Abstract
Four hantaviruses—Hantaan virus (HTNV), Seoul virus (SEOV), Dobrava virus (DOBV) and Puumala virus—are known to cause hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia. HTNV causes the most severe form of HFRS (5 to 15% case-fatality rate) and afflicts tens of thousands of people annually. Previously, we demonstrated that DNA vaccination with a plasmid expressing the SEOV M gene elicited neutralizing antibodies and protected hamsters against infection with SEOV and HTNV. Here, we report the construction and evaluation of a DNA vaccine that expresses the HTNV M gene products, G1 and G2. DNA vaccination of hamsters with the HTNV M gene conferred sterile protection against infection with HTNV, SEOV, and DOBV. DNA vaccination of rhesus monkeys with either the SEOV or HTNV M gene elicited high levels of neutralizing antibodies. These are the first immunogenicity data for hantavirus DNA vaccines in nonhuman primates. Because a neutralizing antibody response is considered a surrogate marker for protective immunity in humans, our protection data in hamsters combined with the immunogenicity data in monkeys suggest that hantavirus M gene-based DNA vaccines could protect humans against the most severe forms of HFRS.
Hantaan virus (HTNV) (genus Hantavirus, family Bunyaviridae) is the causative agent of the most severe form of a rodent-borne disease known as hemorrhagic fever with renal syndrome (HFRS). Other hantaviruses that are known to cause HFRS include Seoul virus (SEOV), which causes disease primarily in Asia, and Dobrava virus (DOBV) and Puumala virus (PUUV), which cause disease in Europe, Scandinavia, and western Russia (19). In addition, several other hantaviruses have been associated with outbreaks of a highly lethal disease, hantavirus pulmonary syndrome (HPS), in the Americas (20). Because hantaviruses can cause epidemics with high morbidity, or outbreaks with high case-fatality rates, and because there is no proven therapy for hantaviral disease, a safe and effective vaccine(s) against hantaviruses is needed.
Hantavirus virions are enveloped particles that contain a tripartite genome consisting of three negative-sense RNA segments (21). The large (L) segment encodes the RNA-dependent RNA polymerase the small (S) segment encodes the nucleocapsid protein (N) and the medium (M) segment encodes a polypeptide that is cotranslationally cleaved to yield two membrane-associated glycoproteins, G1 and G2. G1 and G2 form oligomers that comprise the surface morphologic units of the virion and are the targets of neutralizing antibodies (1, 9, 29). Passive transfer of neutralizing antibodies protects newborn rats (29), suckling mice (2), or hamsters (24) against infection. N-specific antibodies are neither neutralizing nor protective.
We previously demonstrated that DNA vaccination with a plasmid containing a cDNA representing the SEOV M segment (pWRG/SEO-M) elicited neutralizing antibody responses in mice and hamsters (11). In a hamster infection model, we demonstrated that gene gun vaccination with pWRG/SEO-M, but not with a plasmid containing a cDNA representing the SEOV S segment, protected hamsters against infection with SEOV and HTNV (11, 12).
Here we report the development of a HTNV M DNA vaccine. This vaccine expresses the G1 and G2 proteins of HTNV, elicits neutralizing antibodies, and protects hamsters against infection with three of the four hantaviruses known to cause HFRS. More importantly, we demonstrate for the first time that both the SEOV M and HTNV M DNA vaccines elicit high-titer neutralizing antibody responses in rhesus macaques. These nonhuman primate data are an important contribution to the development of a DNA vaccine to protect humans against hantaviral disease.
MATERIALS AND METHODS
Viruses, cells, medium, and MAbs.
HTNV strain 76-118 (14), SEOV strain SR-11 (13), DOBV (4) and PUUV strain K27 (26) were propagated in Vero E6 cells (Vero C1008; ATCC CRL 1586). Transient-expression experiments were performed with COS cells (COS-7; ATTC CRL1651). Both cell types were maintained in Eagle minimal essential medium with Earle's salts (EMEM) containing 10% fetal bovine serum, 10 mM HEPES (pH 7.4), and the antibiotics penicillin (100 U/ml), streptomycin (100 μg/ml), and gentamicin (50 μg/ml) (cEMEM) at 37°C in a 5% CO2 incubator.
The HTNV G1-specific monoclonal antibody (MAb) 6D4 and the G2-specific MAb 23G10 were described previously (1).
Construction of hantavirus M gene DNA vaccine plasmids.
Construction of the SEOV M DNA vaccine plasmid, pWRG/SEO-M, was as described previously (11).
To make pWRG/SEO-M(x), DNA encoding the SEOV G1 and G2 proteins was amplified by PCR from pWRG/SEO-M by using a forward primer (primer1-24, 5′-GGCCGCGGCCGCGGATCTGCAGGAATTCGGCACGAGAGTAGTAGACTCCGCAAGAAACAGCA) and a reverse primer (SEOMX, 5′-GCGCGGATCCAGATTGGGAGATAGAAGAGAG). The PCR product was cut with NotI and BamHI and then ligated into NotI-BglII-cut pWRG7077 vector. This clone was made to remove undesirable cloning artifact DNA (∼100 nucleotides of the simian immunodeficiency virus nef gene) found between the BamHI and BglII sites of pWRG7077. Removing this sequence had no effect on expression of cloned genes (data not shown).
The HTNV M DNA vaccine plasmid, pWRG/HTN-M, was constructed essentially as follows. First, DNA encoding HTNV G1 and G2 was cut from pTZ19RHTNMm (22) as a BglII fragment and ligated into BamHI-cut pWRG7077 vector. This plasmid expressed G2 but not G1 (data not shown). Next, the NotI-PshAI fragment of this plasmid, which contained the 5′ end of the M gene, was excised and replaced with DNA amplified by PCR (from a pUC18 plasmid containing a full-length HTNV M gene cloned by reverse transcriptase-PCR cloning from viral RNA), using primer1-24 (see above) and a reverse primer (M5B, 5′-TCAGGACTCCTGTCATGCAATAAGATCTC). The reverse primer included silent nucleotide changes in the HTNV M gene that created a BglII site used for diagnostic purposes. The PCR product was cut with NotI and PshAI and ligated into the NotI-PshAI-cut plasmid to create pWRG/HTN-M.
pWRG/HTN-M(x) was constructed by using primer1-24 and a reverse primer (HTNMX, 5′-GCGCGGATCCGTTTGTGGTTAGAAAGCTAC) to PCR amplify the HTNV M gene from pWRG/HTN-M. PCR product was cut with NotI and BamHI and ligated into the NotI-BglII-cut pWRG7077 vector. This clone was identical to pWRG/HTN-M; however, a portion of the 3′-untranslated region of the gene and the vector sequence between BamHI and BglII was removed.
Plasmid DNA was purified by using Qiagen Maxiprep DNA purification kits according to the manufacturer's directions.
Immunoprecipitation.
COS cells grown in T-25 cell culture flasks were transfected with 5 μg of plasmid DNA with Fugene6 (Boehringer Mannheim). After 24 h, expression products were radiolabeled with Promix ([35S]methionine and [35S]cysteine; Amersham) and immunoprecipitated as described previously (25). Reduced samples were run on 4 to 12% bis-Tris sodium dodecyl sulfate polyacrylamide gel electrophoresis gradient gels with morpholinepropanesulfonic acid running buffer (NuPage), at a 200-V constant voltage.
Vaccinations.
Gene gun cartridges (∼0.5 μg of plasmid DNA coated on 0.5 mg of gold) were prepared, and outbred golden Syrian hamsters were gene gun vaccinated as described previously (11). Briefly, each vaccination consisted of four gene gun (Powderject-XR Delivery Device; Powderject Vaccines, Inc.) administrations (four cartridges) at nonoverlapping sites on the shaved abdominal epidermis using 400 lb/in2 of helium pressure. Hamsters were vaccinated three times at 3-week intervals. Rhesus macaques were vaccinated with the same type of cartridges and the same gene gun conditions used to vaccinate the hamsters; however, the monkeys received eight administrations per vaccination, rather than four. Hamsters and monkeys were anesthetized during the nonpainful gene gun procedure, which only results in mild erythema.
Rhesus macaques were vaccinated with a recombinant vaccinia virus, rVV/HTN-M+S, by the method used to vaccinate humans in a phase II clinical trial (17). The vaccine (3.4 × 107 PFU in 0.5 ml of PBS) was injected subcutaneously into the right lateral upper arm with a 26G 3/8-in. needle. After 42 days, the monkeys received an identical vaccination on the left arm.
Plaque reduction neutralization tests (PRNT).
Neutralization assays were performed essentially as previously described (7, 10). Heat-inactivated (56°C, 30 min) serum samples were diluted in cEMEM and then combined with an equal volume (111 μl) of cEMEM containing ∼75 PFU of virus and 10% guinea pig complement (catalog no. ACL-4051; Accurate Chemical and Scientific Corp.). This mixture was incubated overnight at 4°C, and then a plaque assay was performed exactly as described previously, using 7-day-old Vero E6 monolayers in six-well plates (11). HTNV and SEOV PRNT were stained with neutral red (Gibco-BRL) after 1 week and PUUV and DOBV PRNT were stained after 9 days. Plaques were counted 2 days (37°C) after staining.
Challenge with hantaviruses.
Adult, female, Syrian hamsters (Charles River) were injected intramuscularly (i.m.; caudal thigh, 25-gauge needle) with the indicated hantavirus diluted in 0.2 ml of sterile PBS (pH 7.4). The challenge dose for each virus was 2,000 PFU. This dose is ∼1,000 50% infective doses (ID50) for HTNV and SEOV, ∼100 ID50 for DOBV, and ∼10 ID50 for PUUV (J. W. Hooper, unpublished data). At 28 days after challenge, the hamsters were anesthetized and exsanguinated by cardiac puncture. Pre- and postchallenge sera were evaluated for the presence of N-specific antibodies by enzyme-linked immunosorbent assay (ELISA) and for the presence of neutralizing antibodies by PRNT. Detecting postchallenge N-specific antibody indicated that the hamster was infected with the challenge virus.
This animal research was conducted in accordance with procedures described in the 1996 Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Md.). The facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
N-specific ELISA.
The ELISA used to detect N-specific antibodies was previously described (10, 11). The antigen consisted of a truncated SEOV N (amino acids 1 to 117) or truncated PUUV N (amino acids 1 to 117) expressed as a histidine-tagged fusion protein by using the pRSET plasmid (Invitrogen) in Escherichia coli BL21(DE3) (Novagen, Inc.) and purified by affinity chromatography on Ni-nitrilotriacetic acid columns (Qiagen). A negative control antigen (pET19B plasmid expressing the Ebola virus nucleocapsid protein) was prepared by the same affinity chromatography method. The secondary antibody was horseradish peroxidase-labeled goat anti-hamster antibody (catalog no. 14-22-06; Kirkegaard & Perry Laboratories). The substrate was tetramethylbenzidine substrate (catalog no. 50-76-04; Kirkegaard & Perry Laboratories). The colorimetric reaction was stopped by adding Stop solution (catalog no. 50-85-04, Kirkegaard & Perry Laboratories), and the optical density (OD) at 450 nm was determined. Nonspecific binding was controlled for by subtracting OD values obtained on negative control antigen from OD values obtained on the hantavirus N antigen. Endpoint titers were determined as the highest dilution with an OD greater than the mean OD value of serum samples from negative control serum sample wells plus three standard deviations. The SEOV N antigen was used to detect HTNV N-, DOBV N-, and SEOV N-specific antibodies. The PUUV N was used to detect PUUV N-specific antibodies.
RESULTS
Expression of G1 and G2 from HTNV M DNA vaccine.
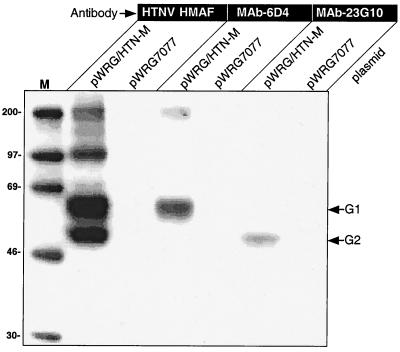
cDNA representing the HTNV M genome segment was cloned into a cytomegalovirus promoter-based expression plasmid, pWRG7077, to create pWRG/HTN-M. Radioimmunoprecipitation assay (RIPA) experiments using polyclonal antibodies and MAbs indicated that both the G1 and G2 proteins were transiently expressed in cells transfected with pWRG/HTN-M (Fig. 1).
FIG. 1.
Transient expression of HTNV G1 and G2. COS cells were transfected with pWRG/HTN-M or a negative control plasmid (pWRG7077) and, after 24 h, radiolabeled cell lysates were prepared for analysis by RIPA. Expression products were immunoprecipitated with a polyclonal mouse hyperimmune ascitic fluid against HTNV (HTN HMAF), a G1-specific MAb (MAb 6D4), or a G2-specific MAb (MAb 23G10). Molecular size markers (M) are shown in the first lane and sizes in kilodaltons are indicated to the left. The position of G1 and G2 are shown at the right.
DNA vaccination with pWRG/HTN-M elicits neutralizing antibodies and protects hamsters against infection with HTNV.
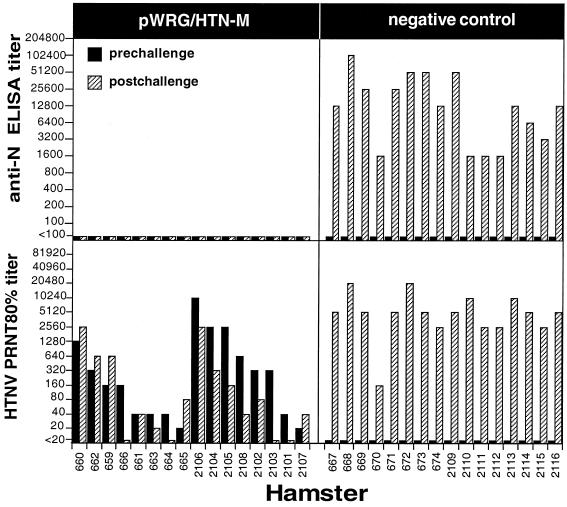
To determine if the HTNV M DNA vaccine plasmid was immunogenic, we used a gene gun to vaccinate hamsters with either pWRG/HTN-M (pWRG/HTN-M or pWRG/HTN-M(x); see Materials and Methods) or a negative control. Three weeks after the final vaccination, the hamsters were bled and sera were evaluated for neutralizing antibodies by PRNT. In two separate experiments, all of the hamsters vaccinated with pWRG/HTN-M developed HTNV-neutralizing antibody responses (Fig. 2). Titers (80% PRNT [PRNT80]) ranged from 20 to 1,280 with a geometric mean titer (GMT) of 104 in the first experiment and from 20 to 10,240 with a GMT of 493 in the second experiment. Negative control groups remained seronegative. Thus, gene gun vaccination with pWRG/HTN-M was immunogenic in hamsters.
FIG. 2.
DNA vaccination with plasmid expressing HTNV G1 and G2 protects against HTNV infection. The results of two independent experiments are combined in this figure. In the first experiment, one group of hamsters (659 to 666) was vaccinated with pWRG/HTN-M, and a second negative control group (667 to 674) was vaccinated with the vector plasmid, pWRG7077. In the second experiment one group of hamsters (2101 to 2108) was vaccinated with a slightly modified plasmid, pWRG/HTN-M(x), and a second negative control group (2109 to 2116) remained unvaccinated. Three weeks after the final vaccination, prechallenge serum samples were obtained, and the hamsters were challenged with HTNV. Postchallenge serum samples were collected 28 days after challenge. The pre- and postchallenge serum samples were tested for N-specific antibodies by anti-N ELISA and for neutralizing antibodies by PRNT. The pre- and postchallenge endpoint antibody titer for each hamster is shown. For each experiment, the prechallenge homologous PRNT80 titers were sorted from highest to lowest (left to right).
To determine the protective efficacy of pWRG/HTN-M, we used an infection model described previously (11). The model involves challenging vaccinated hamsters with virus and, after 4 weeks, using serological assays to detect evidence of infection. Specifically, if a challenged hamster developed antibodies to hantavirus N protein (which is not a component of the vaccine), then that hamster was considered to be infected. On the other hand, if a challenged hamster failed to develop a N-specific antibody response, then that hamster was considered not to be infected (i.e., protected against infection). A >4-fold increase in the neutralizing antibody response after challenge also served as a marker for evidence of infection.
Vaccinated hamsters were challenged with 2,000 PFU (the ID50 is approximately 2 PFU; Hooper, unpublished) of HTNV i.m. At 4 weeks after challenge, blood samples were collected, and sera were tested for N-specific antibodies by ELISA and for neutralizing antibodies by PRNT. All of the hamsters that were vaccinated with pWRG/HTN-M were protected against infection as defined by an absence of a postchallenge N-specific antibody response (Fig. 2). In addition, the pre- and postchallenge PRNT titers differed by ≤4-fold. In contrast, all of the negative control hamsters, whether they were vaccinated with pWRG7077 or remained unvaccinated, were infected, as evidenced by the development of N-specific antibodies and neutralizing antibodies postchallenge (Fig. 2). Thus, gene gun vaccination with pWRG/HTN-M [or pWRG/HTN-M(x)] protected against productive infection with HTNV, even when the prechallenge PRNT80 titer was as low as 20.
DNA vaccination with either pWRG/SEO-M or pWRG/HTN-M cross-protects against challenge with heterologous hantaviruses.
Having determined that our SEOV M (11, 12) and HTNV M gene-based DNA vaccines were capable of protecting hamsters against infection with homologous virus, we wanted to determine if either of these vaccines could cross-protect against other HFRS-associated hantaviruses. We first measured the cross-neutralizing activities of sera from HFRS hantavirus-infected hamsters and of hamsters vaccinated with either the SEOV M or HTNV M vaccine (Table 1). We found that sera from SEOV-infected hamsters had a low level of HTNV neutralizing activity and no detectable DOBV or PUUV neutralizing activity. Sera from HTNV- or DOBV-infected hamsters exhibited a low level of neutralizing antibodies against SEOV, DOBV, and PUUV. Sera from PUUV-infected hamsters failed to neutralize HTNV or SEOV and had a barely detectable DOBV-neutralizing activity. The sera from vaccinated hamsters exhibited greater levels of cross-neutralizing activity than the sera from the infected hamsters. Vaccination with either pWRG/SEO-M or pWRG/HTN-M elicited an antibody response that cross-neutralized SEOV, HTNV, and DOBV, but not PUUV.
TABLE 1.
Cross-neutralization activity in serum from infected or DNA-vaccinated hamsters
| Hamster serum sourcea | PRNT80 or PRNT50d in animals infected with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SEOV
|
HTNV
|
DOBV
|
PUUV
|
|||||
| 80% | 50% | 80% | 50% | 80% | 50% | 80% | 50% | |
| Infectedb with: | ||||||||
| SEOV | 640 | 5,120 | ∗ | 80 | ∗ | ∗ | ∗ | ∗ |
| HTNV | ∗ | 160 | 1,280 | 5,120 | 40 | 320 | ∗ | 20 |
| DOBV | 20 | 320 | 20 | 160 | 2,560 | 10,240 | ∗ | 40 |
| PUUV | ∗ | ∗ | ∗ | ∗ | ∗ | 20 | 5,120 | 20,480 |
| Vaccinatedc with: | ||||||||
| pWRG/SEO-M | 5,120 | 10,240 | 320 | 320 | 160 | 320 | ∗ | ∗ |
| pWRG/HTN-M | 40 | 80 | 5,120 | 5,120 | 160 | 640 | ∗ | ∗ |
A pool of sera from three to four hamsters with a homologous titer of at least 160 was used. Serum was collected 4 weeks after infection or 3 weeks after the final DNA vaccination.
Hamsters were injected i.m. with 2,000 PFU of the indicated virus.
Hamsters were gene gun-vaccinated three times at 3-week intervals with the indicated DNA vaccine plasmid.
PRNT values are the reciprocal serum dilutions that neutralize the indicated virus plaque number by 80% or 50%. Homologous titers are shown in boldface. ∗, titer of <20.
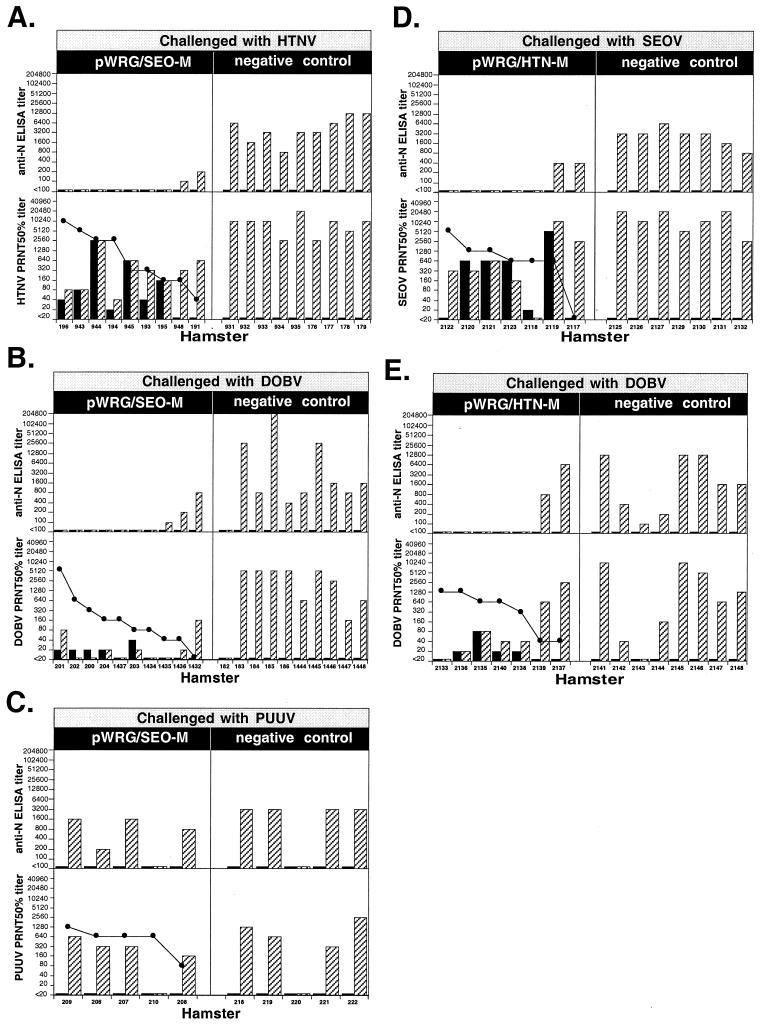
We previously demonstrated that the SEOV M DNA vaccine protected most hamsters not only from SEOV but also from challenge with HTNV (12). To confirm these findings and to further evaluate the capacity of the SEOV M vaccine to cross-protect against other hantaviruses, we tested pWRG/SEO-M for protective efficacy against HTNV, DOBV, and PUUV. The results of this study indicated that most hamsters vaccinated with pWRG/SEO-M were protected against HTNV and DOBV, but not PUUV, as determined by the absence of anti-N antibody response after challenge (Fig. 3). A homologous PRNT80 titer of >160 protected against HTNV, and a titer of ≥80 protected against DOBV, but a titer of as high as 1,280 failed to protect against PUUV (Fig. 3A, B, and C). One vaccinated and one control hamster failed to respond to the PUUV challenge, probably because the PUUV challenge dose was 10 ID50, whereas it was 10 to 100 times higher for the other viruses.
FIG. 3.
Cross-protection. Hamsters were vaccinated with the indicated plasmid [pWRG/SEO-M, pWRG/HTN-M(x), or a negative control], and then challenged with the indicated virus. The negative control hamsters in panels A, B, and C were vaccinated with a pWRG7077-based plasmid; the negative control hamsters in panels D and E remained unvaccinated. Pre- and postchallenge serum samples were tested for anti-N antibodies by anti-N ELISA and for neutralizing antibodies by PRNT. PRNT80 titers for homologous virus and PRNT50 titers for heterologous virus were determined. The prechallenge (■) and postchallenge ( ) endpoint antibody titers for each hamster are shown. Prechallenge homologous PRNT80 titers (sorted from highest to lowest, left to right) are shown as lines with symbols (●). The identification code for each hamster is shown on the x axis. The HTNV PRNT and anti-N ELISA data for hamsters 943, 944, 945, and 948 were published previously (11).
) endpoint antibody titers for each hamster are shown. Prechallenge homologous PRNT80 titers (sorted from highest to lowest, left to right) are shown as lines with symbols (●). The identification code for each hamster is shown on the x axis. The HTNV PRNT and anti-N ELISA data for hamsters 943, 944, 945, and 948 were published previously (11).
We similarly tested the capacity of pWRG/HTN-M to cross-protect against SEOV or DOBV. The results indicated that vaccination with pWRG/HTN-M elicited cross-protective immunity against both SEOV and DOBV (Fig. 3D and E). Homologous PRNT80 titers of ≥640 were associated with protection of hamsters against SEOV, and titers of ≥320 protected against DOBV. We did not measure the capacity of vaccination with pWRG/HTN-M to protect against PUUV infection because of our findings that the SEOV DNA vaccine did not protect (Fig. 3C) and because a vaccinia virus-vectored HTNV vaccine did not protect hamsters against PUUV infection (8). Together, these data indicate that DNA vaccination with either pWRG/SEO-M or pWRG/HTN-M cross-protected against three of the four HFRS-associated hantaviruses: SEOV, HTNV, and DOBV.
DNA vaccination with pWRG/SEO-M or pWRG/HTN-M elicits a high-titer neutralizing antibody response in nonhuman primates.
Our vaccination data with plasmids expressing the SEOV M gene or HTNV M gene suggest that these vaccines might be efficacious in humans. As a further step toward the clinical development of these vaccines, we tested their capacity to elicit antibody responses in nonhuman primates. Two rhesus macaques were vaccinated with the SEOV M DNA vaccine, and three rhesus macaques were vaccinated with the HTNV M DNA vaccine. As negative controls, three monkeys were vaccinated with pWRG7077 expressing irrelevant genes and, as positive controls, three monkeys were vaccinated with a recombinant vaccinia virus expressing the HTNV M and S genes, rVV/HTN-M+S (23). rVV/HTN-M+S was previously shown to elicit HTNV-specific immunity, including neutralizing antibodies, in hamsters (8) and to elicit neutralizing antibodies in humans (17). The DNA vaccines were administered three times at 3-week intervals. The rVV/HTN-M+S vaccine was administered by the dose and schedule used in the human phase II trials (i.e., a primary subcutaneous vaccination followed by a boost at day 42) (17).
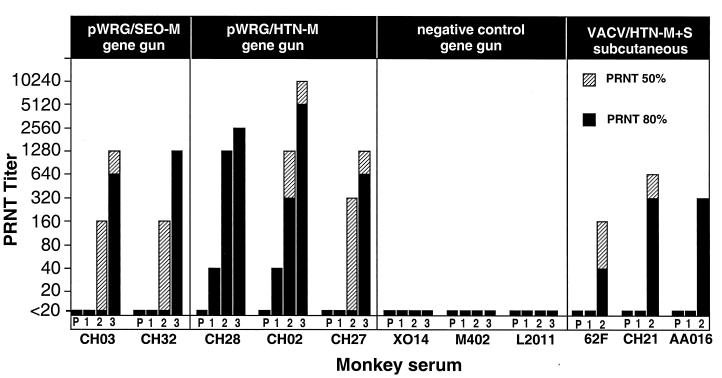
Three weeks after the first vaccination, two of the three monkeys vaccinated with pWRG/HTN-M(x) demonstrated neutralizing antibodies (Fig. 4). Three weeks after the second gene gun vaccination, all of the monkeys vaccinated with either pWRG/SEO-M(x) or pWRG/HTN-M(x) demonstrated detectable levels of neutralizing antibodies. Three weeks after the third vaccination, high titers of neutralizing antibodies were detected in all of the monkeys vaccinated with pWRG/SEO-M(x) or pWRG/HTN-M(x). Negative control-vaccinated monkeys did not develop neutralizing antibodies. Monkeys vaccinated with rVV/HTN-M+S failed to develop a neutralizing antibody response after one vaccination but did develop neutralizing antibodies after the 42-day boost. Three weeks after the final vaccination, the PRNT80 GMTs of the monkeys vaccinated with pWRG/SEO-M(x), pWRG/HTNM(x), or rVV/HTN-M+S, were 905, 2,032, and 160, respectively. These data demonstrate, for the first time, that DNA vaccines expressing hantaviral M gene products are immunogenic in nonhuman primates and elicit very high levels of neutralizing antibodies.
FIG. 4.
DNA vaccination with plasmid expressing SEOV or HTNV G1 and G2 elicits high-titer neutralizing antibody responses in rhesus monkeys. Rhesus monkeys were vaccinated with either pWRG/SEO-M(x), pWRG/HTN-M(x), negative control DNA, or rVV/HTN-M+S by the indicated route as described in Materials and Methods. Serum samples were obtained before vaccination (column P) and then 3 weeks after the first (column 1), second (column 2), and third (column 3) vaccinations. The PRNT titer represents the reciprocal serum dilution that reduced virus plaque number by 80% or 50%. The live virus vaccinia recombinant vaccine (rVV/HTN-M+S) was administered only two times at a 6- week interval. The identification code for each monkey is shown below its respective plot.
To evaluate the duration of immunity elicited by the DNA vaccine and the recombinant vaccinia virus vaccine, sera from vaccinated monkeys were collected 2, 4, 6, and 8 months after the final vaccination and then tested for neutralizing activity. Titers in individual DNA-vaccinated monkeys dropped two- to fourfold in the first 2 months after the final vaccination and then decreased slowly over the next several months. After 8 months, all of the monkeys vaccinated with pWRG/SEO-M(x) or pWRG/HTN-M(x) still had detectable levels of neutralizing antibodies (PRNT50% GMT = 80). In contrast, monkeys vaccinated with the positive control vaccine, rVV/HTN-M+S, exhibited little or no detectable neutralizing antibodies (i.e., PRNT50 ≤ 20) 4 to 8 months after the final vaccination.
DNA vaccination of nonhuman primates with pWRG/SEO-M or pWRG/HTN-M vaccines elicits antibody responses that cross-neutralize DOBV.
We tested the sera from the vaccinated monkeys for cross-neutralizing activity by PRNT (Table 2). All of the monkeys vaccinated with either pWRG/SEO-M(x) or pWRG/HTNV-M(x) had antibodies that cross-neutralized DOBV. rVV/HTN-M+S vaccinated monkeys also had DOBV-cross-neutralizing antibodies, albeit at lower titers. Monkeys vaccinated with pWRG/SEO-M(x) had weak or no HTNV-neutralizing antibody responses and monkeys vaccinated with pWRG/HTN-M(x) had weak or no SEOV-neutralizing antibody responses. Only one monkey (monkey CH32), which was DNA vaccinated with pWRG/SEO-M(x), demonstrated a detectable level of PUUV-neutralizing antibodies.
TABLE 2.
Cross-neutralization activity in serum from vaccinated monkeys
| Monkey serum sourcea | Monkeye | PRNT80 or PRNT50d in animals infected with:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SEOV
|
HTNV
|
DOBV
|
PUUV
|
||||||
| 80% | 50% | 80% | 50% | 80% | 50% | 80% | 50% | ||
| Vaccinatedb with pWRG/SEO-M(x) | CH03 | 640 | 1,280 | ∗ | ∗ | 40 | 160 | ∗ | ∗ |
| CH32 | 1,280 | 1,280 | ∗ | 80 | 320 | 640 | ∗ | 40 | |
| Vaccinatedb with pWRG/HTN-M(x) | CH28 | ∗ | ∗ | 2,560 | 2,560 | ∗ | 320 | ∗ | ∗ |
| CH02 | ∗ | ∗ | 5,120 | 10,240 | 160 | 320 | ∗ | ∗ | |
| CH27 | ∗ | 20 | 640 | 1,280 | 80 | 320 | ∗ | ∗ | |
| Vaccinatedc with rVV/HTN-M+S | 62F | ∗ | ∗ | 40 | 160 | ∗ | 80 | ∗ | ∗ |
| CH21 | ∗ | ∗ | 320 | 640 | ∗ | 160 | ∗ | ∗ | |
| AA016 | ∗ | ∗ | 320 | 320 | ∗ | 20 | ∗ | ∗ | |
Monkey serum was collected 3 weeks after the final vaccination.
Monkeys were gene gun-vaccinated three times at 3-week intervals with the indicated DNA vaccine plasmid.
Monkeys were injected subcutaneously with recombinant vaccinia virus vaccine two times at a 42-day interval.
PRNT values are the reciprocal serum dilutions that neutralize the indicated virus plaque number by 80% or 50%. Homologous titers are shown in boldface. ∗, Titer of <20.
Monkey identification code.
DISCUSSION
Our previous results with the SEOV M DNA vaccine (11), along with the results reported here demonstrating immunogenicity and protective efficacy of the HTNV M DNA vaccine, make a strong case for the use of a full-length M gene in DNA vaccines against other hantaviruses. The HTNV M DNA vaccine is only the second hantavirus DNA vaccine that has been shown to express both the G1 and G2 proteins (the other was the SEOV M DNA vaccine plasmid [11]). Difficulties in either cloning intact hantavirus M genes, expressing intact G1 and G2, or eliciting an immune response in DNA vaccinated animals have befallen us and been reported by others (5). It remains unclear whether or not G1 alone, G2 alone, or fragments of the glycoproteins can elicit neutralizing antibodies and protect against infection. Vaccination with recombinant baculovirus-infected cell lysates containing G1 or G2 alone, and recombinant vaccinia viruses expressing G1 or G2 alone, failed to elicit neutralizing antibody and exhibited incomplete protection in a hamster infection model (24). These data suggest that a full-length M gene capable of expressing G1 and G2 may be required for protective immunity. In contrast, Bharadwaj et al. reported low levels (1:10 to 1:20) of neutralizing antibodies after i.m. needle injection of mice with DNA vaccine plasmids containing short (∼166-amino-acid) sections of the M gene of Sin nombre virus (SNV), an HPS-associated hantavirus (5). This finding suggests that eliciting a neutralizing antibody response not only does not require a full-length M gene but also occurs when only fragments of G1 or G2 are expressed. Further studies are needed to clarify the importance of the conformational integrity of G1 and G2 for immunogenicity.
There are four hantaviruses known to cause HFRS and at least six are known to cause HPS, so information on cross-neutralization and cross-protection among hantaviruses is important for the rational design of cross-protective vaccines. Investigators have evaluated the capacity of sera from various species (including humans) infected with hantaviruses to cross-neutralize other hantaviruses (3, 7, 15, 16, 18). Different species, and different individuals within a species, appear to exhibit differing levels of cross-neutralizing antibodies. In general, data from these experiments indicate that sera from HTNV-, SEOV-, or DOBV-infected individuals share cross-neutralizing antibodies, albeit with a >4-fold difference in titer among the serotypes, whereas sera from PUUV-infected individuals exhibit few or no SEOV-, HTNV-, or DOBV-cross-neutralizing antibodies. Our data obtained with sera from infected or DNA-vaccinated hamsters are consistent with earlier findings. We observed a greater level of cross-neutralizing antibodies in the pooled sera from the DNA-vaccinated hamsters than in the pooled sera from infected hamsters. This may reflect a qualitative difference between the antibodies elicited after DNA vaccination and the antibodies elicited by infection or it may simply reflect a quantitative difference in antibody levels. It is noteworthy that high homologous neutralizing antibody levels in vaccinated hamsters did not necessarily correlate with cross-neutralizing activity (Fig. 3).
The cross-neutralization tests with the hamster serum pools suggested that if neutralizing antibodies could predict protective immunity, then vaccination with either pWRG/SEO-M or pWRG/HTN-M would protect against SEOV, HTNV, and DOBV, but not PUUV and this we found, in general, to be true. The data in Fig. 3 indicate that the presence of cross-neutralizing antibodies correlated with a protective effect with a single exception. Hamster 2119 exhibited cross-neutralizing antibodies, but was not protected by definition because there was a detectable anti-N response after challenge. However, the absence of detectable levels of cross-neutralizing antibodies did not necessarily predict a lack of protection. Examples of the latter case can be found in Fig. 3, hamsters 1437, 1434, 2122, and 2133. It is possible that cross-neutralizing antibodies are present but are not detected due to limitations of the assay or, more likely, that responses other than neutralizing antibody are also protective.
Whether the neutralizing antibodies elicited by DNA vaccination is necessary and/or sufficient to confer protection, or only a surrogate marker to predict protection, will require passive-transfer experiments involving sera from DNA-vaccinated animals. We have not evaluated the cell-mediated immune response elicited by DNA vaccination with pWRG/SEO-M or pWRG/HTN-M, but we suspect that there is a response that plays a role in protection. In support of this, another study demonstrated that a lymphoproliferative response was elicited in mice injected i.m. with DNA encoding ∼166 amino acids of the SNV G1 or G2 proteins, indicating that a cell-mediated response to epitopes of both G1 and G2 can occur (5). The cell-mediated response to DNA vaccination with the full-length G1 and G2 proteins and its role in protection remain to be determined.
In this study, we demonstrated that vaccination with either pWRG/SEO-M or pWRG/HTN-M cross-protected against SEOV, HTNV, and DOBV. Other experimental hantavirus vaccines can cross-protect. For example, a vaccinia virus recombinant expressing the HTNV G1, G2, and N (rVV/HTN-M+S) cross-protected against infection with SEOV but not PUUV (8), and vaccinia virus recombinants expressing the SEOV G1 and G2, or N, cross-protected against HTNV (27). Ours is the first report of any vaccine protecting against DOBV.
All previous data concerning the immunogenicity and protective efficacy of DNA vaccines against hantaviruses have been performed in rodents. Here, were report for the first time, the results of experiments performed in nonhuman primates. The hantavirus M gene-based DNA vaccines administered by gene gun not only elicited positive responses in rhesus macaques but also elicited levels of neutralizing antibodies that were very high. When we combined the serological data from the SEOV and HTNV M gene DNA thrice-vaccinated monkeys, the PRNT80 GMT was 1,470. This neutralizing antibody response was almost 10 times greater than that elicited by the recombinant vaccinia virus vaccine (PRNT80 GMT = 160, PRNT50 GMT = 320), which was similar to the neutralizing antibody responses previously reported for humans (PRNT50 GMT = 160) (17). In China, where several killed virus vaccines against hantaviruses have been developed and tested in humans, the PRNT assay is used to evaluate the potency of the vaccine (28, 30). Most of the inactivated virus vaccines made in cell culture elicit neutralizing antibodies in 90 to 100% of the human vaccinees after three doses (GMT ≤ 100). and the percentage of seropositive individuals drops to ∼50% 6 months after the final boost.
Eight months after the final gene gun vaccination, neutralizing antibodies could still be detected in all of the monkeys vaccinated with either pWRG/SEO-M(x) or pWRG/HTN-M(x). In comparison, monkeys vaccinated with the recombinant vaccinia virus vaccine had low or undetectable levels of neutralizing antibodies after 4 months (Fig. 5). We plan to monitor the kinetics of the decline in antibody levels of the vaccinated monkeys to determine if a low level of neutralizing antibodies is maintained beyond 8 months or if the levels fall below our level of detection in a definable period of time.
FIG. 5.
Neutralizing antibodies elicited by DNA vaccination are still detected in rhesus monkeys 8 months after the final vaccination. Rhesus monkeys vaccinated with the indicated vaccine were bled 3 weeks after each vaccination and then at 2, 4, 6, and 8 months after the final vaccination (weeks 14, 22, 30, and 38, respectively). The homologous neutralizing antibody response for the indicated week after the first vaccination (week 0) was evaluated by PRNT. Each line represents an individual monkey. The week 9 data are also presented in Fig. 4.
We have directed our efforts toward the development of a recombinant hantavirus vaccine(s) that is intended to be safer and more immunogenic than the killed-virus vaccines currently being tested in Asia. Our data suggest that it might be possible to elicit a more robust and longer-lasting neutralizing antibody response using a vaccine platform that entails the expression of G1 and G2 within the cells of the vaccinee (e.g., DNA vaccine) rather than as exogenous proteins (e.g., beta-propiolactone-treated virions combined with adjuvant). In support of this, others have reported that a DNA vaccine containing the Japanese encephalitis virus (JEV) envelope gene elicted a stronger and longer-lasting anti-envelope antibody response than the currently used inactivated JEV vaccine (6).
In summary, we have demonstrated that gene gun vaccination of hamsters with DNA vaccines containing the hantavirus M gene confers sterilizing immunity against three of the four hantaviruses that cause HFRS and elicits very high levels of neutralizing antibodies in monkeys. Because a neutralizing antibody response is considered a surrogate marker for protective immunity in humans, our protection data in hamsters combined with the immunogenicity data in monkeys suggest that hantavirus M gene-based DNA vaccines could protect humans against the most severe forms of HFRS.
ACKNOWLEDGMENTS
The gene gun (Powderject Delivery Device) and pWRG7077 were kindly provided by Powderject Vaccine, Inc. The plasmids used to prepare N-specific ELISA antigen, pSEOSXdelta and pPUUSXdelta, were kindly provided by Fredrik Elgh. The negative control ELISA antigen was kindly provided by Colleen Jonsson.
REFERENCES
- 1.Arikawa J, Schmaljohn A L, Dalrymple J M, Schmaljohn C S. Characterization of Hantaan virus envelope glycoprotein antigenic determinants defined by monoclonal antibodies. J Gen Virol. 1989;70:615–624. doi: 10.1099/0022-1317-70-3-615. [DOI] [PubMed] [Google Scholar]
- 2.Arikawa J, Yao J S, Yoshimatsu K, Takashima I, Hashimoto N. Protective role of antigenic sites on the envelope protein of Hantaan virus defined by monoclonal antibodies. Arch Virol. 1992;126:271–281. doi: 10.1007/BF01309700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asada H, Balachandra K, Tamura M, Kondo K, Yamanishi K. Cross-reactive immunity among different serotypes of virus causing haemorrhagic fever with renal syndrome. J Gen Virol. 1989;70:819–25. doi: 10.1099/0022-1317-70-4-819. [DOI] [PubMed] [Google Scholar]
- 4.Avsic-Zupanc T, Xiao S Y, Stojanovic R, Gligic A, van der Groen G, LeDuc J W. Characterization of Dobrava virus: a hantavirus from Slovenia, Yugoslavia. J Med Virol. 1992;38:132–137. doi: 10.1002/jmv.1890380211. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj M, Lyons C R, Wortman I A, Hjelle B. Intramuscular inoculation of Sin Nombre hantavirus cDNAs induces cellular and humoral immune responses in BALB/c mice. Vaccine. 1999;17:2836–2843. doi: 10.1016/s0264-410x(99)00096-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen H W, Pan C H, Liau M Y, Jou R, Tsai C J, Wu H J, Lin Y L, Tao M H. Screening of protective antigens of Japanese encephalitis virus by DNA immunization: a comparative study with conventional viral vaccines. J Virol. 1999;73:10137–10145. doi: 10.1128/jvi.73.12.10137-10145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu Y K, Jennings G, Schmaljohn A, Elgh F, Hjelle B, Lee H W, Jenison S, Ksiazek T, Peters C J, Rollin P, et al. Cross-neutralization of hantaviruses with immune sera from experimentally infected animals and from hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome patients. J Infect Dis. 1995;172:1581–1584. doi: 10.1093/infdis/172.6.1581. [DOI] [PubMed] [Google Scholar]
- 8.Chu Y K, Jennings G B, Schmaljohn C S. A vaccinia virus-vectored Hantaan virus vaccine protects hamsters from challenge with Hantaan and Seoul viruses but not Puumala virus. J Virol. 1995;69:6417–6423. doi: 10.1128/jvi.69.10.6417-6423.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dantas J R, Okuno Y, Asada H, Tamura M, Takahashi M, Tanishita O, Takahashi Y, Kurata T, Yamanishi K. Characterization of glycoproteins of viruses causing hemorrhagic fever with renal syndrome (HFRS) using monoclonal antibodies. Virology. 1986;151:379–384. doi: 10.1016/0042-6822(86)90058-9. [DOI] [PubMed] [Google Scholar]
- 10.Elgh F, Lundkvist A, Alexeyev O A, Stenlund H, Avsic-Zupanc T, Hjelle B, Lee H W, Smith K J, Vainionpaa R, Wiger D, Wadell G, Juto P. Serological diagnosis of hantavirus infections by an enzyme-linked immunosorbent assay based on detection of immunoglobulin G and M responses to recombinant nucleocapsid proteins of five viral serotypes. J Clin Microbiol. 1997;35:1122–1130. doi: 10.1128/jcm.35.5.1122-1130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper J W, Kamrud K I, Elgh F, Custer D, Schmaljohn C S. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against seoul virus infection. Virology. 1999;255:269–278. doi: 10.1006/viro.1998.9586. [DOI] [PubMed] [Google Scholar]
- 12.Kamrud K I, Hooper J W, Elgh F, Schmaljohn C S. Comparison of the protective efficacy of naked DNA, DNA-based Sindbis replicon, and packaged Sindbis replicon vectors expressing Hantavirus structural genes in hamsters. Virology. 1999;263:209–219. doi: 10.1006/viro.1999.9961. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T, Morita C, Komatsu T, Sugiyama K, Arikawa J, Shiga S, Takeda H, Akao Y, Imaizumi K, Oya A, Hashimoto N, Urasawa S. Isolation of virus causing hemorrhagic fever with renal syndrome (HFRS) through a cell culture system. Jpn J Med Sci Biol. 1983;36:17–25. doi: 10.7883/yoken1952.36.17. [DOI] [PubMed] [Google Scholar]
- 14.Lee H W, Lee P W, Johnson K M. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Lee P W, Gibbs C J, Gajdusek D C, Yanagihara R. Serotypic classification of hantaviruses by indirect immunofluorescent antibody and plaque reduction neutralization tests. J Clin Microbiol. 1985;22:940–944. doi: 10.1128/jcm.22.6.940-944.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundkvist A, Hukic M, Horling J, Gilljam M, Nichol S, Niklasson B. Puumala and Dobrava viruses cause hemorrhagic fever with renal syndrome in Bosnia-Herzegovina: evidence of highly cross-neutralizing antibody responses in early patient sera. J Med Virol. 1997;53:51–59. [PubMed] [Google Scholar]
- 17.McClain D J, Summers P L, Harrison S A, Schmaljohn A L, Schmaljohn C S. Clinical evaluation of a vaccinia-vectored Hantaan virus vaccine. J Med Virol. 2000;60:77–85. doi: 10.1002/(sici)1096-9071(200001)60:1<77::aid-jmv13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Niklasson B, Jonsson M, Lundkvist A, Horling J, Tkachenko E. Comparison of European isolates of viruses causing hemorrhagic fever with renal syndrome by a neutralization test. Am J Trop Med Hyg. 1991;45:660–665. doi: 10.4269/ajtmh.1991.45.660. [DOI] [PubMed] [Google Scholar]
- 19.Peters C J, Simpson G L, Levy H. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med. 1999;50:531–45. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- 20.Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmaljohn C S. Molecular biology of hantaviruses. In: Elliott R M, editor. The Bunyaviridae. New York, N.Y: Plenum Press, Inc.; 1996. pp. 63–90. [Google Scholar]
- 22.Schmaljohn C S, Arikawa J, Dalrymple J M, Schmaljohn A L. Expression of the envelope glycoproteins of Hantaan virus with vaccinia and baculovirus recombinants. In: Kolakofsky D, Mahy B, editors. Genetics and pathogenicity of negative strand viruses. Amsterdam, The Netherlands: Elsevier Press; 1989. pp. 58–66. [Google Scholar]
- 23.Schmaljohn C S, Hasty S E, Dalrymple J M. Preparation of candidate vaccinia-vectored vaccines for haemorrhagic fever with renal syndrome. Vaccine. 1992;10:10–13. doi: 10.1016/0264-410x(92)90412-d. [DOI] [PubMed] [Google Scholar]
- 24.Schmaljohn C S, Chu Y K, Schmaljohn A L, Dalrymple J M. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J Virol. 1990;64:3162–3170. doi: 10.1128/jvi.64.7.3162-3170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmaljohn C, Vanderzanden L, Bray M, Custer D, Meyer B, Li D, Rossi C, Fuller D, Fuller J, Haynes J, Huggins J. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J Virol. 1997;71:9563–9569. doi: 10.1128/jvi.71.12.9563-9569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tkachenko E A, Bashkirtsev V N, van der Groen G, Dzagurova T K, Ivanov A P, Ryltseva E V. Isolation in Vero-E6 cells of hantavirus from Clethrionomys glareolus captured in the Bashkiria area of the U.S.S.R. Ann Soc Belg Med Trop. 1984;64:425–426. [PubMed] [Google Scholar]
- 27.Xu X, Ruo S L, McCormick J B, Fisher-Hoch S P. Immunity to hantavirus challenge in Meriones unguiculatus induced by vaccinia-vectored viral proteins. Am J Trop Med Hyg. 1992;47:397–404. doi: 10.4269/ajtmh.1992.47.397. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Zhu Z, Yao Z, Dong G. Inactivated cell-culture Hantavirus vaccine developed in China. In: Saluzzo J F, Dodet B, editors. Factors in the emergence and control of rodent-borne viral diseases (hantavirus and arenal diseases). Paris, France: Elsevier Press; 1999. pp. 157–161. [Google Scholar]
- 29.Zhang X-K, Takashima I, Hashimoto N. Characteristics of passive immunity against hantavirus infection in rats. Arch Virol. 1989;105:235–246. doi: 10.1007/BF01311360. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z Y, Tang H Y, Li Y J, Weng J Q, Yu Y X, Zeng R F. Investigation on inactivated epidemic hemorrhagic fever tissue culture vaccine in humans. Chin Med J. 1994;107:167–170. [PubMed] [Google Scholar]