 with human nPKC-epsilon and nPKC-eta, Drosophila PKC98-PA and C. elegans nPKC-delta/theta TPA-1A, and mutation sites of ttx-4. Prefix: d, Drosophila; h, human. TTX-4 shares 56% overall identity with human nPKC-epsilon and 52% identity with human nPKC-eta. Black highlights identical residues, and gray highlights similar residues. The bar patterns above the amino-acid alignment correspond to those marking domains in the structure of TTX-4. The alanine in the pseudosubstrate (PS) region was changed to aspartate to construct the ttx-4 gain-of-function form (ttx-4gf) (Dekker et al, 1993). (C) Unrooted dendrogram of two C. elegans nPKCs, TTX-4 and TPA-1A, Drosophila PKC98-PA, and four human PKCs. Human cPKC-alpha and aPKC-zeta were used as the representation of cPKC and aPKC, respectively. The dendrogram was generated by bootstrap analysis and neighbor-joining method using a freely available program, Clustal W (Thompson et al, 1994) (http://www.ddbj.nig.ac.jp/search/clustalw-j.html). The numbers at the branches indicate the percentage bootstrap values from 1000 replicates.
F57F5.5/ttx-4 gene encodes an nPKC-epsilon/eta ortholog, which has been designated as kin-13 (Figure 2B and C; Land et al, 1994). nj1 and nj4 have a missense mutation of a conserved amino acid in the catalytic loop (D502N) and the ATP-binding site (G390D) in the kinase domain, respectively. nj3 has a nonsense mutation in the C1 domain (W218stop) (Figure 2B). Since the nj3 mutation probably causes a truncated protein, the kinase activity should be completely lost in nj3 mutants. nj1 mutants exhibit a severer phenotype than do nj3 mutants, which suggests that the missense mutation nj1 might have a dominant negative effect. However, the nj1 mutation was recessive in our behavioral analysis (data not shown).
TTX-4 nPKC-epsilon/eta is expressed and functions in sensory neurons
Cells expressing the ttx-4 gene were identified by using a reporter construct in which the green fluorescence protein (GFP) gene was fused to the 3′ end of the wild-type ttx-4 genomic fragment (Figure 2A). A previous immunohistochemical study reported that KIN-13/TTX-4 protein was expressed mainly in sensory neurons and interneurons, although the specific cells had not been identified (Land et al, 1994). We found that the ttx-4::GFP reporter construct was expressed in many sensory neurons, including neurons that sense temperature (AFD), odorants (AWA and AWC), NaCl (ASE), or high osmolality (ASH) (Figure 3A and data not shown). The reporter construct was also expressed in many interneurons including AIY and AIZ, which play critical roles in thermotaxis (Figure 3A; Mori and Ohshima, 1995).
Figure 3.
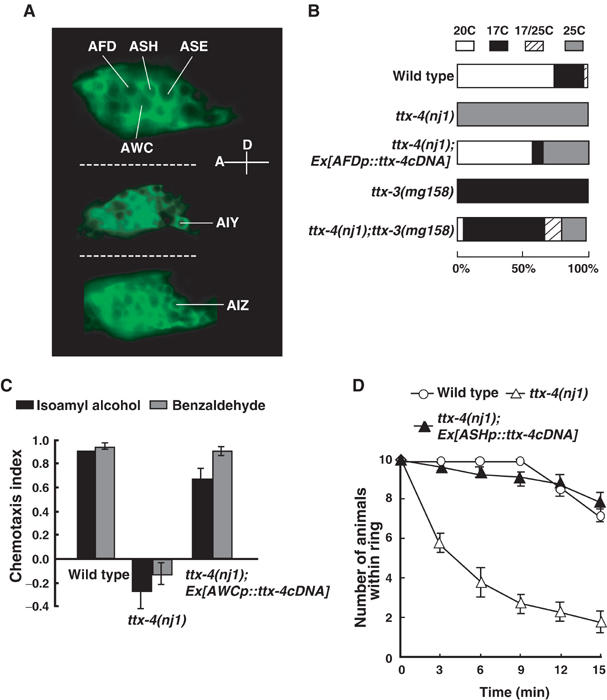
Expression and cell-autonomous functions of TTX-4 in sensory neurons. (A) TTX-4 expression in sensory neurons and interneurons. Lateral view of L2 larva showing the cell body of several neurons. Anterior is to the left and dorsal is up. TTX-4 protein was localized to not only cell body but also dendrite and axon (data not shown). TTX-4 protein was also expressed in other posterior neurons and some motor neurons (data not shown). (B) Rescue of the thermophilic phenotype by specific expression of ttx-4 cDNA in AFD thermosensory neurons and the thermophilic phenotype of ttx-4 mutants was suppressed by the ttx-3 mutation, which leads to the inactivation of AIY interneurons. The gcy-8 promoter was used for AFD expression (Yu et al, 1997). gcy-8p::ttx-4 cDNA was injected at 2 ng/μl. For each genotype, 60–100 animals were individually assayed for thermotaxis. (C) Rescue of the chemotaxis phenotype by specific expression of TTX-4 in AWC olfactory neurons. The odr-3 promoter was used for AWC expression (Roayaie et al, 1998). odr-3p::ttx-4 cDNA was injected at 20 ng/μl. A total of 10 animals were placed on each assay plate in this behavioral analysis. The bars represent the means of more than three independent assays. (D) Rescue of defective osmotic avoidance by specific expression of TTX-4 in ASH nociceptive neurons. The sra-6 promoter was used for ASH expression (Troemel et al, 1995). sra-6p::ttx-4 cDNA was injected at 20 ng/μl. A total of 10 animals were placed on each assay plate in this time-course assay for osmotic avoidance. The bars represent the means of more than three independent assays with an error bar showing s.e.m.
To identify the cells that require TTX-4 activity for their appropriate sensory behavior, the ttx-4 cDNA was expressed by using cell-specific promoters. Specific expression of the ttx-4 cDNA in the AFD thermosensory neurons of ttx-4(nj1) mutants rescued the thermophilic phenotype (Figure 3B), but not other behavioral defects such as chemotaxis to NaCl in ttx-4 mutants (data not shown). Furthermore, specific expression of ttx-4 cDNA in the AWC olfactory neurons or in the ASH nociceptive neurons rescued the abnormal chemotaxis to AWC-sensed odorants (Figure 3C) or the abnormal avoidance response to high osmolality (Figure 3D), respectively. These results suggest that TTX-4 nPKC-epsilon/eta acts cell-autonomously in the sensory neurons.
Genetic epistasis analysis also indicates that the thermophilic phenotype of the ttx-4 mutant is caused by abnormal AFD function. The AIY interneurons are thought to receive signals directly from the AFD neurons (White et al, 1986). Laser ablation of the AIY interneurons or mutations in ttx-3, a transcription factor that specifies AIY cell differentiation, lead to cryophilic phenotypes in which the animals always migrate to a temperature lower than the cultivation temperature (Mori and Ohshima, 1995; Hobert et al, 1997; Altun-Gultekin et al, 2001). We examined ttx-4;ttx-3 double mutants to show that the inactivation of AIY by the ttx-3 mutation suppresses the thermophilic phenotype of ttx-4 mutants. The majority of ttx-4;ttx-3 double mutants showed a cryophilic phenotype (Figure 3B), which resembles the phenotype of ttx-3 mutants. This result is consistent with the idea that the thermophilic phenotype of ttx-4 mutants is due to abnormal function of the AFD neurons (Figure 3B). Thus, we conclude that TTX-4 functions in the AFD thermosensory neurons. The incomplete suppression of the thermophilic phenotype of ttx-4 by the ttx-3 mutation might, however, implicate other neuron(s), where TTX-4 nPKC-epsilon/eta plays a minor role in thermosensation.
TTX-4 nPKC-epsilon/eta negatively regulates the function of AFD neurons
We asked how TTX-4 nPKC-epsilon/eta regulates thermosensory signal transduction in the AFD neurons. Inactivation of the AFD thermosensory neurons by a mutation in the AFD-specific transcription factor ttx-1 gene causes a cryophilic phenotype (Satterlee et al, 2001), which is opposite to the thermophilic phenotype of the ttx-4 mutants (Figure 1A). Laser ablation of the AFD neurons also causes a cryophilic, athermotactic, or cryophilic/athermotactic synthetic phenotype on a radial temperature gradient (Mori and Ohshima, 1995). To elucidate whether AFD neurons are hyperactivated in ttx-4 mutants, a gain-of-function form (TTX-4 gf) (Dekker et al, 1993) was expressed specifically in the AFD neurons of the wild type and ttx-4 mutants. Wild-type animals and ttx-4 mutants expressing TTX-4 gf in AFD neurons showed the cryophilic phenotype (Figure 4A), suggesting that abnormal activation of TTX-4 inactivates AFD neurons. Considering this result and the thermophilic phenotype in ttx-4 mutants, the AFD neurons may be hyperactivated in ttx-4 mutants.
Figure 4.
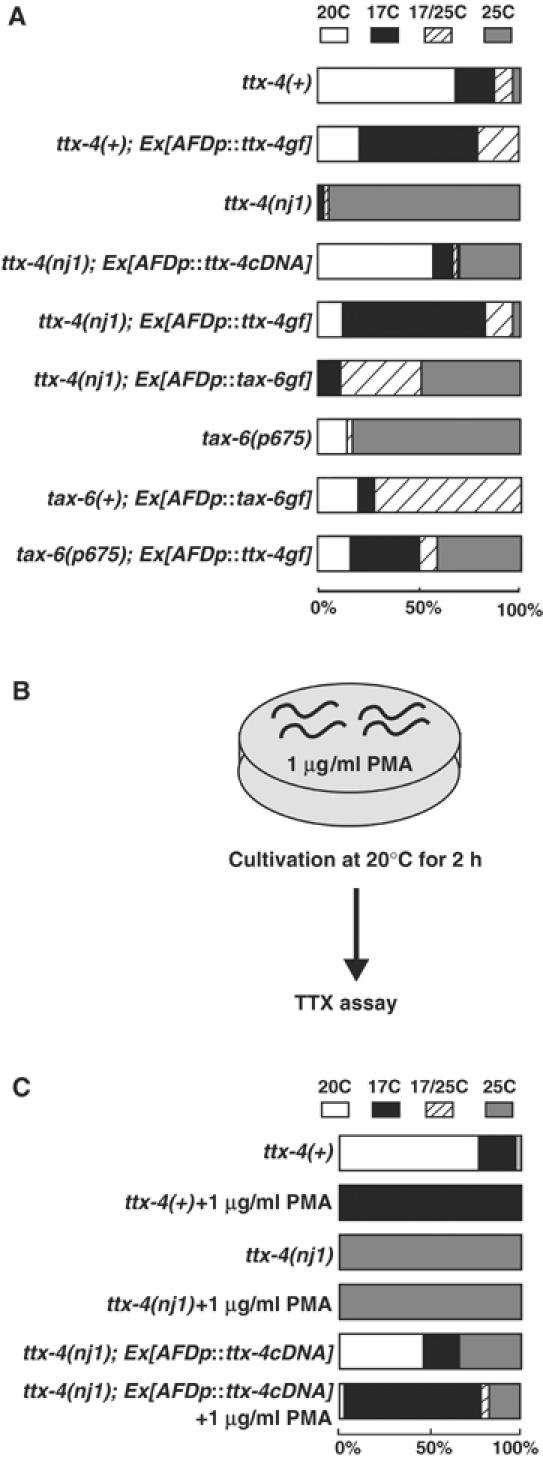
Effect of TTX-4 gf in AFD neurons and PMA treatment in thermotaxis. (A) Expression of the TTX-4 gf form caused inactivation of the AFD neurons, and TTX-4 and TAX-6 interact genetically in the AFD neurons. Gain-of-function forms of ttx-4 or tax-6 (Kuhara et al, 2002) were expressed in wild type and tax-6 and ttx-4 mutants using the AFD-specific gcy-8 promoter. The expression of gcy-8p::ttx-4gf did not affect morphology of the AFD neurons (data not shown). gcy-8p::ttx-4gf or gcy-8p::tax-6gf was injected at 2 ng/μl. For each genotype, 60–140 animals were individually assayed. (B) Schematic view of PMA treatment. (C) PMA inactivated AFD neurons through TTX-4 activation. gcy-8p::ttx-4 cDNA was injected at 2 ng/μl. For each genotype, 30–90 animals were individually assayed.
In tax-6 lf mutants, the AFD neurons are also hyperactivated, resulting in the thermophilic phenotype (Kuhara et al, 2002). TAX-6 encodes calcineurin, a calcium/calmodulin-dependent protein phosphatase (Kuhara et al, 2002). To investigate whether TTX-4 and TAX-6 interact in the AFD neurons, TTX-4 gf form or TAX-6 gf form was expressed specifically in the AFD neurons of tax-6 or ttx-4 mutants, respectively. AFD-specific expression of TTX-4 gf in tax-6 mutants partially suppressed the thermophilic phenotype (Figure 4A). The thermophilic phenotype in ttx-4 mutants was also suppressed partially by AFD-specific expression of TAX-6 gf that completely inactivates AFD neurons in wild-type animals (Figure 4A; Kuhara et al, 2002). These results did not show clear genetic epistatic dominance, but suggest that TTX-4 and TAX-6 interact genetically (see Discussion).
Activation of TTX-4 by DAG in adult animals causes inactivation of the AFD neurons
We addressed whether the activity of TTX-4 is required in the developed mature AFD neurons or in the developing AFD neurons. In this case, pharmacological analysis provides better temporal resolution than genetic analysis to understand the physiological role of the molecules. The mammalian nPKC subtype is activated by DAG. Therefore, TTX-4 nPKC-epsilon/eta in wild-type animals may be activated by exogenous phorbol-12 myristate 13-acetate (PMA), an analog of DAG, thereby leading to the cryophilic phenotype as observed in transgenic animals expressing TTX-4 gf in the AFD neurons. To investigate the effect of PMA on thermotaxis, we treated wild-type and ttx-4 mutant adult animals with PMA (1 μg/ml) for 2 h at 20°C, and then subjected them to the thermotaxis assay (Figure 4B). PMA-treated wild-type animals showed the cryophilic phenotype (Figure 4C), suggesting that PMA treatment in adulthood can behaviorally mimic the activation of TTX-4 in vivo. In contrast to the wild-type animals, PMA-treated ttx-4 mutants showed the thermophilic phenotype as did the PMA-untreated ttx-4 mutants (Figure 4C). This result suggests that the cryophilic phenotype of the PMA-treated wild-type animals is due to the activation of TTX-4, not to the side effect of the drug. We further asked whether TTX-4 in AFD neurons is the target of PMA. Most of the PMA-treated ttx-4 mutants expressing ttx-4 cDNA only in the AFD neurons showed the cryophilic phenotype (Figure 4C), which suggests that TTX-4 in the AFD neurons is the main target of PMA. Taken together, DAG likely activates TTX-4 to regulate thermosensation in the mature AFD neurons.
TTX-4 nPKC-epsilon/eta may function in the downstream of DGK-1 and in the upstream of TAX-4 channel
What molecules regulate the activation of TTX-4? Phospholipase C (PLC) produces DAG, which leads to various cellular responses probably through PKC activation (Nishizuka, 1995; Rhee, 2001). PLC-beta is required for taste response in mouse and phototransduction in Drosophila (Bloomquist et al, 1988; Zhang et al, 2003). To investigate whether PLC-beta supplies DAG to activate TTX-4, we tried to test the thermotaxis behavior of egl-8 mutants lacking PLC-beta (Lackner et al, 1999). We, however, could not evaluate the thermotaxis behavior of egl-8 mutants, because they did not move on the assay plate owing to their locomotion defect (data not shown). Phospholipase D (PLD) is also involved in the supply of DAG indirectly (Liscovitch et al, 2000). pld-1 encodes the sole PLD gene in the C. elegans genome. We found that pld-1 mutants lacking a base sequence encoding a catalytic motif in the PLD-1 gene (N Hisamoto and K Matsumoto, personal communication) showed normal thermotaxis behavior (Figure 5). DGK, which exchanges DAG into phosphatidic acid (PA), is a possible candidate for the regulatory molecule of PKC (van Blitterswijk and Houssa, 2000). dgk-1 encodes DGK-theta (Nurrish et al, 1999), which is expressed in the neurons involved in thermotaxis (data not shown). If DGK-1 regulates TTX-4 activity in the AFD neuron, we expected that DAG would accumulate in the AFD neurons of dgk-1 mutants, which might lead to the excess activation of TTX-4, thereby causing the cryophilic phenotype. Consistent with our hypothesis, dgk-1 mutants showed the cryophilic phenotype (Figure 5). We then examined a ttx-4;dgk-1 double mutant to show that the ttx-4 mutation suppresses the cryophilic phenotype of dgk-1 mutants. Most ttx-4;dgk-1 double mutants showed a thermophilic phenotype (Figure 5). This is also consistent with the model that TTX-4 functions in the downstream of DGK-1.
Figure 5.
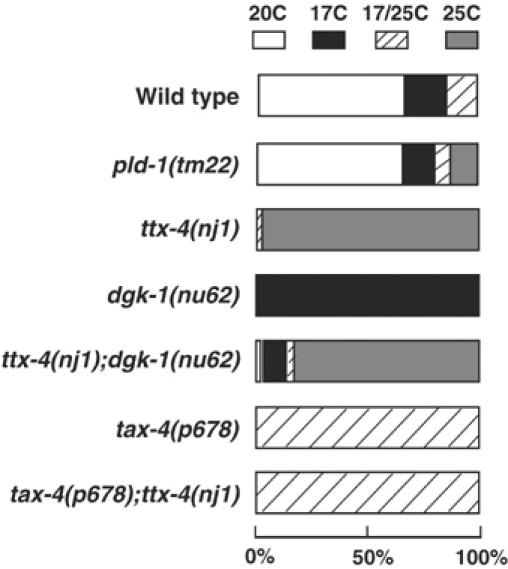
Thermotaxis behavior of pld-1, ttx-4;dgk-1 or tax-4;ttx-4 mutants. For each genotype, 60–90 animals were individually assayed on thermotaxis assay plates.
We next addressed whether TTX-4 regulates primary sensory transduction in AFD neurons. tax-4 encodes the alpha subunit of the CNG channel that is required for thermosensation in AFD neurons, and tax-4 mutants show an athermotactic phenotype (Figure 5; Komatsu et al, 1996). We examined the tax-4;ttx-4 double mutant to see if the tax-4 mutation would suppress the thermophilic phenotype of the ttx-4 mutant. tax-4;ttx-4 double mutants showed an athermotactic phenotype, which is similar to the phenotype of the tax-4 mutants (Figure 5). This result is consistent with the possibility that TTX-4 functions in the upstream of TAX-4. Thus, one plausible molecular model is that TTX-4 nPKC-epsilon/eta may be under the negative regulation by DGK-1 and negatively regulate the thermosensory signal transduction pathway by inhibiting the activity of the TAX-4 channel directly or indirectly.
TTX-4 nPKC-epsilon/eta positively regulates the function of ASH neurons
Although the ttx-4 lf mutation leads to hyperactivation of AFD neurons, it causes defects in the sensation of high osmolality, implicating inactivation of ASH neurons. To elucidate whether ASH neurons are inactivated in ttx-4 mutants, the TTX-4 gf form was expressed specifically in ASH neurons of wild-type and ttx-4 mutant animals. ASH-specific expression of TTX-4 gf resulted in hypersensitivity to high osmolality in the transgenic animals (Figure 6A). More than 80% of transgenic animals expressing TTX-4 gf in the ASH neurons stayed within the glycerol ring for 21 min, while about 50% of the wild-type animals moved out of the ring within the same period (Figure 6A), suggesting that TTX-4 gf causes hyperactivation of ASH neurons. Thus, it is likely that TTX-4 positively regulates the sensation of high osmolality in the ASH neurons.
Figure 6.
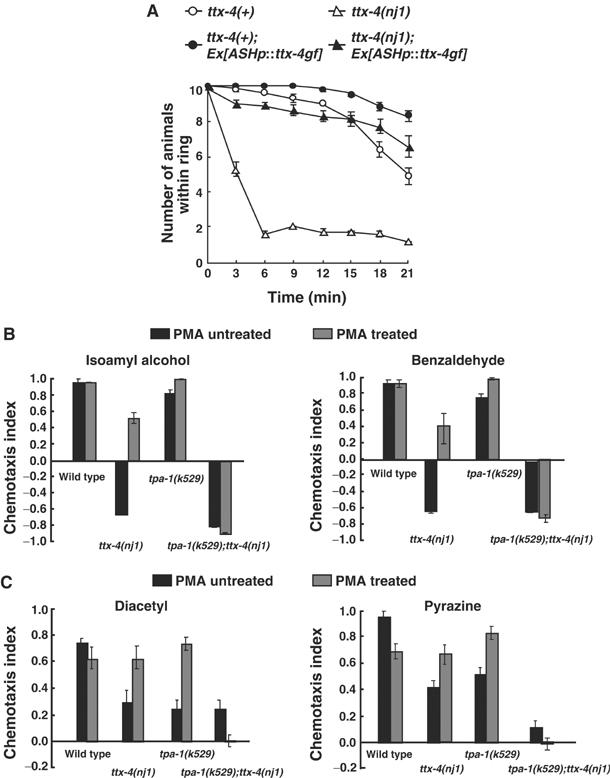
Effects of TTX-4 gf in ASH neurons and PMA treatment in chemotaxis. (A) Expression of the TTX-4 gf form caused hyperactivation of the ASH neurons. Animals were grown at 23°C. A total of 10 animals were placed on the assay plate for osmotic avoidance. The bars represent the means of more than three independent assays with an error bar showing s.e.m. For each assay, the numbers of animals remaining within the ring were scored after 3, 6, 9, 12, 15, 18, and 21 min. (B, C) PMA treatment rescued the defects in AWC and AWA olfactory neurons of ttx-4 mutants presumably by activating another PKC, TPA-1 nPKC-delta/theta. For each assay, 30–150 animals were analyzed. The bars represent the means of three independent assays with an error bar showing s.e.m.
TTX-4 and another nPKC function in olfactory neurons
We next investigated the role of TTX-4 in olfactory neurons. The chemotaxis defect for odorants sensed by AWC neurons in ttx-4 mutants could be caused by the inactivation of AWC neurons as in the case of ASH neurons. To elucidate whether TTX-4 positively regulates olfactory signaling also in AWC olfactory neurons, the TTX-4 gf form or excess TTX-4 was expressed specifically in the AWC neurons of wild-type animals. The transgenic animals showed normal olfactory responses to high and low concentrations of benzaldehyde (data not shown). We then investigated the effect of PMA on chemotaxis to odorants. In wild-type animals, PMA treatment did not affect the chemotaxis behavior significantly (Figure 6B and C). However, in the ttx-4 mutants, PMA treatment completely or partially rescued the defects in the chemotaxis behaviors mediated by AWA or AWC sensory neurons, respectively. This is surprising because the ttx-4 mutation completely abolished the effect of PMA in the thermotaxis behavior (Figure 4C). The results suggest that other protein(s) can substitute for TTX-4 upon PMA treatment in olfactory neurons to regulate olfactory sensation.
A candidate for the other PMA-activated protein is TPA-1 nPKC-delta/theta, which was identified by a genetic analysis of a PMA-resistant mutant (Tabuse et al, 1989). tpa-1 mutants showed a slight defect in chemotaxis to AWC-sensed odorants, and a severe or mild defect to AWA-sensed diacetyl or pyrazine, respectively (Figure 6B and C). The PMA treatment of tpa-1 mutants rescued chemotaxis to odorants sensed by either of these neurons (Figure 6B and C). These results led us to think that when treated with PMA, TPA-1 nPKC-delta/theta was activated in ttx-4 mutants and TTX-4 nPKC-epsilon/eta was activated in tpa-1 mutants, thereby achieving better or normal olfactory responses in both mutants. Consistent with this possibility, PMA-treated tpa-1;ttx-4 double mutants remained defective in the olfactory responses as were PMA-untreated tpa-1;ttx-4 double mutants (Figure 6B and C). These results indicate that two nPKCs, TTX-4 nPKC-epsilon/eta and TPA-1 nPKC-delta/theta, function in the AWA and AWC sensory neurons to regulate olfactory sensation in a partially redundant manner.
Discussion
TTX-4 nPKC-epsilon/eta negatively or positively regulates the neural activity in various sensory neurons of C. elegans
In this study, we demonstrated that C. elegans nPKC-epsilon/eta TTX-4 is required for the proper responses to environmental stimuli such as temperature, volatile and soluble compounds, and noxious stimulus. In addition, we showed that TTX-4 functions cell-autonomously in developed sensory neurons for these stimuli. These results suggest that TTX-4 nPKC-epsilon/eta regulates sensory signal transduction in the sensory neurons. Our results indicate that the TTX-4 lf mutation causes hyperactivation of AFD thermosensory neurons, whereas the TTX-4 gf form causes their inactivation. In contrast, TTX-4 lf causes inactivation of ASH nociceptive neurons and TTX-4 gf causes their hyperactivation. Taken together, we provide functional evidence to show that the activation of nPKC-epsilon/eta causes the opposite effects on the activities of different sensory neurons.
Two distinct nPKCs function in olfactory neurons
Severe defects in chemotaxis to odorants in ttx-4 mutants were rescued by exogenous PMA, suggesting that TTX-4 nPKC-epsilon/eta is a regulator rather than an essential component of the signal transduction pathway in AWA and AWC olfactory neurons. In addition, pharmacological analysis revealed that another nPKC, TPA-1 nPKC-delta/theta, functions redundantly in the two olfactory neurons. In mammals, nPKC-epsilon and nPKC-delta are often coexpressed in neurons such as photoreceptor cells and sympathetic neurons (Scholze et al, 2002; Sokal et al, 2003). We showed that TPA-1 or TTX-4 activation by PMA can rescue the chemotaxis defect for odorants sensed by AWA and AWC neurons in ttx-4 or tpa-1 mutants, respectively. These results may imply that these two nPKCs share common target molecules in these neurons.
TTX-4 nPKC-epsilon/eta-mediated sensory signalings
DGK exchanges DAG into PA (van Blitterswijk and Houssa, 2000). dgk-1 encodes DGK-theta (Nurrish et al, 1999), which is expressed in the neurons involved in thermotaxis (data not shown). We found that dgk-1 mutants show a cryophilic phenotype and that this cryophilic phenotype is suppressed by the ttx-4 mutation. In addition, we showed that the thermophilic phenotype of ttx-4 mutants is suppressed by the tax-4 mutation. These results are consistent with the possibilities that TTX-4 functions in the downstream of DGK-1 and in the upstream of TAX-4.
In AFD neurons, TAX-4 and TAX-2 CNG channels play an essential role in thermosensation (Coburn and Bargmann, 1996). Electrophysiological studies showed that the TAX-4 CNG channel exhibits much higher affinity to cGMP than to cAMP (Komatsu et al, 1999). Higher affinity of TAX-4 to cGMP (Komatsu et al, 1999) also suggests that guanylyl cyclase is involved in the signal transduction in AFD neurons. In mammals, the cGMP-gated (CNG) channel is inhibited by nPKC-dependent phosphorylation in bovine photoreceptor cells, and phosphorylation of guanylyl cyclase by PKC in cultured smooth muscle cells resulted in its desensitization (Hamad and Knox, 1999; Muller et al, 2001). These data suggest that the TAX-4 CNG channel or unidentified guanylyl cyclase may be the targets of TTX-4 phosphorylation in AFD neurons. Altogether, we propose the following molecular mechanism: TTX-4 nPKC-epsilon/eta, which may be under the negative control of DGK-1, negatively regulates the thermosensory transduction pathway by inhibiting the activity of the TAX-4 CNG channel in AFD neurons.
PKC is reported to phosphorylate calcineurin (Hashimoto and Soderling, 1989). If TAX-6 calcineurin is the target of TTX-4, it is reasonable to expect that AFD-specific expression of the TTX-4 gf form in tax-6 mutants fails to suppress the thermophilic phenotype of tax-6 mutants, while the expression of the TAX-6 gf form in ttx-4 mutants suppresses the thermophilic phenotype of ttx-4 mutants. Our results showed that there is no clear genetic epistatic dominance between TTX-4 and TAX-6, suggesting that TTX-4 does not phosphorylate TAX-6. The weak genetic interaction between TTX-4 and TAX-6, however, implies that TTX-4 and TAX-6 may coregulate thermosensory signal transduction in AFD neurons.
In ASH neurons, OSM-9 and OCR-2 TRPV channel homologs are required for nociception (Colbert et al, 1997; Tobin et al, 2002). Previous studies reported the involvement of nPKC-epsilon in the nociceptive neurons of mammals; nPKC-epsilon could phosphorylate the TRPV1 channel (Numazaki et al, 2002), and it induces the inflammatory peptide bradykinin-dependent activation of TRPV1 in rat nociceptive neurons (Premkumar and Ahern, 2000). It is thus quite possible that mammalian nociceptive neurons and C. elegans ASH neurons share the evolutionarily conserved nPKC-epsilon-mediated regulatory mechanism for their nociceptive signaling.
In summary, our results imply that TTX-4 nPKC-epsilon/eta phosphorylates different substrates in different sensory neurons. We suggest that the difference in PKC substrates implicated by various experimental approaches derives from the diverse functional nature of nPKC-epsilon/eta per se. Further genetic analysis will help reveal additional molecular components involved in the regulation of different TTX-4 nPKC-epsilon/eta-mediated sensory signalings.
Materials and methods
Strains and genetics
The techniques used for culturing C. elegans were essentially as described by Brenner (1974). The following strains were used in this work: wild-type C. elegans variety Bristol strain (N2), KM22 pld-1(km22) II, CB1372 daf-7(e1372) III, FK127 tax-4(p678) III, CB40 daf-1(m40) IV, IK17 tax-6(p675) IV, IK438 tpa-1(k529) IV, IK105 ttx-4(nj1) V, IK130 ttx-4(nj3) V, IK174 ttx-4(nj4) V, CB4123 lon-3(e2175) V, SP1493 sma-1(e30) unc-76(e911)/vab-8(e1017) V, MT3773 unc-42(e270) vab-8(e1017) V, ttx-3(mg158) X, IK570 dgk-1(nu62) X. ttx-4(nj1) and ttx-4(nj3) were isolated in genetic screens for mutants that bypassed temperature-dependent dauer formation of daf-1(m40) or daf-7(e1372) backgrounds (Hobert et al, 1997). The ttx-4 mutation was mapped using the thermotaxis defect and chemotaxis defect to NaCl. By linkage analysis, ttx-4(nj1) and ttx-4(nj3) were found to be linked to lon-3(e2175) on chromosome V. From the following three-factor crosses, ttx-4 was mapped on the region between sma-1 and vab-8. From sma-1 unc-76/ttx-4 hermaphrodites, 42/43 non-Sma Unc recombinant progenies segregated ttx-4. From unc-42 vab-8/ttx-4 hermaphrodites, 34/38 Unc non-Vab and 1/21 non-Unc Vab recombinant progenies segregated ttx-4. The ttx-4 mutants were backcrossed three (nj4) or six times (nj1 and nj3) before the behavioral analyses.
Analysis of sensory behaviors
The animals were grown at 20°C for all behavioral assays in this study, unless especially described. The procedure for the thermotaxis assay using radial temperature gradients was according to Mori and Ohshima (1995) with a slight modification. Evaluation of thermotaxis was essentially according to Mohri et al (2005). Thermotaxis of individual animals on a radial temperature gradient was evaluated by using four phenotypic categories: the animal that moved to the cold region (the center of the plate) was classified as ‘17C,' the animal that moved to the 20°C region was classified as ‘20C,' the animal that moved to the warm region (the periphery of the plate) was classified as ‘25C,' and the animal that moved to the cold and warm regions was classified as ‘17/25C.' Chemotaxis to odorants was assayed according to Bargmann et al (1993), except that the assay plates contained a slightly different medium (2% agar, 1 mM MgSO4, 1 mM CaCl2, 25 mM potassium phosphate (pH6)). The dilutions of odorants with ethanol were as follows: 1:1000 diacetyl, 10 mg/ml pyrazine, 1:1000 2,4,5-trimethylthiazole, 1:100 isoamyl alcohol, 1:200 benzaldehyde, and 1:1000 butanone. The chemotaxis index was calculated according to Bargmann et al (1993). The osmotic avoidance assay with a high concentration glycerol ring was essentially according to Culotti and Russell (1978). Chemotaxis to NaCl was essentially as described previously (Komatsu et al, 1996). Briefly, when the animal migrated repeatedly to the concentration peak or stayed at the peak during the assay, the animal was scored as ‘Normal.' When the animal migrated toward the peak only once or stayed very briefly at the peak, the animal was scored as ‘Partially defective.' When the animal failed to migrate to the peak, the animal was scored as ‘Defective.'
Germline transformation
Germline transformation was performed by coinjecting test DNA at a concentration of 2–50 ng/μl and an injection marker pKDK66 (ges-1p::NLS-GFP) (Fukushige et al, 1996) at a concentration of 20–50 ng/μl or ofm-1p::GFP (Miyabayashi et al, 1999) at a concentration of 20 ng/μl into the gonad of ttx-4(nj1) or wild-type animals (Mello et al, 1991). The ofm-1p::GFP was used as an injection marker together with sra-6p::ttx-4 cDNA or sra-6p::ttx-4gf. The small body size of the tax-6 mutant was rescued by expressing tax-6 cDNA only in body wall muscle by using the myo-3 promoter to improve movement of the tax-6 mutants (Kuhara et al, 2002). Transgenic animals were recognized with the intestinal or coelomocyte's GFP fluorescence that was due to ges-1::NLS-GFP or ofm-1p::GFP expression, respectively. Multiple independent transgenic lines were established for each test DNA. The transgenic rescue of ttx-4 defects was evaluated with regard to behaviors.
Molecular biology
A subclone of cosmid C38F2 was generated as follows: a 10 kb fragment of C38F2, which contains the whole ttx-4 gene and a 4 kb upstream sequence, was cloned into pBluescript SK+ using HindIII and SphI sites to generate pYOS4. pYOS7 was constructed by deleting the F10C2.3 gene in pYOS4, which contains two predicted genes, F57F5.5/ttx-4 and F10C2.3. The ttx-4::gfp fusion gene was constructed as follows: the stop codon of the ttx-4 gene was removed and modified by PCR, and the gfp gene from the GFP vector pPD95.75 was inserted between the end and the 3′UTR of the ttx-4 gene in pYOS4 using the KpnI and EcoRI sites. The identification of cells expressing ttx-4::gfp was carried out with the light microscope AxioPlan2 (Carl Zeiss).
The full-length ttx-4 cDNA was constructed as follows: yk248a5 contained all the ttx-4 cDNA except for 4 bp of the 5′ end in the first exon. The 5′ end of ttx-4 cDNA was amplified from pYOS4 by PCR, and the PCR product and the rest of ttx-4 cDNA derived from yk248a5 was ligated into pPD49.26 to generate pYOS70. The gcy-8 promoter DNA, odr-3 promoter DNA, or sra-6 promoter DNA was inserted into pYOS70. The ttx-4 gf form was generated by using specific PCR primers to create the mutation that exchanged alanine to asparate in the pseudosubstrate region (Dekker et al, 1993). The ges-1 promoter region (Fukushige et al, 1996) was ligated into GFP vector pPD95.67 to generate pKDK66. The odr-3 promoter or sra-6 promoter was PCR-amplified to make a modified promoter that did not contain its own initial codon and was inserted into pBluescript SK+.
PMA treatment
Cultivation plates containing PMA were made according to a previous report (Miwa et al, 1982). PMA treatment of animals was carried out as follows: before the thermotaxis assay, animals were picked up from the usual cultivation plate and transferred onto the cultivation plate containing PMA. After 2 h, animals were moved onto the assay plate containing no PMA and tested for thermotaxis. Before the chemotaxis assay for odorants, animals were collected with M9 buffer containing 1 μg/ml PMA and transferred onto a cultivation plate containing PMA. After 2 h, animals were washed three times with M9 buffer and once with water, and then transferred onto the assay plate containing no PMA to test for chemotaxis to odorants. Although PMA treatment affected the movement of the animals, we were able to evaluate the effect of PMA on thermotaxis and chemotaxis to odorants, because the animals were still able to move toward a preferred stimulus. With regard to the evaluation of chemotaxis to odorants, we divided the assay plate into left and right halves, where the attractive odor was spotted on the left side, and the chemotaxis index was calculated as ((number of the animals on the left side)−(number of the animals on the right side))/(total number of the animals). In contrast, for osmotic avoidance behavior, it was difficult to distinguish whether a high avoidance index of PMA-treated animals was due to decreased movement or enhanced osmotic sensation. Thus, we did not observe the effect of PMA on osmotic avoidance behavior.
Supplementary Material
Supplementary data
Acknowledgments
We are grateful to M Okumura for sharing all the efforts to screen novel thermotaxis-defective mutants. We thank Y Kohara for yk clones; A Fire for pPD plasmids; D Garbars, B Wedel, C Bargmann, and J McGhee for promoter plasmids; P Sengupta for ofm-1p::GFP; N Hisamoto and K Matsumoto for pld-1 mutant; H Inada, A Kuhara and SB Miwa for critical reading of the manuscript; and Mori lab members for stimulating discussions. The Caenorhabditis Genetic Center provided some of the strains used in this study. KDK was supported by CREST of the Japan Science and Technology Corporation. This work was supported by research grants from the Ministry of Education, Culture, Science and Sports of Japan and the Human Frontier Science Program Organization (to IM).
References
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O (2001) A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128: 1951–1969 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR (1993) Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527 [DOI] [PubMed] [Google Scholar]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH (2000) A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155: 85–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL (1988) Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54: 723–733 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824 [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P (1996) A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA 93: 15435–15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CM, Bargmann CI (1996) A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706 [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI (1997) OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17: 8259–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotti JG, Russell RL (1978) Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics 90: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Payne R (1999) Protein kinase C activators inhibit the visual cascade in Limulus ventral photoreceptors at an early stage. J Neurosci 19: 10262–10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker LV, McIntyre P, Parker PJ (1993) Mutagenesis of the regulatory domain of rat protein kinase C-eta. A molecular basis for restricted histone kinase activity. J Biol Chem 268: 19498–19504 [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y (2001) Vertebrate photoreceptors. Prog Retin Eye Res 20: 49–94 [DOI] [PubMed] [Google Scholar]
- Firestein S, Zufall F, Shepherd GM (1991) Single odor-sensitive channels in olfactory receptor neurons are also gated by cyclic nucleotides. J Neurosci 11: 3565–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T, Schroeder DF, Allen FL, Goszczynski B, McGhee JD (1996) Modulation of gene expression in the embryonic digestive tract of C. elegans. Dev Biol 178: 276–288 [DOI] [PubMed] [Google Scholar]
- Hamad AM, Knox AJ (1999) Mechanisms involved in desensitization of particulate guanylyl cyclase in human airway smooth muscle: the role of protein kinase C. Biochem Biophys Res Commun 266: 152–155 [DOI] [PubMed] [Google Scholar]
- Hart AC, Sims S, Kaplan JM (1995) Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82–85 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Soderling TR (1989) Regulation of calcineurin by phosphorylation. Identification of the regulatory site phosphorylated by Ca2+/calmodulin-dependent protein kinase II and protein kinase C. J Biol Chem 264: 16524–16529 [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL (1975) Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 72: 4061–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G (1997) Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron 19: 345–357 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Jin YH, L'Etoile N, Mori I, Bargmann CI, Akaike N, Ohshima Y (1999) Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res 821: 160–168 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y (1996) Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718 [DOI] [PubMed] [Google Scholar]
- Kuhara A, Inada H, Katsura I, Mori I (2002) Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron 33: 751–763 [DOI] [PubMed] [Google Scholar]
- Lackner MR, Nurrish SJ, Kaplan JM (1999) Facilitation of synaptic transmission by EGL-30 Gqα and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346 [DOI] [PubMed] [Google Scholar]
- Land M, Islas-Trejo A, Freedman JH, Rubin CS (1994) Structure and expression of a novel, neuronal protein kinase C (PKC1B) from Caenorhabditis elegans. PKC1B is expressed selectively in neurons that receive, transmit, and process environmental signals. J Biol Chem 269: 9234–9244 [PubMed] [Google Scholar]
- L'Etoile ND, Bargmann CI (2000) Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron 25: 575–586 [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X (2000) Phospholipase D: molecular and cell biology of a novel gene family. Biochem J 345 (Part 3): 401–415 [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI (1995) Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 378: 78–81 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J, Tabuse Y, Furusawa M, Yamasaki H (1982) Tumor promoters specifically and reversibly disturb development and behavior of Caenorhabditis elegans. J Cancer Res Clin Oncol 104: 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabayashi T, Palfreyman MT, Sluder AE, Slack F, Sengupta P (1999) Expression and function of members of a divergent nuclear receptor family in Caenorhabditis elegans. Dev Biol 215: 314–331 [DOI] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Koike M, Mizuno T, Mori I (2005) Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I (1999) Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu Rev Genet 33: 399–422 [DOI] [PubMed] [Google Scholar]
- Mori I, Ohshima Y (1995) Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348 [DOI] [PubMed] [Google Scholar]
- Muller F, Vantler M, Weitz D, Eismann E, Zoche M, Koch KW, Kaupp UB (2001) Ligand sensitivity of the 2 subunit from the bovine cone cGMP-gated channel is modulated by protein kinase C but not by calmodulin. J Physiol 532: 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614 [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9: 484–496 [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M (2002) Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cɛ and identification of two target serine residues. J Biol Chem 277: 13375–13378 [DOI] [PubMed] [Google Scholar]
- Nurrish S, Segalat L, Kaplan JM (1999) Serotonin inhibition of synaptic transmission: Gαo decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242 [DOI] [PubMed] [Google Scholar]
- Pace U, Hanski E, Salomon Y, Lancet D (1985) Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature 316: 255–258 [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP (2000) Induction of vanilloid receptor channel activity by protein kinase C. Nature 408: 985–990 [DOI] [PubMed] [Google Scholar]
- Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI (1998) The Gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20: 55–67 [DOI] [PubMed] [Google Scholar]
- Satterlee JS, Sasakura H, Kuhara A, Berkeley M, Mori I, Sengupta P (2001) Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron 31: 943–956 [DOI] [PubMed] [Google Scholar]
- Scholze T, Moskvina E, Mayer M, Just H, Kubista H, Boehm S (2002) Sympathoexcitation by bradykinin involves Ca2+-independent protein kinase C. J Neurosci 22: 5823–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal I, Hu G, Liang Y, Mao M, Wensel TG, Palczewski K (2003) Identification of protein kinase C isozymes responsible for the phosphorylation of photoreceptor-specific RGS9-1 at Ser475. J Biol Chem 278: 8316–8325 [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Nishiwaki K, Miwa J (1989) Mutations in a protein kinase C homolog confer phorbol ester resistance on Caenorhabditis elegans. Science 243: 1713–1716 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C (2002) Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI (1995) Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218 [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Houssa B (2000) Properties and functions of diacylglycerol kinases. Cell Signal 12: 595–605 [DOI] [PubMed] [Google Scholar]
- Varkevisser B, Kinnamon SC (2000) Sweet taste transduction in hamster: role of protein kinases. J Neurophysiol 83: 2526–2532 [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous-system of the nematode Caenorhabditis elegans. Philos Trans R Soc London B 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL (1997) Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci USA 94: 3384–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


