Abstract
Objective: High-density lipoprotein cholesterol efflux function may prevent brain amyloid beta deposition and neurodegeneration. However, the relevance of this finding has not been established in the diverse middle-aged population. Methods: We examined 1826 adults (47% Black adults) who participated in the Dallas Heart Study to determine associations between high-density lipoprotein (HDL) measures and brain structure and function. White matter hyperintensities (WMH) and whole-brain grey matter volume (GMV) were measured using brain MRI, and the Montreal Cognitive Assessment (MoCA) was used to measure neurocognitive function. HDL cholesterol efflux capacity (HDL-CEC) was assessed using fluorescence-labeled cholesterol efflux from J774 macrophages, and HDL particle size measures were assessed using nuclear magnetic resonance (NMR) spectroscopy (LipoScience). Multivariable linear regressions were performed to elucidate associations between HDL-CEC and brain and cognitive phenotypes after adjustment for traditional risk factors such as age, smoking status, time spent in daily physical activity, and education level. Results: Higher HDL-CEC and small HDL particle (HDL-P) concentration were positively associated with higher GMV normalized to total cranial volume (TCV) (GMV/TCV) after adjustment for relevant risk factors (β = 0.078 [95% CI: 0.029, 0.126], p = 0.002, and β = 0.063 [95% CI: 0.014, 0.111], p = 0.012, respectively). Conversely, there were no associations between HDL measures and WMH or MoCA (all p > 0.05). Associations of HDL-CEC and small HDL-P with GMV/TCV were not modified by ApoE-ε4 status or race/ethnicity. Interpretation: Higher HDL cholesterol efflux and higher plasma concentration of small HDL-P were associated with higher GMV/TCV. Additional studies are needed to explore the potential neuroprotective functions of HDL.
Keywords: high-density lipoprotein cholesterol, brain grey matter, neurodegeneration, neurocognitive function
1. Introduction
Several epidemiological studies have demonstrated associations of plasma lipids during early and midlife with the development of cognitive decline and dementia in late life [1,2,3,4]. Notably, low levels of high-density lipoprotein cholesterol (HDL-C) have been associated with impaired cognitive function [5,6,7] and the presence of amyloid beta (β) deposits [8] and parahippocampal atrophy [9] in the brain that are associated with dementia [10]. In contrast, some studies have observed no association between HDL-C and impaired cognitive function [11,12,13]. Importantly, HDL particles exert antioxidant and anti-inflammatory effects and play an important role in the removal of excess cholesterol from vascular cells through reverse cholesterol transport [14]. Greater HDL cholesterol efflux capacity (HDL-CEC), a measure of HDL reverse cholesterol transport functionality, associates linearly with reduced cardiovascular disease risk independently from HDL-C and of Apolipoprotein A-I (ApoA-I), the main constituent protein of HDL particles [15,16]. Excess levels of cholesterol in the central nervous system promote neurotoxicity and the development of Alzheimer’s disease (AD) by enhancing amyloid β deposition [17], and this is reflected in the reduced HDL-CEC levels found in the cerebrospinal fluid of individuals with mild cognitive impairment (MCI) and AD [18,19,20].
However, the association between plasma HDL-CEC or HDL composition and brain structure has not been investigated. Although one prior study investigated the association between plasma HDL-CEC and cognitive function, it involved a small sample of participants that included 50 cognitively normal adults [18]. The small sample size limited the ability to account for the confounding influence of relevant variables. To address this, we used a large, multiethnic cohort of middle-aged adults from the Dallas Heart Study to evaluate the associations between HDL measures and brain structure and function.
2. Materials and Methods
All investigations were approved by the Institutional Review Board at the University of Texas Southwestern Medical Center and conducted in participants of the Dallas Heart Study after informed consent was obtained. In summary, the Dallas Heart Study (DHS) is a multiethnic, population-based, probability sample of Dallas County, Texas, U.S.A. The details of the Dallas Heart Study (DHS) participant selection criteria, overall study design, and detailed methods, have been previously described [21]. As DHS was a population-based cohort study of Dallas County adults, there were no exclusion criteria, while all adults aged 35–70 years were eligible for enrollment. Participants of the DHS phase-1 (DHS-1) that occurred between 2000 and 2002, and the longitudinal follow-up DHS phase-2 (DHS-2) that occurred between 2007 and 2009, were eligible to be included in the present study (NCT00344903). All participants had fasting lipoprotein measures assessed by nuclear magnetic resonance (NMR). High-density lipoprotein (HDL) cholesterol efflux capacity (CEC) was assessed using J774 macrophages, fluorescence-labeled cholesterol, and Apolipoprotein B-depleted plasma during both phases (DHS-1 and DHS-2). Participants underwent cognitive testing (Montreal Cognitive Assessment; MoCA) and brain magnetic resonance imaging (MRI) during a single visit of DHS-2. Race was self-reported by all participants of this study, and race categories were defined based on the United States Office of Management and Budget’s Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity.
2.1. Lipoprotein Characterization and HDL-Cholesterol Efflux Capacity (HDL-CEC)
Fasting blood samples collected by venipuncture was placed in ethylenediaminetetraacetic acid tubes, centrifuged, and the plasma was removed and stored at −70 °C [22]. Plasma lipids, including HDL cholesterol, were assessed as previously described [22]. HDL particle concentration and size were likewise described previously [15]. HDL cholesterol efflux capacity was assessed by measuring fluorescence-labeled cholesterol efflux from J774 macrophages to apolipoprotein B-depleted plasma, as previously described [23].
2.2. Brain Magnetic Resonance Imaging (Brain MRI)
The DHS brain MRI protocol was performed on a 3.0 Tesla MR imaging unit (Achieva; Philips Medical Systems, Memphis, TN, USA) with acquisition of two-dimensional, fluid attenuated inversion recovery (FLAIR) images obtained with an echo time of 130 ms, a repetition time of 11,000 ms, and an inversion time of 2800 ms; a sensitivity encoding factor of 2; a echo train length of 44; a field of view of 250 × 250 mm; and 4 mm sections spaced at 5 mm centers. Three-dimensional magnetization prepared rapid acquisition with gradient echo (MPRAGE) images that were obtained with an echo time of 5.8 and a repetition time of 9.6 ms; a sensitivity encoding factor of 2; a flip angle of 12°; a field of view of 260 × 260 mm; and 2 mm sections spaced at 1 mm centers. Further details are as previously described [24]. The morphologic variables selected for the present analysis were total cranial volume (TCV), white matter hyperintensities (WMH), and whole-brain grey matter volume (GMV). Structural brain imaging measures used for this analysis were acquired through regional quantification of brain volumes using the freely available Functional MRI of the Brain Software Library (FSL v6.0) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, accessed on 2 September 2024) [24,25,26].
2.3. The Montreal Cognitive Assessment (MoCA)
Total MoCA score is a 30-point screening tool to assess cognitive function [27], which includes assessment of attention, verbal memory, orientation, language, visuospatial, and executive function [28]. The MoCA was administered by trained research personnel in the DHS-2 and double-checked for accuracy, as previously described [27].
2.4. Physical Activity Assessment
A wrist-based accelerometer (Actical, Philips Respironics, Bend, OR, USA) was used to measure time spent in moderate-to-vigorous physical activity (MVPA) for participants enrolled in the Dallas Heart Study between 2008 and 2009, as previously described [21].
2.5. Apolipoprotein E (ApoE) Genotyping
TaqMan single-nucleotide polymorphism genotyping assays (Applied Biosystems, Foster City, CA, USA) were used to genotype c.388T> C (rs429358) and c.526C > T (rs7412), that define three haplotypes ε2(388 T–526 T), ε3(388 T-526C), and ɛ4(388C-526C) from genomic DNA that was extracted from circulating leukocytes. Positive carrier status for ApoE-ε4 allele was defined as ε2/ε4, ε3/ε4, or ε4/ε4 and negative as ε2/ε2, ε2/ε3, or ε3/ε3.
2.6. Statistical Analysis
Statistical analyses were performed using SAS version 9.4 (Carey, NC, USA). Participant characteristics are reported as the mean ± SD (standard deviation), or mean with 95% SD, for continuous data. Frequency with percentage (%) are reported for categorical data. The Mann–Whitney U test and the two-sample t-test were used to compare continuous baseline measurements while the Chi-squared test was used to compare categorical baseline measurements. Multivariable linear regression analyses were conducted to assess associations between lipid exposures and brain and cognitive outcomes. Standardized beta estimates with 95% confidence intervals (CI) were reported. The standardized beta predicted the change in the dependent variable for 1 SD of change in the independent variable.
Multivariable linear regression analyses were performed to assess the associations between HDL and lipoprotein measures averaged from both DHS phases and total MoCA score, WMH/TCV, and GMV/TCV from DHS-2. Models were performed with a single HDL parameter and were adjusted for the following covariates: age, sex, race/ethnicity, smoking status, education level, and MVPA from DHS-2; and mean systolic blood pressure (SBP), fasting plasma glucose, body mass index (BMI), and estimated glomerular filtration rate averaged from DHS-1 and DHS-2. The use of mean exposure variables as well as the mean levels of covariates during the follow-up between DHS-1 and DHS-2 allow for assessment of the cumulative impact of cardiovascular risk factors on the brain structure and function outcomes, which was used previously in longitudinal studies [29,30]. Education levels were categorized into four groups: (1) less than high school education, (2) high school education, (3) college education, and (4) above college degree. All modeling assumptions were verified utilizing diagnostic tools (residual versus predicted value plot, Cook’s distance, and Q–Q plot). Similar models were performed to test interactions between race/ethnicity and risk factors by including multiplicative interaction terms. Since GMV and WMH data were skewed, analyses were performed after log transformation. Restricted cubic splines of association between cardiac and brain variables were constructed. Statistical significance was set a priori as p-value < 0.05.
3. Results
3.1. Participant Characteristics
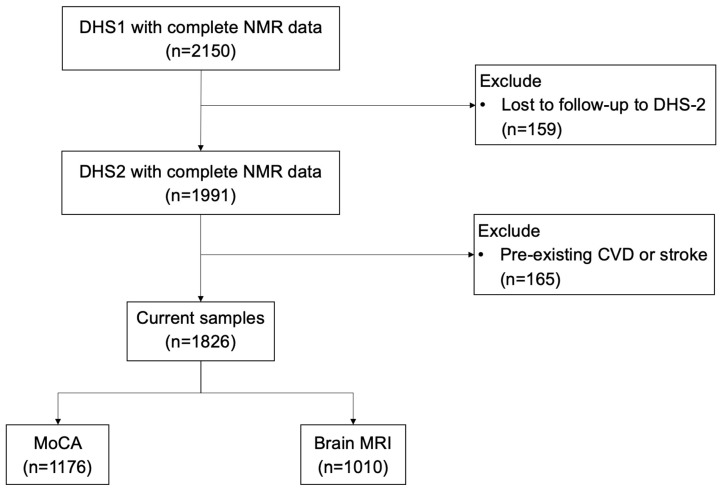
As shown in Figure 1, there were 2150 participants in DHS-1 that had complete HDL particle measurement by NMR, of whom 159 were lost to follow-up and did not participate in DHS-2. Of the participants in DHS-1, there were 1991 who had participated in both phases (DHS-1 and DHS-2). Among those, 165 were removed due to prior myocardial infarction, cardiac arrest, or stroke, leaving 1826 adults included for analyses. Among these remaining participants, 1010 underwent brain MRI and 1176 underwent the Montreal Cognitive Assessment (MoCA).
Figure 1.
Study Flow Chart (CVD: cardiovascular disease; DHS: Dallas Heart Study; MRI: magnetic resonance imaging; NMR: nuclear magnetic resonance; MoCA: Montreal Cognitive Assessment).
The characteristics of the study participants are shown in Table 1. Overall, the mean age at the DHS-2 visit was 51.0 ± 9.7 years, with the cohort being 58% female, and 47% Black adults. Approximately 21% were current smokers and 32% were ApoE-ε4 carriers. Postsecondary education levels (i.e., college level and above) were reported by 63% of participants. The average lipid measures were as follows: mean HDL-C was 53.4 ± 12.8 mg/dL, total HDL particle (HDL-P) was 21.8 ± 3.2 μmol/L, small HDL-P was 15.0 ± 3.0 μmol/L, medium HDL-P was 4.7 ± 2.4 μmol/L, large HDL-P was 2.2 ± 1.4 μmol/L, and mean HDL-CEC was 0.93 ± 0.21 AU. The mean time spent engaging in MVPA was 39 (95% CI 37.2–40.7) minutes per day and the average systolic blood pressure was 128 ± 16 mmHg.
Table 1.
Participant characteristics in each phase of the Dallas Heart Study.
| Variable | DHS-1 | DHS-2 | Average (DHS-1 and DHS-2) |
|---|---|---|---|
| Female (%) | 1060 (58%) | ||
| Black participants | 863 (47%) | ||
| Age (years old) | 43.7 ± 9.8 | 51.0 ± 9.7 | |
| BMI (kg/m2) | 30.5 ± 7.4 | 31.2 ± 7.4 | 30.9 ± 7.2 |
| Fasting plasma glucose | 100.2 ± 36.0 | 102.0 ± 34.0 | 101.1 ± 30.8 |
| eGFR (mL/min per 1.73 m2) | 99.2 ± 22.7 | 93.1 ± 26.5 | 96.2 ± 22.7 |
| Systolic blood pressure (mm Hg) | 123 ± 18 | 132 ± 19 | 128 ± 16 |
| HDL particle, μmol/L | 20.9 ± 3.9 | 22.7 ± 3.6 | 21.8 ± 3.2 |
| Small HDL particle, μmol/L | 15.0 ± 3.5 | 15.0 ± 3.6 | 15.0 ± 3.0 |
| Medium HDL particle, μmol/L | 3.7 ± 2.8 | 5.6 ± 2.6 | 4.7 ± 2.4 |
| Large HDL particle, μmol/L | 2.2 ± 1.6 | 2.1 ± 1.5 | 2.2 ± 1.4 |
| HDL cholesterol, mg/dL | 51.1 ± 14.7 | 55.7 ± 13.2 | 53.4 ± 12.8 |
| Apolipoprotein A-I, mg/dL | 127.6 ± 29.6 | 142.9 ± 26.4 | 135.3 ± 25.3 |
| HDL cholesterol efflux capacity (AU) | 1.03 ± 0.32 | 0.83 ± 0.24 | 0.93 ± 0.21 |
| Current smoker (%) | 440 (24.1%) | 384 (21.3%) | |
| MVPA (min/day) | 39 (95% CI 37.2–40.7) | ||
| ApoE-ε4 carrier (%) | 566 (31.5%) | ||
| Education Status | |||
| Less than high school | 257 (14%) | ||
| High school | 434 (24%) | ||
| College level | 957 (53%) | ||
| Above college | 172 (10%) | ||
Data are presented as the mean ± SD, mean with 95% SD, or frequency with percentage. BMI: body mass index; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; MVPA: moderate-to-vigorous physical activity.
3.2. Association between HDL Measures and Brain Structure
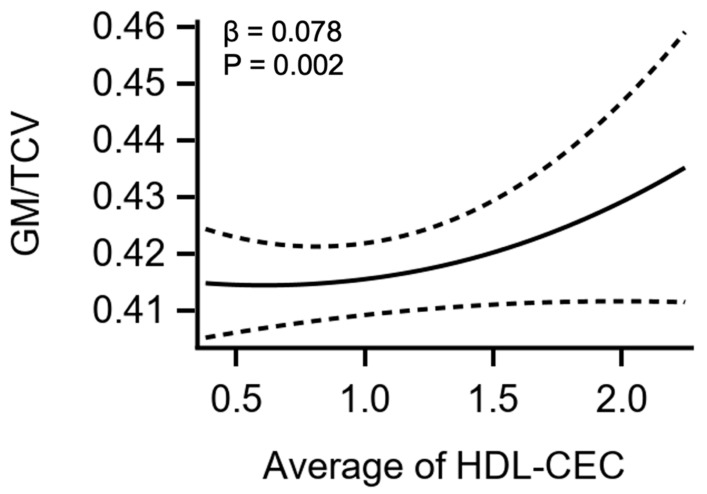
On multivariable regression, higher HDL cholesterol efflux capacity and higher circulating levels of small HDL-P were positively associated with greater GMV after normalization by TCV and adjustment for cardiovascular risk factors including age, smoking status, MVPA, and education (β = 0.078 [95% CI: 0.029, 0.126], p = 0.002, and β = 0.063 [95% CI: 0.014, 0.111], p = 0.012, respectively, Table 2, Figure 2). Conversely, there were no associations seen between total HDL-P, medium HDL-P, large HDL-P, HDL-C, or ApoAI with GMV after normalization by TCV and full adjustment in the whole cohort on multivariable regression analysis. The positive associations between HDL cholesterol efflux capacity and small HDL-P with GMV/TCV were not modified by ApoE-ε4 carrier status by race/ethnicity (all p > 0.05).
Table 2.
Multivariable associations between high-density lipoprotein measures and grey matter volume, white matter hyperintensities, and total Montreal Cognitive Assessment score.
| Outcome: Log GMV/TCV for Whole Cohort (n = 1010) | |||
|---|---|---|---|
| Variable | Β a | 95% CI | p-value |
| Total HDL-P | 0.011 | −0.039, 0.060 | 0.668 |
| Small HDL-P | 0.063 | 0.014, 0.111 | 0.012 b |
| Medium HDL-P | −0.045 | −0.095, 0.004 | 0.072 |
| Large HDL-P | −0.041 | −0.094, 0.012 | 0.130 |
| HDL-C | −0.040 | −0.092, 0.014 | 0.149 |
| ApoAI | −0.020 | −0.070, 0.035 | 0.516 |
| HDL-CEC * | 0.078 | 0.029, 0.126 | 0.002 b |
| Outcome: Log WMH/TCV for Whole Cohort (n = 1010) | |||
| HDL-P | 0.003 | −0.055, 0.061 | 0.920 |
| Small HDL-P | −0.014 | −0.072, 0.043 | 0.623 |
| Medium HDL-P | 0.0001 | −0.058, 0.058 | 0.997 |
| Large HDL-P | 0.042 | −0.020, 0.105 | 0.181 |
| HDL-C | 0.018 | −0.045, 0.081 | 0.573 |
| ApoAI | 0.012 | −0.050, 0.073 | 0.713 |
| HDL-CEC * | 0.033 | −0.024, 0.091 | 0.256 |
| Outcome: MoCA for Whole Cohort (n = 1176) | |||
| HDL-P | 0.021 | −0.028, 0.070 | 0.408 |
| Small HDL-P | −0.005 | −0.054, 0.044 | 0.844 |
| Medium HDL-P | 0.017 | −0.032, 0.067 | 0.498 |
| Large HDL-P | 0.033 | −0.020, 0.086 | 0.218 |
| HDL-C | 0.044 | −0.009, 0.097 | 0.103 |
| ApoAI | 0.036 | −0.016, 0.088 | 0.171 |
| HDL-CEC * | −0.009 | −0.059, 0.042 | 0.737 |
a β (standardized regression coefficients) and 95% confidence intervals. b Indicates statistical significance. All exposures are an average from DHS 1 and DHS 2. Model: Multivariable models were performed with a single HDL parameter and adjusted for age, sex, race/ethnicity, smoking status, education level, and MVPA from DHS-2; and BMI, systolic BP, fasting plasma glucose, and eGFR averaged from DHS-1 and DHS-2. * Includes HDL-C as covariate in the model plus the above adjustments. Abbreviations: ApoAI: apolipoprotein AI; BMI: body mass index; BP: blood pressure; CEC: cholesterol efflux capacity; DHS: Dallas Heart Study; eGFR: estimated glomerular filtration rate; GMV: grey matter volume; HDL: high-density lipoprotein; MVPA: moderate-to-vigorous physical activity; TCV: total cranial volume; WMH: white matter hyperintensities.
Figure 2.
Cubic Spline of HDL-CEC with GM Volume. Restricted cubic spline and 95% confidence bands relating the average HDL-CEC with GMV in the whole cohort after adjustment for age, sex, race, MVPA, smoking status, education levels, and the average across DHS-1 and DHS-2 of BMI, systolic BP, plasma glucose, eGFR, and average HDL-C from both DHS phases. (BMI: body mass index; CEC: cholesterol efflux capacity; eGFR: estimated glomerular filtration rate; GMV: grey matter volume; HDL: high-density lipoprotein; MVPA: moderate-to-vigorous physical activity; BP: blood pressure; β: standardized regression coefficient.)
There were no associations between mean HDL-P, small HDL-P, medium HDL-P, large HDL-P, HDL-C, ApoAI, or HDL cholesterol efflux capacity with white matter hyperintensities normalized by total cranial volumes for the whole cohort (all p > 0.05, Table 2).
3.3. Association Between HDL Measures and Cognitive Function
No significant associations were observed between mean HDL-P, small HDL-P, medium HDL-P, large HDL-P, HDL-C, ApoAI, or HDL cholesterol efflux capacity with total MoCA score after adjustment for cardiovascular risk factors, education, and MVPA (all p > 0.05, Table 2).
4. Discussion
The main findings from our study are threefold. First, higher levels of HDL cholesterol efflux capacity, a measure of HDL reverse cholesterol transport functionality, was positively associated with greater grey matter volume after normalization to total cranial volume. Second, higher levels of circulating small HDL particles in the plasma were associated with greater grey matter volume. Third, the positive associations between HDL cholesterol efflux capacity and small HDL-P with grey matter volume were not modified by race/ethnicity or ApoE-ε4 carrier status.
Prior studies and meta-analyses have shown an inconsistent relationship between circulating plasma HDL-C and cognitive function or risk of dementia [5,6,7,13,31,32,33,34]. However, imaging studies have not been performed to investigate the impact of HDL composition or function on brain structure. Our study demonstrated that higher levels of HDL-CEC and plasma concentration of small HDL-P were positively associated with grey matter volume. This is significant, as previous studies have shown associations between GMV loss and the development of mild cognitive impairment (MCI) and the conversion of MCI to Alzheimer’s disease (AD) [35]. These findings may be explained by the observation that the majority of HDL (~95%) particles that crosses the blood–brain barrier are small HDL particles [36,37]. Once in cerebrospinal (CSF) fluid, small HDL particles play important roles in cholesterol biosynthesis, reducing inflammation, maintaining neural membrane integrity, and neuronal regeneration [38,39,40]. In the context of our findings, high HDL cholesterol efflux capacity and high levels of small HDL-P in the peripheral circulation likely reflect a metabolic milieu that is protective against neuronal loss and brain atrophy that are characteristic of neurodegenerative diseases.
Despite the significant association between HDL-CEC and small HDL with grey matter, no associations with MoCA score or white matter hyperintensities (WMH) were identified in our study. Previous studies have demonstrated plasma levels of small HDL are significantly lower in patients with AD when compared to healthy older adults, which aligns with our findings [36,41]. Moreover, the presence of high WMH volume generally represent cerebral small vessel disease, which is common among older adults with cardiovascular disease risk factors such as elevated blood pressure or stroke [42]. Thus, the inclusion of cognitively normal, younger participants (~50 years of age), and those free of clinical cardiovascular disease in our study likely contributed to the lack of association between our exposures and WMH. Reduced grey matter volume is a recognized risk marker for cognitive decline and the development of dementia in multiple populations [9,35,43]. Taken together, our findings support a beneficial role of HDL efflux capacity and circulating levels of small HDL in preserving grey matter volume and potential prevention of brain atrophy in healthy, diverse, middle-aged adults.
While some population studies have implicated plasma HDL-C in the pathogenesis of impaired cognitive function and dementia [44,45], the exact relationship remains controversial [11,12]. Our data did not identify associations between HDL-C with the MoCA, WMH, or GMV in the overall cohort. However, there were major differences in assessment of lipid measures, statistical design, and study population characteristics for the present study when compared to previous investigations [11,12,45]. Indeed, our study uniquely included serial measurements of lipoprotein variables to assess the cumulative impact of these risk factors in a middle-age and diverse cohort. Moreover, our study adjusted for several important confounders including race/ethnicity, smoking status, fasting plasma glucose, and time spent in daily physical activity, that were not consistently accounted for in prior studies, which may explain differences in results.
Our study had several strengths. The associations of the present study were observed in a large, population-based cohort of middle-aged adults, who were free from prior CVD or stroke, and appeared independent of traditional risk factors including age, smoking status, time spent in daily physical activity, and education level. Secondly, the focus of our study was placed on healthy, middle-aged individuals, where early-stage dementia risk may be present. Moreover, our study included serial measurements of HDL-CEC and lipoprotein concentrations and size that were averaged in the same participants across two visits to account for the cumulative exposure to these parameters [29,30]. Our study is limited by the use of the MoCA as the only tool for assessment of cognitive function, and lack of CSF collection for participants in the present study. Therefore, future studies are needed to characterize association between HDL structure/function with more comprehensive neurocognitive assessment in all domains. However, the use of CSF samples in populations with normal cognition as a screening tool is impractical. Nevertheless, changes in brain structure in cognitively intact adults, such as reduced GMV as a process of normal aging, are thought to be related to downstream changes in cognitive function later in life [46]. Therefore, having higher GMV in mid-life may aid in preventing cognitive decline associated with brain atrophy as part of normal aging in late life.
5. Conclusions
Our data suggest that higher levels of HDL cholesterol efflux capacity and higher levels of small circulating HDL particles are positively associated with greater grey matter volume independent of traditional risk factors such as age, smoking status, time spent in daily physical activity, and education level. Future studies should focus on determining if adopting strategies to maintain optimal HDL functionality and HDL composition during midlife results in the preservation of gray matter volume into late life.
Author Contributions
Conception and design of this study, interpreted study findings, and wrote this manuscript, J.M.G., A.R. and W.V.; contributed to planning analysis and data analysis, J.W., W.V. and J.M.G.; contributed to data collection and storage, R.Z., B.J.K., I.H., F.F.Y. and B.P.T.; provided critical feedback and helped shape the research, analysis, and manuscript, J.A.d.L., A.R. and W.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board at the University of Texas Southwestern Medical Center (ClinicalTrials.gov ID: NCT00344903, date of approval 17 November 2006).
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The Dallas Heart Study was funded by the Donald W. Reynolds Foundation and was partially supported by the National Center for Advancing Translational Sciences of the NIH (UL1TR001105). W.V. is supported by the UT Southwestern O’Brien Kidney Center, the Charles and Jane Pak Center for Mineral Metabolism, and Clinical Research R01 AG057571.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yaffe K., Vittinghoff E., Pletcher M.J., Hoang T.D., Launer L.J., Whitmer R.A., Coker L.H., Sidney S. Early Adult to Midlife Cardiovascular Risk Factors and Cognitive Function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman R.F., Schneider A.L.C., Zhou Y., Coresh J., Green E., Gupta N., Knopman D.S., Mintz A., Rahmim A., Sharrett A.R., et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivipelto M., Helkala E.-L., Laakso M.P., Hänninen T., Hallikainen M., Alhainen K., Soininen H., Tuomilehto J., Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon A., Kivipelto M., Wolozin B., Zhou J., Whitmer R.A. Midlife Serum Cholesterol and Increased Risk of Alzheimer’s and Vascular Dementia Three Decades Later. Dement. Geriatr. Cogn. Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates K.A., Sohrabi H.R., Rainey-Smith S.R., Weinborn M., Bucks R.S., Rodrigues M., Beilby J., Howard M., Taddei K., Martins G., et al. Serum high-density lipoprotein is associated with better cognitive function in a cross-sectional study of aging women. Int. J. Neurosci. 2016;127:243–252. doi: 10.1080/00207454.2016.1182527. [DOI] [PubMed] [Google Scholar]
- 6.Crichton G.E., Elias M.F., Davey A., Sullivan K.J., Robbins M.A. Higher HDL Cholesterol Is Associated with Better Cognitive Function: The Maine-Syracuse Study. J. Int. Neuropsychol. Soc. 2014;20:961–970. doi: 10.1017/S1355617714000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal G., Braidy N., Ahmed T. Blood-Based Biomarkers for Predictive Diagnosis of Cognitive Impairment in a Pakistani Population. Front. Aging Neurosci. 2020;12:223. doi: 10.3389/fnagi.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed B., Villeneuve S., Mack W., DeCarli C., Chui H.C., Jagust W. Associations Between Serum Cholesterol Levels and Cerebral Amyloidosis. JAMA Neurol. 2014;71:195–200. doi: 10.1001/jamaneurol.2013.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong N.M., An Y., Beason-Held L., Doshi J., Erus G., Ferrucci L., Davatzikos C., Resnick S.M. Predictors of neurodegeneration differ between cognitively normal and subsequently impaired older adults. Neurobiol. Aging. 2018;75:178–186. doi: 10.1016/j.neurobiolaging.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turri M., Marchi C., Adorni M.P., Calabresi L., Zimetti F. Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids. 2022;1867:159123. doi: 10.1016/j.bbalip.2022.159123. [DOI] [PubMed] [Google Scholar]
- 11.Peters R., Xu Y., Antikainen R., Beckett N., Gussekloo J., Jagger C., Jukema J.W., Keinanen-Kiukaanniemi S., Rydén L., Skoog I., et al. Evaluation of High Cholesterol and Risk of Dementia and Cognitive Decline in Older Adults Using Individual Patient Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2021;50:318–325. doi: 10.1159/000519452. [DOI] [PubMed] [Google Scholar]
- 12.Iwagami M., Qizilbash N., Gregson J., Douglas I., Johnson M., Pearce N., Evans S., Pocock S. Blood cholesterol and risk of dementia in more than 1·8 million people over two decades: A retrospective cohort study. Lancet Health. Longev. 2021;2:e498–e506. doi: 10.1016/S2666-7568(21)00150-1. [DOI] [PubMed] [Google Scholar]
- 13.Schilling S., Tzourio C., Soumaré A., Kaffashian S., Dartigues J.-F., Ancelin M.-L., Samieri C., Dufouil C., Debette S. Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study. PLoS Med. 2017;14:e1002265. doi: 10.1371/journal.pmed.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A., Westerterp M., von Eckardstein A., Remaley A., Rye K.-A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation. 2021;143:2293–2309. doi: 10.1161/CIRCULATIONAHA.120.044221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohatgi A., Khera A., Berry J.D., Givens E.G., Ayers C.R., Wedin K.E., Neeland I.J., Yuhanna I.S., Rader D.R., de Lemos J.A., et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye H., Xu G., Ren L., Peng J. Cholesterol efflux capacity in coronary artery disease: A meta-analysis. Coron. Artery Dis. 2020;31:642–649. doi: 10.1097/MCA.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 17.Abramov A.Y., Ionov M., Pavlov E., Duchen M.R. Membrane cholesterol content plays a key role in the neurotoxicity of β-amyloid: Implications for Alzheimer’s disease. Aging Cell. 2011;10:595–603. doi: 10.1111/j.1474-9726.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 18.Ko Y.-A., Billheimer J.T., Lyssenko N.N., Kueider-Paisley A., Wolk D.A., Arnold S.E., Leung Y.Y., Shaw L.M., Trojanowski J.Q., Kaddurah-Daouk R.F., et al. ApoJ/Clusterin concentrations are determinants of cerebrospinal fluid cholesterol efflux capacity and reduced levels are associated with Alzheimer’s disease. Alzheimer’s Res. Ther. 2022;14:1–12. doi: 10.1186/s13195-022-01119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchi C., Adorni M.P., Caffarra P., Ronda N., Spallazzi M., Barocco F., Galimberti D., Bernini F., Zimetti F. ABCA1- and ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer’s disease. J. Lipid Res. 2019;60:1449–1456. doi: 10.1194/jlr.P091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yassine H.N., Feng Q., Chiang J., Petrosspour L.M., Fonteh A.N., Chui H.C., Harrington M.G. ABCA1-Mediated Cholesterol Efflux Capacity to Cerebrospinal Fluid Is Reduced in Patients With Mild Cognitive Impairment and Alzheimer’s Disease. J. Am. Hear. Assoc. 2016;5 doi: 10.1161/JAHA.115.002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakoski S.G., Kozlitina J. Ethnic Differences in Physical Activity and Metabolic Risk. Med. Sci. Sports Exerc. 2014;46:1124–1132. doi: 10.1249/MSS.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 22.Victor R.G., Haley R.W., Willett D.L., Peshock R.M., Vaeth P.C., Leonard D., Basit M., Cooper R.S., Iannacchione V.G., Visscher W.A., et al. The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am. J. Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Sankaranarayanan S., Kellner-Weibel G., de la Llera-Moya M., Phillips M.C., Asztalos B.F., Bittman R., Rothblat G.H. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 2011;52:2332–2340. doi: 10.1194/jlr.D018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta M., King K.S., Srinivasa R., Weiner M.F., Hulsey K., Ayers C.R., Whittemore A., McColl R.W., Rossetti H.C., Peshock R.M. Association of 3.0-T Brain Magnetic Resonance Imaging Biomarkers With Cognitive Function in the Dallas Heart Study. JAMA Neurol. 2015;72:170–175. doi: 10.1001/jamaneurol.2014.3418. [DOI] [PubMed] [Google Scholar]
- 25.Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C.F., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 26.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Rossetti H.C., Lacritz L.H., Hynan L.S., Cullum C.M., Van Wright A., Weiner M.F. Montreal Cognitive Assessment Performance among Community-Dwelling African Americans. Arch. Clin. Neuropsychol. 2016;32:238–244. doi: 10.1093/arclin/acw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahinrad S., Kurian S., Garner C.R., Sedaghat S., Nemeth A.J., Moscufo N., Higgins J.P., Jacobs D.R., Jr., Hausdorff J.M., Lloyd-Jones D.M., et al. Cumulative Blood Pressure Exposure During Young Adulthood and Mobility and Cognitive Function in Midlife. Circulation. 2020;141:712–724. doi: 10.1161/CIRCULATIONAHA.119.042502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navar-Boggan A.M., Peterson E.D., D’agostino R.B., Neely B., Sniderman A.D., Pencina M.J. Hyperlipidemia in Early Adulthood Increases Long-Term Risk of Coronary Heart Disease. Circulation. 2015;131:451–458. doi: 10.1161/CIRCULATIONAHA.114.012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson V.W., Guthrie J.R., Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. J. Neurol. Neurosurg. Psychiatry. 2003;74:1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitz C., Tang M.-X., Manly J., Schupf N., Mayeux R., Luchsinger J.A. Plasma Lipid Levels in the Elderly Are Not Associated with the Risk of Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2008;25:232–237. doi: 10.1159/000115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sáiz-Vazquez O., Puente-Martínez A., Ubillos-Landa S., Pacheco-Bonrostro J., Santabárbara J. Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis. Brain Sci. 2020;10:386. doi: 10.3390/brainsci10060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reitz C., Tang M.-X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. Association of Higher Levels of High-Density Lipoprotein Cholesterol in Elderly Individuals and Lower Risk of Late-Onset Alzheimer Disease. Arch. Neurol. 2010;67:1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Mortel L.A., Initiative F.T.A.D.N., Thomas R.M., van Wingen G.A. Grey Matter Loss at Different Stages of Cognitive Decline: A Role for the Thalamus in Developing Alzheimer’s Disease. J. Alzheimer’s Dis. 2021;83:705–720. doi: 10.3233/JAD-210173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez A.E., Weissberger G., Kuklenyik Z., He X., Meuret C., Parekh T., Rees J.C., Parks B.A., Gardner M.S., King S.M., et al. The small HDL particle hypothesis of Alzheimer’s disease. Alzheimer’s Dement. 2022;19:391–404. doi: 10.1002/alz.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borràs C., Mercer A., Sirisi S., Alcolea D., Escolà-Gil J.C., Blanco-Vaca F., Tondo M. HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2022;23:9356. doi: 10.3390/ijms23169356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckert G., Cairns N., Maras A., Gattaz W., Müller W. Cholesterol Modulates the Membrane- Disordering Effects of Beta-Amyloid Peptides in the Hippocampus: Specific Changes in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2000;11:181–186. doi: 10.1159/000017234. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Kulas J.A., Wang C., Holtzman D.M., Ferris H.A., Hansen S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. USA. 2021;118:e2102191118. doi: 10.1073/pnas.2102191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koudinov A.R., Koudinova N.V. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 2001;15:1858–1860. doi: 10.1096/fj.00-0815fje. [DOI] [PubMed] [Google Scholar]
- 41.Pedrini S., Hone E., Gupta V.B., James I., Teimouri E., Bush A.I., Rowe C.C., Villemagne V.L., Ames D., Masters C.L., et al. Plasma High Density Lipoprotein Small Subclass is Reduced in Alzheimer’s Disease Patients and Correlates with Cognitive Performance. J. Alzheimer’s Dis. 2020;77:733–744. doi: 10.3233/JAD-200291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wardlaw J.M., Hernández M.C.V., Muñoz-Maniega S. What are White Matter Hyperintensities Made of? J. Am. Hear. Assoc. 2015;4:001140. doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karas G., Scheltens P., Rombouts S., Visser P., van Schijndel R., Fox N., Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. NeuroImage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 44.European Alzheimer’s & Dementia Biobank Mendelian Randomization (EADB-MR) Collaboration. Luo J., Thomassen J.Q., Bellenguez C., Grenier-Boley B., de Rojas I., Castillo A., Parveen K., Küçükali F., Nicolas A., et al. Genetic Associations Between Modifiable Risk Factors and Alzheimer Disease. JAMA Netw. Open. 2023;6:e2313734. doi: 10.1001/jamanetworkopen.2023.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson E.L., Zimmerman S.C., Jiang C., Choi M., Swinnerton K., Choudhary V., Meyers T.J., Hoffmann T.J., Gilsanz P., Oni-Orisan A., et al. Low- and High-Density Lipoprotein Cholesterol and Dementia Risk Over 17 Years of Follow-up Among Members of a Large Health Care Plan. Neurology. 2023;101:E2172–E2184. doi: 10.1212/WNL.0000000000207876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neufeld N., Parker A.F., Kwan H., Mazerolle E.L., Gawryluk J.R. Longitudinal changes in grey matter and cognitive performance over four years of healthy aging. Neuroimage Rep. 2022;2:100140. doi: 10.1016/j.ynirp.2022.100140. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.