Abstract
Borna disease virus (BDV)-induced immunopathology in mice is most prominent in strains carrying the major histocompatibility complex H-2k allele and is mediated by CD8+ T cells that are directed against the viral nucleoprotein p40. We now identified the highly conserved octamer peptide TELEISSI, located between amino acid residues 129 and 136 of BDV p40, as a potent H-2Kk-restricted cytotoxic T-cell (CTL) epitope. When added to the culture medium of L929 target cells, TELEISSI conferred sensitivity to lysis by CTLs isolated from brains of BDV-infected MRL mice with acute neurological disease. Vaccinia virus-mediated expression of a p40 variant with mutations in the two Kk-specific anchor residues of the TELEISSI peptide (p40E130K,I136T) did not sensitize L929 target cells for lysis by BDV-specific CTLs, whereas expression of wild-type p40 did. Furthermore, unlike vaccination with wild-type p40, vaccination of persistently infected symptomless B10.BR mice with p40E130K,I136T did not result in central nervous system inflammation and neurological disease. These results demonstrate that TELEISSI is the immunodominant CTL epitope of BDV p40 in H-2k mice.
The highly neurotropic Borna disease virus (BDV) is the causative agent of a nonpurulent meningoencephalitis predominantly observed in horses and sheep in central Europe (23, 40, 45). BDV is an enveloped virus with a single-stranded RNA genome of negative polarity that replicates and transcribes its genome in the nuclei of infected cells (3, 6). A large number of warm-blooded animal species is susceptible to experimental infection with BDV (40). BDV is noncytolytic in vitro (18, 24) and in vivo (12, 42), and it can readily establish a persistent infection of the central nervous system (CNS). In naturally infected hosts and in experimentally infected rodents, neurological disease and behavioral abnormalities seem to result mainly from immunopathological processes (2, 16, 29). Strong perivascular and parenchymal infiltrations of CD4+ and CD8+ T cells were observed, and their appearance in the brain correlates with the onset of disease symptoms (29, 33, 47). Studies in rodent model systems and in naturally infected horses indicated that immunopathology is mediated by CD8+ T cells, which require help from the CD4+ T-cell subset (2, 16, 30, 44, 46).
The mouse strain MRL is highly susceptible to BDV-induced neurological disease (16). Its high susceptibility is determined by the H-2k haplotype and by additional, unidentified, genetic traits. BDV-infected mice of strain B10.BR, which also carry the H-2k haplotype, are resistant to spontaneous neurological disease due to immunological ignorance of BDV antigens (17). However, these persistently infected mice quickly develop neurological disease after vaccination with recombinant vaccinia virus expressing BDV p40 (17). The nucleoprotein p40 is encoded by the first gene of the BDV genome. It is present in large amounts in the brains of infected animals (23). We and others have recently shown that BDV p40 is the major viral target recognized by disease-inducing cytotoxic T cells (CTLs) in the brains of diseased mice (17) and rats (34). We report here that the highly conserved octameric peptide TELEISSI is the immunodominant H-2Kk-restricted CTL epitope of BDV p40.
MATERIALS AND METHODS
Mice.
MRL/MpJ and B10.BR mice were originally purchased from The Jackson Laboratory (Bar Harbor, Maine). Breeding colonies of both strains were maintained in our local animal facility.
Viruses.
A rat-adapted strain of BDV was adapted to the mouse by four consecutive passages through brains of MRL mice. This virus, which was originally assumed to be derived from strain He/80 (16), has recently been identified as strain RW 98 (9). For virus passage, mice were infected intracerebrally at 4 weeks of age. Brains of animals showing strong neurological disease were collected and used to prepare new virus stocks. Stocks obtained from the fourth mouse passage were amplified once in brains of 5-week-old rats. A 10% (wt/vol) rat brain homogenate was prepared (stock no. 82) and used throughout this study. The viral titer of stock no. 82 was approximately 100 focus-forming units/ml when determined by a standard fluorescence focus assay on Vero cells.
Vaccinia virus expressing BDV p40 (VV-p40) or influenza virus A/FPV/ Rostock/34 neuraminidase (VV-NA) was described earlier (17). Recombinant vaccinia viruses expressing FLAG-tagged wild-type and mutated versions of BDV p40 were produced by standard procedures (26) using vaccinia virus strain WR and plasmid pSC11-derived constructs for recombination. Plain pSC11 plasmid (4) was used to produce a control vaccinia virus expressing β-galactosidase (VV-β-gal).
Animal infections.
MRL/MpJ mice were infected intracerebrally under ether anesthesia at an age of 10 to 17 days with 10-μl samples of mouse-adapted BDV stock no. 82 (100 focus-forming units/ml). Injections into the thalamic region were done by using a Hamilton syringe. For vaccination experiments with vaccinia viruses, B10.BR mice were infected as newborns by the intracerebral route with 10-μl samples of mouse-adapted BDV and challenged 7 to 10 weeks later by intravenous injection of 5 × 106 PFU of the indicated recombinant vaccinia viruses.
Plasmid constructs and site-directed mutagenesis.
PCR fragments reflecting full-length and C-terminally truncated versions of p40 were generated with a common 5′ primer introducing a BamHI restriction site, followed by a FLAG tag and individual 3′ primers introducing a BamHI site. The 3′ primers were complementary to nucleotide positions 642 to 665, 843 to 865, and 1090 to 1113 of the p40 open reading frame. PCR products were cut with BamHI and ligated into BglII-digested plasmid pSC11.
Site-directed mutagenesis of p40 was done by the overlap extension method using PCR (19). Mutations leading to amino acid changes E130K and I136T were introduced by using oligonucleotides 5′-CAGCGTGATCTCACCAAGCTGGAGATATCCTCTACATTCAGCCATTGTTGC-3′ and 5′-GCAACAATGGCTGAATGTAGAGGATATCTCCAGCTTGGTGAGATCACGCTG-3′. In addition, these primers introduced a silent mutation that resulted in a new EcoRV restriction site which allowed convenient selection of a PCR product harboring the mutation. The BamHI-digested PCR product was subsequently cloned into the BglII site of plasmid pSC11.
Northern blot analysis.
Total RNA from CV-1 or L929 cells infected for 4 h with the various vaccinia virus recombinants was prepared by using 1 ml of TRIZOL reagent for 106 infected cells. Samples (10 μg) of RNA were subjected to electrophoresis through a 1.2% agarose–formaldehyde gel, transferred to a nylon membrane, and hybridized under standard conditions to a radiolabeled cDNA fragment corresponding to nucleotides 1 to 264 of the BDV p40 coding region. To control for possible variation in gel loading, the blots were stripped and rehybridized with a radiolabeled rat glyceraldehyde-3-phosphate dehydro-genase cDNA probe (42). After stringent washing, Kodak Biomax MR films (Kodak, Rochester, N.Y.) were exposed to the membranes for 1 day to visualize the radioactive signals.
Western blot analysis.
L929 cells were infected with the various recombinant vaccinia viruses at a multiplicity of infection of 0.5, and whole-cell lysates were prepared 18 h postinfection by adding 200 μl of lysis buffer (20 mM Tris HCl [pH 8.0], 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 μg of pepstatin per ml) to 5 × 106 cells. Samples (35 μl) of the lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes, and probed with a monoclonal antibody to BDV p40 (Bo18) (15) or a monoclonal antibody to the FLAG epitope (M2; Sigma, Deisenhofen, Germany). The blots were developed with horseradish peroxidase-conjugated goat anti-mouse serum and subsequent incubation with 4-chloro-1-naphthol substrate (Fluka, Buchs, Switzerland).
Peptides.
Peptides were purchased from Neosystem (Strasbourg, France) at a purity of >65% (immunograde). They were dissolved in dimethyl sulfoxide at a concentration of 10 mM. For incubation with cells, peptides were diluted in medium to the indicated concentrations. All of the peptides used in this study are listed in Table 1.
TABLE 1.
Candidate H-2k-restricted T-cell epitopes in BDV p40
| Peptide sequence | Restriction element | Protein | Position | Score
|
|
|---|---|---|---|---|---|
| BIMASa | SYFPEITHIb | ||||
| TELEISSI | Kk | BDV p40 | 129–136 | 1,000 | 23 |
| RDLTELEI | Kk | BDV p40 | 126–133 | 50 | 22 |
| IRHPDAIKL | Dk | BDV p40 | 286–294 | —c | —c |
| IRQNAVALL | Dk | BDV p40 | 53–61 | —c | —c |
| LTELEISSI | Kk | BDV p40 | 128–136 | <10 | 11 |
| DLTELEISSI | Kk | BDV p40 | 127–136 | 10 | —d |
| TELEISSIF | Kk | BDV p40 | 129–137 | 20 | 13 |
| LTELEISSIF | Kk | BDV p40 | 128–137 | <10 | —d |
| Control peptides | |||||
| TEMEKGEKI | Kk | HIV-1 RTe | 206–214 | 1,000 | 25 |
| FEANGNLI | Kk | FLU HAf | 259–266 | 3,000 | 24 |
| KAVYNFATM | Db | LCMV GPg | 33–41 | 936 | 29 |
Scores of at least 1,000 (BIMAS) have good predictive value in this program.
Scores of at least 20 have good predictive value in this program.
—, no computer-based prediction was available for Dk-restricted peptides.
—, a search for Kk decamers is not possible in SYFPEITHI.
HIV-1 RT, human immunodeficiency virus type 1 reverse transcriptase.
FLUHA, HA of influenza virus strain A/PR/8/34 (H1N1).
LCMV GP, glycoprotein of LCMV.
Isolation of brain lymphocytes.
Brain lymphocytes were isolated essentially as previously described (20). Briefly, brains of diseased mice were gently pressed through a metal grid (60 mesh) in 10 ml of Hanks balanced salt solution containing 0.05% collagenase D (Roche, Mannheim, Germany), 0.1 μg of the trypsin inhibitor Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK; Sigma) per ml, 10 μg of DNase I (Roche) per ml, and 10 mM HEPES buffer, pH 7.3. This tissue suspension was incubated on a roller shaker for 1 h at room temperature and then allowed to stand for 30 min at room temperature without agitation. Cells in the supernatant were pelleted and suspended in 5 ml of phosphate-buffered saline. This suspension was layered on a 10-ml gradient composed of 75% Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) and 25% RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). After centrifugation for 30 min at 500 × g, the cell pellet was suspended in Iscove's modified Dulbecco's medium supplemented with 10 μg of gentamicin per ml, 2× 10−5 M β-mercaptoethanol, and 10% FCS at a concentration of 2 × 106 cells per ml. This suspension was used as the effector cell population for in vitro cytotoxicity assays.
In vitro cytotoxicity assay.
Ex vivo cytolytic activity of spleen cells and brain lymphocytes from uninfected or BDV-infected animals was determined by two types of 51Cr release assays. Unless stated otherwise, cytotoxicity assays were performed as previously described (17). Briefly, 5 × 106 L929 (H-2k) cells were labeled in suspension with 200 μCi of 51Cr (NEN, Cologne, Germany) for 2 h at 37°C. After three washings, 106 L929 cells labeled with 51Cr were infected with the respective recombinant vaccinia viruses for 2 to 4 h at a multiplicity of infection of 5. They were then diluted to a final concentration of 4 × 104 cells/ml, dispensed into 96-well round-bottom microtiter plates at 4 × 103 cells per well and coincubated with different numbers of effector cells in a total volume of 200 μl. For simultaneous testing of various peptides at one or more concentrations, the second type of 51Cr release assay, termed mini-killer, was used (30a). Briefly, 0.3 × 106 to 1 × 106 labeled cells were loaded with the indicated peptides at final concentrations ranging from 10−4 to 10−8 M as described above and diluted to a final concentration of 4 × 104 cells per ml. Aliquots (50 μl) of these cells were then dispensed into V-bottom 96-well plates and coincubated with various effector cell numbers in a total volume of 100 μl (30a). Incubation of target cells with effectors was done for 6 h at 37°C for both assays. The percentage of specific 51Cr release was calculated according to the following formula: 100 × [(test release − spontaneous release)/(total release − spontaneous release)].
Histology.
Complete brain hemispheres from sacrificed animals were preserved in Zamboni's fixative (4% paraformaldehyde and 15% picric acid in 0.25 M sodium phosphate, pH 7.5) and embedded in paraffin. Sagittal sections (4 μm) were stained with hematoxylin-eosin and viewed and photographed under a Leitz Dialux 20 EB microscope. The degree of encephalitis was scored on an arbitrary scale of 0 to 3 (0, no infiltrates; 1, up to three perivascular infiltrates per brain section with one or two layers of cells; 2, up to six perivascular infiltrates per brain section with multilayer appearance and one or two parenchymal infiltrates; 3, more than six perivascular infiltrates per brain section with multiple layers of cells and strong infiltration of the parenchyma at multiple sites).
H-2Kk stabilization assay and peptide dissociation assay.
T2-Kk cells (kindly provided by J. Haurum, Copenhagen, Denmark) were maintained in RPMI 1640 medium supplemented with 10% FCS at 37°C. Before loading with peptide, the cells were incubated at 29°C in serum-free AIM-V medium (Life Technologies, Karlsruhe, Germany) for 24 h. We incubated 106 cells per assay point overnight at 29°C with the indicated peptide concentrations in 500 μl of AIM-V medium. Cells were washed once with phosphate-buffered saline–2% FCS–0.1% NaN3, and H-2Kk surface expression was measured by flow cytometry using monoclonal antibody 36-7-5, which is specific for murine H-2Kk (BD Pharmingen, Heidelberg, Germany).
RESULTS
Mapping of CTL epitopes in BDV p40 by C-terminal deletion analysis.
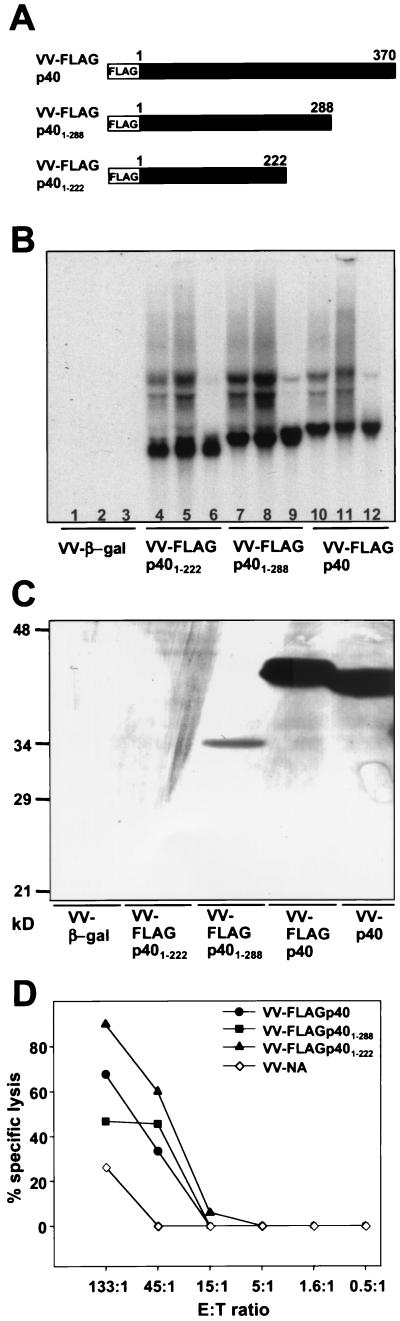
To identify regions in BDV p40 that may carry CTL epitopes, vaccinia virus recombinants were generated that express C-terminal deletion mutant forms of p40. We successfully rescued recombinant vaccinia viruses expressing N-terminally flagged, full-length p40 (VV-FLAGp40) and deletion mutant forms lacking 82 (VV-FLAGp401–288) and 148 (VV-FLAGp401–222) amino acids at the C terminus of p40, respectively (Fig. 1A). For unknown reasons, it was not possible to rescue recombinant vaccinia viruses expressing shorter versions of p40. When CV-1 or L929 cells infected with the various recombinant vaccinia viruses were analyzed for p40-specific transcripts by Northern blotting, RNAs of the expected sizes were found to be abundantly present (Fig. 1B). However, analysis of p40 expression by immunofluorescence (data not shown) or immunoblotting (Fig. 1C) using monoclonal antibody Bo18 (which detects a linear epitope close to the N terminus of p40) revealed that only infection with VV-FLAGp40 yielded easily detectable levels of BDV antigen. This protein migrated slightly slower on SDS-PAGE than authentic p40 due to the presence of the FLAG tag at the N terminus. Surprisingly, cells infected with VV-FLAGp401–288 contained only low levels of p40 and no p40 antigen was detectable in CV-1 or L929 cells infected with VV-FLAGp401–222 (Fig. 1C and data not shown). Western blot analysis of such cell extracts with a monoclonal antibody that detects the FLAG epitope yielded comparable results: again, the truncated versions of p40 were not or only barely detectable (data not shown). Since truncated p40 mRNAs were abundantly present in infected cells (Fig. 1B) and since sequencing reconfirmed the integrity of the open reading frames in the recombinant vaccinia viruses, these findings strongly indicated that the half-lives of C-terminally truncated versions of BDV p40 were short.
FIG. 1.
At least one CTL epitope is contained in the N-terminal 222 amino acid residues of BDV p40. (A) Schematic drawing showing the structures of C-terminally truncated versions of BDV p40 expressed by the indicated recombinant vaccinia viruses. (B) Northern blot analysis of RNA from CV-1 cells (lanes 3, 6, 9, and 12) or L929 cells (lanes 1, 2, 4, 5, 7, 8, 10, and 11) infected for 4 h with the indicated vaccinia virus recombinants at a multiplicity of infection of 5 (lanes 1, 3, 4, 6, 7, 9, 10, and 12) or a multiplicity of infection of 10 (lanes 2, 5, 8, and 11). VV-β-gal served as a negative control. A radiolabeled probe specific for BDV p40 was used for hybridization. (C) Western blot analysis of lysates from L929 cells infected with the indicated recombinant vaccinia viruses. BDV p40-specific monoclonal antibody Bo18 was used for detection. Note the weak staining of p40 mutants, presumably resulting from the poor in vivo stability of these truncated proteins. (D) Lysis of target cells infected with the various vaccinia virus recombinants by lymphocytes from brains of BDV-infected MRL mice with acute neurological disease. Lysis observed with L929 cells infected with a control vaccinia virus recombinant (VV-NA) at the highest effector-to-target (E:T) ratio represents the background lytic activity of brain lymphocyte preparations toward vaccinia virus-infected target cells.
Recombinant vaccinia viruses expressing the various p40 variants were used to infect L929 cells, which then served as targets in a 51Cr release assay. As effector cells, lymphocyte preparations from brains of BDV-infected MRL mice in the acute phase of neurological disease were used throughout this study without further restimulation in vitro. Animals were inoculated intracerebrally at an age of 2 weeks with 10-μl aliquots of mouse-adapted stock no. 82 corresponding to an infectious dose of 1 FFU per mouse. This inoculum size always resulted in persistent CNS infection, indicating an underestimation of the viral titer by the standard focus-forming assay. Animals were euthanatized when they showed significant weight loss, severe ataxia, paraparesis, and apathy. Brain lymphocyte preparations from such animals are subsequently referred to as BDV-specific CTLs. On average, one brain yielded about 3 × 106 lymphocytes. We found that full-length p40 and truncation mutant forms FLAGp401–288 and FLAGp401–222 sensitized L929 target cells equally well for lysis by BDV-specific CTLs (Fig. 1D). L929 cells infected with a control virus (VV-NA) were not lysed noticeably at low effector-to-target ratios, and lysis remained low at the highest effector-to-target ratio. Weak nonspecific background activity of brain-derived lymphocytes toward vaccinia virus-infected target cells has previously been observed (17). Collectively, these results indicated that at least one prominent CTL epitope was contained within the N-terminal 222 amino acids of p40. They further indicated that truncated p40 antigen was indeed synthesized and efficiently presented on major histocompatibility complex (MHC) class I molecules by cells infected with VV-FLAGp40(1–288) or VV-FLAGp40(1–222).
Evaluation of computer-predicted CTL epitopes.
As we failed to rescue recombinant vaccinia viruses expressing short N-terminal fragments of BDV p40, we analyzed the p40 sequence for H-2k-restricted T-cell epitopes by using two different computer programs, namely, the HLA peptide binding prediction program of BIMAS (http://bimas.dcrt.nih.gov/molbio /hla_bind/index.html) (31) and the SYFPEITHI epitope prediction program (http://www.uni-tuebingen.de/uni/kxi/) (35). Two overlapping octamers, TELEISSI and RDLTELEI, located in the N-terminal moiety of p40 emerged as candidate epitopes (Table 1). They both conform to the minimal consensus sequence of Kk binding, which is XD/EX5–6I/V (36). Peptide TELEISSI got top scores in both prediction programs, whereas peptide RDLTELEI scored well in only one of them (Table 1).
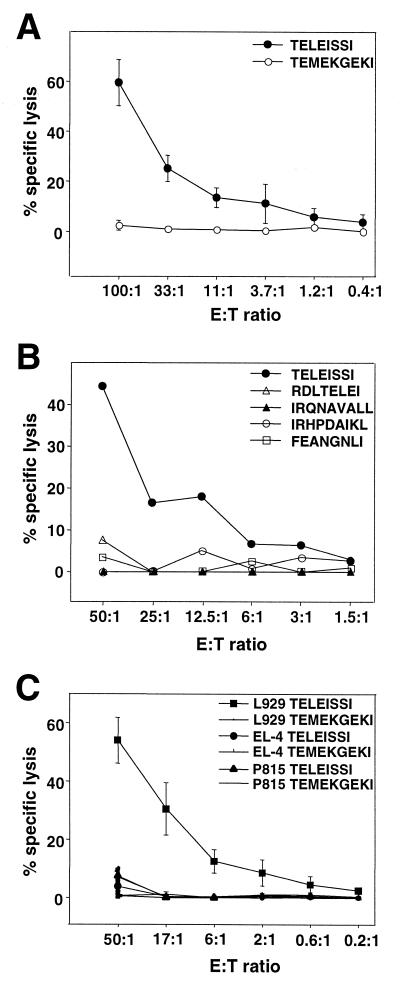
We chemically synthesized these two candidate peptides and tested them for the ability to sensitize L929 cells for cytotoxic activity of BDV-specific effector cells in a standard 51Cr release assay. TELEISSI reproducibly sensitized target cells for lysis by lymphocytes from brains of BDV-infected mice with acute neurological disease (Fig. 2), whereas RDLTELEI did not (Fig. 2B). Similarly, L929 cells pulsed with the peptides IRQNAVALL and IRHPDAIKL, which conform to sequence motifs determined for Dk-binding peptides (Table 1) (7, 25), were not lysed by BDV-specific brain lymphocytes. To verify that lysis of peptide-loaded target cells was H-2k restricted, we pulsed L929 cells (H-2k), EL-4 cells (H-2b), and P815 cells (H-2d) with TELEISSI and determined target cell sensitization by using BDV-specific brain lymphocytes as effectors. TELEISSI sensitized L929 cells but not EL-4 or P815 cells (Fig. 2C).
FIG. 2.
The peptide TELEISSI represents the major Kk-restricted CTL epitope of BDV p40. (A) L929 target cells (H-2k) were loaded with TELEISSI or TEMEKGEKI (representing a Kk-restricted CTL epitope of human immunodeficiency virus type 1 reverse transcriptase) at a concentration of 10−4 M. Peptide-loaded target cells were incubated with lymphocytes from brains of BDV-infected MRL mice with acute neurological disease. Results shown represent the means of four independent experiments. (B) Peptides representing candidate Kk- and Dk-restricted CTL epitopes with lower scores than TELEISSI were tested for the ability to sensitize L929 target cells at a concentration of 10−4 M in a standard 51Cr release assay using lymphocytes from brains of BDV-infected MRL mice with acute neurological disease. For a description of the various BDV p40-derived peptides, see Table 1. The octamer peptide FEANGNLI (corresponding to a well-characterized Kk-restricted epitope of influenza virus HA) was used as a negative control. (C) L929 (H-2k), EL-4 (H-2b), and P815 (H-2d) cells were loaded with TELEISSI or control peptide TEMEKGEKI at a concentration of 10−4 M before they were used as target cells in a standard 51Cr release assay with lymphocytes from brains of BDV-infected MRL mice with acute neurological disease. Results shown represent the means of three independent experiments. E:T ratio, effector-to-target ratio.
N- and C-terminally elongated versions of TELEISSI are recognized less efficiently by BDV-specific CTLs.
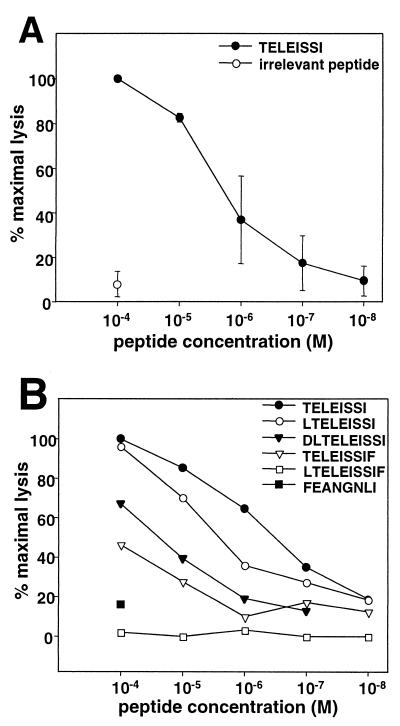
The concentration of peptide TELEISSI required to sensitize L929 target cells for half-maximal lysis by BDV-specific CTLs was approximately 10−6 M (Fig. 3). We determined if C- or N-terminal extensions of TELEISSI (Table 1) would result in more efficient target cell sensitization. Figure 3B shows that this was not the case. The nonamer peptide LTELEISSI was slightly less efficient than TELEISSI, whereas the performance of the decamer DLTELEISSI was reduced by more than 1 order of magnitude. The C-terminally elongated nonamer TELEISSIF had to be used at 10−4 M to reach half-maximal sensitization of target cells for lysis by BDV-specific CTLs (Fig. 3B). A decamer peptide carrying one extra amino acid at each terminus (LTELEISSIF) was virtually inactive in the CTL assay, as was the negative control peptide FEANGNLI (Fig. 3B).
FIG. 3.
N- and C-terminally elongated versions of TELEISSI are recognized less efficiently by BDV-specific CTLs. (A) Titration of the TELEISSI concentration necessary to sensitize L929 cells for BDV-specific lysis. L929 cells pulsed with the indicated peptide concentrations were incubated with lymphocytes from brains of BDV-infected MRL mice with acute neurological disease. Results shown are the averages of two independent titration experiments. (B) Mutant versions of TELEISSI were used at the indicated concentrations to sensitize L929 target cells for lysis by BDV-specific CTLs in a mini-killer 51Cr release assay. The Kk-restricted peptide FEANGNLI from the influenza virus HA served as a negative control. For a detailed description of the peptides used, see Table 1. Values are expressed as percentages of the maximal activity observed with the highest concentration of TELEISSI.
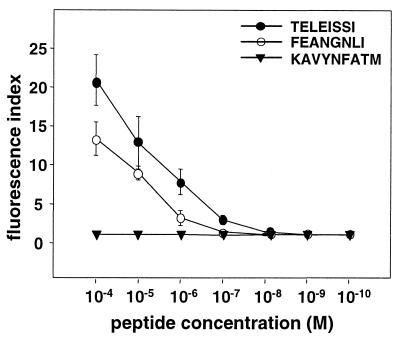
To directly determine the binding affinity of TELEISSI for the H-2Kk molecule, we used an MHC class I surface stabilization assay which is based on the T2 cell line. T2 cells have a defect in peptide transport into the endoplasmic reticulum and display greatly reduced surface expression of MHC class I molecules which can be reversed by addition of exogenous peptide (41). The T2 cell clone used here was stably transfected with a construct expressing the murine Kk molecule. When these cells were incubated with 10−4 M TELEISSI, their surface staining by a Kk-specific monoclonal antibody increased strongly (Fig. 4). Kk surface expression of TELEISSI- treated cells was actually more pronounced than that of cells treated with 10−4 M influenza A/PR8/34 virus hemagglutinin (HA)-derived peptide FEANGNLI, which strongly binds Kk (13). A well-characterized Db-restricted CD8+ T-cell epitope with the sequence KAVYNFATM from the lymphocytic choriomeningitis virus (LCMV) glycoprotein (32) was not able to induce upregulation of Kk cell surface expression, demonstrating the specificity of the assay (Fig. 4). Titration showed that the concentration of TELEISSI required for half-maximal surface expression of Kk was about 3 × 10−5 M, while that for FEANGNLI was about 5 × 10−4 M (Fig. 4). These data suggested that TELEISSI binds Kk with an affinity comparable to or higher than that of a well-known Kk interaction partner.
FIG. 4.
TELEISSI shows a Kk-binding affinity comparable to that of a well-characterized Kk-restricted peptide. T2-Kk cells were incubated for 20 h in AIM-V medium at 29°C and then loaded with the indicated concentrations of peptide TELEISSI, the Kk-restricted influenza virus HA peptide FEANGNLI, the H-2Db-restricted peptide KAVYNFATM derived from the LCMV glycoprotein (32), or no peptide for a further 16 h in AIM-V medium at 29°C. MHC class I cell surface expression was then measured by using monoclonal antibody 36-7-5 (BD PharMingen), which is directed against Kk. Relative peptide affinity is expressed as a fluorescence index (mean fluorescence with peptide/mean fluorescence without peptide). The results shown represent the mean values of three independent experiments.
TELEISSI is the immunodominant CTL epitope in BDV p40.
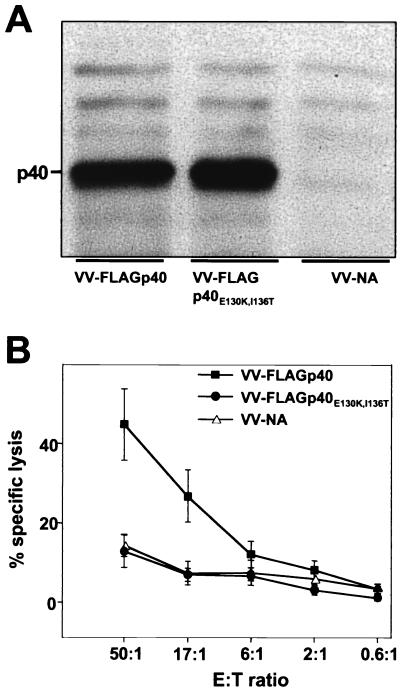
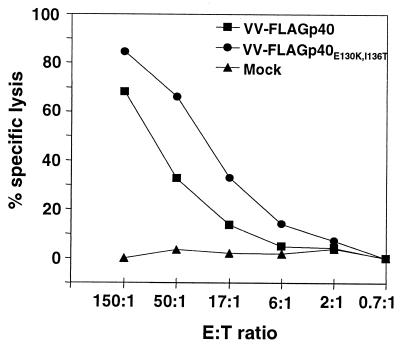
To determine whether TELEISSI indeed represents the immunodominant epitope, we introduced two point mutations into BDV p40. The two anchor residues, glutamate at position 130 and isoleucine at position 136, were changed to lysine and threonine, respectively, in order to destroy the H-2k-binding capacity of TELEISSI, and the resulting cDNA was used to construct recombinant vaccinia virus VV-FLAGp40E130K,I136T. Expression studies with infected CV-1 (Fig. 5A) and L929 (data not shown) cells demonstrated that FLAGp40E130K,I136T was expressed equally as well as its flagged wild-type counterpart. When VV-FLAGp40E130K,I136T was used to infect L929 target cells, CTL activity was at background levels and did not exceed that observed with control cells infected with VV-NA (Fig. 5B). In contrast, L929 cells infected with VV-FLAGp40, which directs the synthesis of wild-type p40, were good CTL targets (Fig. 5B). Thus, BDV-specific CTLs mainly recognized TELEISSI, which identified this peptide as the immunodominant epitope of p40.
FIG. 5.
Lysis by BDV-specific CTLs of target cells expressing p40 but not the anchorless mutant FLAGp40E130K,I136T. (A) The second and last residues of TELEISSI, predicted to represent critical amino acids for binding to Kk, were changed to K and T, respectively, and the resulting cDNA, encoding mutant protein p40E130K,I136T, was inserted into a recombinant vaccinia virus (VV-FLAGp40E130K,I136T). Lysates of CV-1 cells infected with vaccinia viruses expressing either the wild-type or the mutant form of BDV p40 were analyzed by SDS-PAGE and Western blotting using monoclonal antibody Bo18. A recombinant vaccinia virus expressing influenza virus neuraminidase (VV-NA) served as a negative control. (B) Vaccinia virus-infected target cells expressing p40E130K,I136T, wild-type p40, or influenza virus NA were incubated with lymphocytes from brains of BDV-infected MRL mice with acute neurological disease, and specific cell lysis was monitored in a standard 51Cr release assay. Results shown represent the mean values of three independent experiments. E:T ratio, effector-to-target ratio.
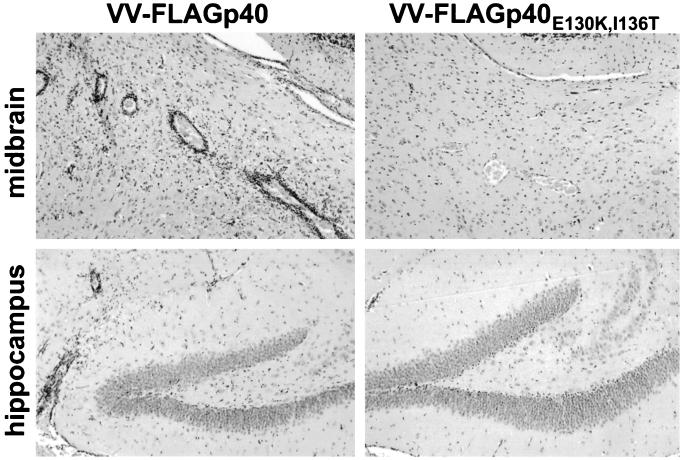
To answer the question of whether TELEISSI also represents the major determinant for recognition by disease-inducing CD8+ T cells in vivo, we took advantage of the fact that vaccinia virus-mediated immunization with wild-type p40 can drive symptomless persistently BDV-infected B10.BR mice into fatal neurological disease (17). Although they harbor a high number of virus-infected neurons in the CNS, as determined by immunohistochemical analysis (data not shown), these mice remain healthy in the absence of immunization as a result of immunological ignorance. The disease-inducing effect of p40 immunization in these mice presumably results from the induction of a vigorous CTL response to BDV antigen (17). If TELEISSI were the immunodominant epitope in p40, we would expect immunization with p40E130K,I136T to have no deleterious effect. This was indeed the case. When persistently infected B10.BR mice were immunized by infection with wild-type p40-expressing vaccinia virus, four of the five challenged animals developed severe neurological disease within 7 to 10 days (Table 2). Histological analysis of the CNS revealed the abundant presence of lymphocytes and prominent perivascular cuffs in the hippocampus and midbrain of diseased animals (Fig. 6), as well as in the cortex and thalamus (data not shown). By contrast, when five persistently infected B10.BR mice were immunized by infection with the vaccinia virus recombinant expressing p40E130K,I136T, no disease was observed (Table 2). Histological examination of the brains of these animals showed no detectable infiltrates of inflammatory cells (Table 2 and Fig. 6), demonstrating that the absence of the TELEISSI motif rendered the p40 immunization ineffective.
TABLE 2.
Vaccinia virus-mediated expression of BDV p40E130K,I136T does not induce CNS inflammation and disease in persistently infected B10.BR mice
| Protein | No. of diseased animals/total no. of animals | Degree of meningoencephalitisa |
|---|---|---|
| FLAGp40 | 4/5 | 3/3/0/3/3 |
| FLAGp40E130K,I136T | 0/5 | 0/0/0/0/0 |
Ratings of inflammation ranged from 0 (no detectable lymphocytic infiltration) to 3 (very severe lymphocytic infiltration) See Materials and Methods for details.
FIG. 6.
Vaccinia virus expressing p40, but not mutant FLAGp40E130K,I136T, induces encephalitis in BDV-infected B10.BR mice. Mice were infected with a mouse-adapted variant of BDV as newborns in order to establish a symptomless persistent infection of the CNS. At the age of 8 to 10 weeks, the animals were infected with recombinant vaccinia virus expressing wild-type p40 or mutant protein p40E130K,I136T. The animals were sacrificed when severe neurological symptoms occurred (7 to 10 days after challenge) or at day 10 post vaccinia virus infection if no disease symptoms were observed. Brain hemispheres were removed and processed for paraffin embedding. Thin sections were stained with hematoxylin and eosin to visualize infiltrating lymphocytes in the midbrain (upper panels) and hippocampus (lower panels) of mice infected with VV-FLAGp40 (left panels) or VV-FLAGp40E130K,I136T (right panels).
To control for the possibility that immunization by infection with VV-FLAGp40E130K,I136T failed to induce disease simply because it did not replicate well in the infected mice, we examined the spleens of VV-p40E130K,I136T-infected mice for the presence of vaccinia virus-specific CTLs. The experiment shown in Fig. 7 demonstrated that high numbers of vaccinia virus-specific CTLs were present in this organ, regardless of whether p40 wild-type- or p40 mutant-expressing vaccinia viruses were used for the challenge. Taken together, these results showed that the integrity of the TELEISSI epitope in BDV p40 was indispensable for induction of neurological disease in persistently infected B10.BR mice. Moreover, these data made it extremely unlikely that other hypothetical epitopes in BDV p40 played an important role in disease induction. We therefore concluded that TELEISSI is indeed the immunodominant CTL epitope against which the disease-inducing CTLs in BDV-infected H-2k mice are directed.
FIG. 7.
VV-FLAGp40E130K,I136T and VV-FLAGp40 induce comparable vaccinia virus-specific CTL responses in infected mice. Single-cell suspensions from spleens of B10.BR mice infected with 5 × 106 PFU of VV-FLAGp40E130K,I136T and VV-FLAGp40 or from spleens of mock-infected mice were used as effectors in a standard 51Cr release assay on vaccinia virus-infected L929 target cells. E:T ratio, effector-to-target ratio.
DISCUSSION
In the present study, we identified the H-2k-restricted CTL epitope in the p40 protein of BDV. It is an octamer peptide located at positions 129 to 136 of BDV p40 with the sequence TELEISSI that conforms to the consensus sequence motif for Kk-restricted CTL epitopes. By using the anchorless mutant FLAGp40E130K,I136T, we showed that TELEISSI represents the immunodominant epitope in p40 and that this peptide is of crucial importance for induction of the disease-determining T-cell response in BDV-infected mice.
We mapped the CTL epitope in BDV p40 by two complementary experimental approaches. In a first approach, we generated C-terminally truncated versions of p40 that were subsequently introduced into recombinant vaccinia viruses in order to express them in L929 cells. These cells were then used as target cells in cytotoxicity assays with lymphocytes from brains of BDV-infected mice with acute neurological disease. In a second approach, we screened the amino acid sequence of p40 for motifs that conform to the known consensus sequence for H-2k-restricted T-cell epitopes. Chemically synthesized peptides corresponding to candidate p40 epitopes were then added to the culture medium of L929 target cells to achieve their loading onto surface MHC class I complexes. An unexpected difficulty of the first approach was that vaccinia viruses carrying p40 variants that lacked 206 or more C-terminal amino acid residues could not be rescued. A second difficulty was that those C-terminally truncated p40 versions that could be rescued did not accumulate to high levels in infected cells, although the corresponding mRNAs were abundantly present. Since L929 cells infected with these recombinant vaccinia viruses were excellent targets for BDV-specific CTLs, it appears that the observed decreased stability of the mutants actually promoted efficient surface presentation of p40-derived peptides. Similar observations were reported with C-terminal truncation mutant forms of the nucleoprotein of influenza virus A/NT/60/68 (H3N2) (48) and the large T antigen of simian virus 40 (11, 37). It has recently been shown that expression of unstable fragments of influenza virus nucleoprotein resulted in higher intracellular levels of antigenic peptides than expression of the full-length nucleoprotein (1). Enhanced CTL responses were also observed when the nucleoprotein of LCMV was expressed in the form of a ubiquitin fusion protein that is quickly degraded by proteasomes (39), supporting the view that proteins with a reduced half-life are presented most efficiently on MHC class I molecules.
Two different computer programs predicted that TELEISSI is an H-2k-restricted epitope in BDV p40. By contrast, the overlapping peptide RDLTELEI, which also conforms to the consensus sequence, scored well in one program only. Both peptides were initially considered to be reasonable candidates because they reside in the N-terminal moiety of p40, which, according to our results with recombinant vaccinia viruses, carries the critical epitope. Experiments with chemically synthesized peptides loaded onto target cells proved that TELEISSI had the predicted activity, whereas RDLTELEI did not. Due to overlapping of these peptides in the p40 protein, mutation of the anchor residue E130 of TELEISSI also converted the putative epitope RDLTELEI into RDLTKLEI. Therefore, RDLTELEI might have lost its potential to substitute for TELEISSI as the immunodominant epitope in mutant protein FLAGp40E130K,I136T. However, since the mutation did not affect a putative anchor residue in RDLTELEI and, more importantly, since RDLTELEI was incapable of sensitizing target cells for lysis by ex vivo BDV-specific CTLs (Fig. 2B), it is highly unlikely that RDLTELEI represents a CTL epitope or could replace TELEISSI in the disease-inducing CD8+ T-cell response.
Since relatively high concentrations of the TELEISSI peptide were needed to sensitize L929 target cells for lysis by BDV-specific CTLs, the question was raised of whether this peptide has a low affinity for MHC class I Kk molecules or whether some intrinsic properties of our assay system might simply limit the sensitivity of the readout. Studies in other systems had previously shown that N-terminal elongation occasionally increases the affinity of peptides for the respective MHC class I molecules (5, 22), although peptide elongation at the N or C terminus usually has a negative effect, as shown for epitopes in the nucleoproteins of human respiratory syncytial virus (14), influenza virus A/PR/8/34 (13), and hepatitis C virus (21). We found here that N-terminal elongation of TELEISSI by one or two amino acids led to a moderate decrease in target cell sensitization and that C-terminal elongation of TELEISSI had an even more pronounced negative effect. This suggested that of all possible BDV p40-derived peptides, TELEISSI functions best as an H-2k epitope.
Since we showed that TELEISSI can up-regulate cell surface expression of Kk on T2 cells with an efficacy equal to that of the well-characterized CTL epitope FEANGNLI, which was reported to sensitize target cells at concentrations of less than 1 nM (13), we assume that the MHC class I-binding affinity of TELEISSI is probably higher than that estimated by our 51Cr release assays. Possibly, the requirement for high peptide concentrations in our assays resulted from inefficient expression of the MHC class I Kk molecule on the surface of our subline of L929 cells. It is also possible that the origin of the effector T cells could play a decisive role in the observed phenomenon. CTL assays with peptide-sensitized target cells are usually performed with permanent T-cell lines or with primary T cells that are restimulated in vitro before use. These procedures may enrich the effector cell population for CTLs with enhanced affinity for the cognate peptide. It should be noted that the CTLs of the present study originated from inflamed mouse brains and were used directly for 51Cr release assays without in vitro restimulation. Alternatively, the requirement for a high peptide concentration could be explained by assuming that T cells recognizing the TELEISSI-MHC class I complex carry low-affinity receptors. It is reasonable to assume that low-avidity CTLs might dominate in persistent virus infections because activation-induced cell death resulting from prolonged exposure to antigen presented by nonprofessional antigen-presenting cells may primarily affect high-avidity CTLs (27, 43).
To find out whether TELEISSI is the immunodominant epitope, we replaced the anchor residues E130 and I136 in p40 with K and T, respectively, in order to destroy the TELEISSI epitope. We found that effector T cells from MRL mice did not recognize any alternative epitopes on cells expressing the mutant form FLAGp40E130K,I136T, which demonstrated that TELEISSI is indeed the immunodominant epitope in p40. In addition, this mutant p40 was not able to induce meningoencephalitis and disease after vaccination of persistently infected B10.BR mice. This showed that no subdominant epitope(s) existed which could replace TELEISSI in inducing disease-mediating CD8+ T cells. These results indicate that the T-cell repertoire for Kk-restricted p40 epitopes is very limited. They further suggest that the immunodominance of TELEISSI is not based on suppression of T-cell responses to other peptides by the dominant peptide, as seems to be the case for immunodominant epitopes of simian virus 40 T antigen and influenza virus HA (8, 28).
Experiments in the rat model system showed that it is possible to protect against BDV infection by adoptive transfer of BDV-specific CD4+ T cells (38), which are thought to act by inducing an antiviral CD8+ T-cell response (30). Our recent experiments indicated that p40-specific vaccination with the help of recombinant vaccinia viruses can suppress viral spread in the CNSs of MRL mice (K. Schamel and J. Hausmann, unpublished data). These results suggest that protective immunity might be achieved by immunizing mice with the TELEISSI peptide alone. In the LCMV system, it was shown that the hierarchy of the CTL response against different viral proteins does not strictly correlate with protective immunity (10). Thus, the possibility should be taken into account that antigenic peptides derived from other BDV proteins may also mediate protective immunity. We have previously observed a weak CTL response to the viral phosphoprotein p24 in infected MRL mice (17), suggesting that BDV harbors additional H-2k-restricted CTL epitopes. Computer-assisted inspection revealed several candidate peptides for Kk binding in p24, gp18, and the L polymerase of BDV (J. Hausmann, unpublished data). Additional experiments are required to determine whether they represent targets of the antiviral immune response in H-2k mice and could have protective potential as peptide vaccines.
With the knowledge that TELEISSI is the immunodominant peptide of BDV that determines the disease-inducing CTL response in persistently infected H-2k mice, new experimental approaches are becoming available which may eventually lead to a more complete understanding of the disease mechanisms. For example, it should now be possible to generate permanent T-cell lines with disease-inducing potential in infected mice. The new information should further allow the generation of tetramers of peptide-loaded MHC class I complexes for in situ detection of TELEISSI-specific CD8+ T cells, which would help in the identification of the site of T-cell priming and would allow monitoring of the fate of antigen-specific T cells in the brain.
ACKNOWLEDGMENTS
We thank Rosita Frank for excellent technical assistance and John Haurum and Mads Hald Andersen, Copenhagen, Denmark, for supplying T2-Kk cells. We further thank Matthias Regner, Geneva, Switzerland, and Mario Lobigs, Canberra, Australia, for help with the peptide binding assays and Otto Haller for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Anton L C, Yewdell J W, Bennink J R. MHC class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535–2542. [PubMed] [Google Scholar]
- 2.Bilzer T, Planz O, Lipkin W I, Stitz L. Presence of CD4+ and CD8+ T cells and expression of MHC class I and MHC class II antigen in horses with Borna disease virus-induced encephalitis. Brain Pathol. 1995;5:223–230. doi: 10.1111/j.1750-3639.1995.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 3.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Sidney J, Southwood S, Cox A L, Sakaguchi K, Henderson R A, Appella E, Hunt D F, Sette A, Engelhard V H. Naturally processed peptides longer than nine amino acid residues bind to the class I MHC molecule HLA-A2.1 with high affinity and in different conformations. J Immunol. 1994;152:2874–2881. [PubMed] [Google Scholar]
- 6.Cubitt B, Oldstone C, De la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bergeyck V, De Plaen E, Chomez P, Boon T, Van Pel A. An intracisternal A-particle sequence codes for an antigen recognized by syngeneic cytolytic T lymphocytes on a mouse spontaneous leukemia. Eur J Immunol. 1994;24:2203–2212. doi: 10.1002/eji.1830240941. [DOI] [PubMed] [Google Scholar]
- 8.Deng Y, Yewdell J W, Eisenlohr L C, Bennink J R. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J Immunol. 1997;158:1507–1515. [PubMed] [Google Scholar]
- 9.Formella S, Jehle C, Sauder C, Staeheli P, Schwemmle M. Sequence variability of Borna disease virus: resistance to superinfection may contribute to high genome stability in persistently infected cells. J Virol. 2000;74:7878–7883. doi: 10.1128/jvi.74.17.7878-7883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallimore A, Dumrese T, Hengartner H, Zinkernagel R M, Rammensee H G. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooding L R, O'Connell K A. Recognition by cytotoxic T lymphocytes of cells expressing fragments of the SV40 tumor antigen. J Immunol. 1983;131:2580–2586. [PubMed] [Google Scholar]
- 12.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995;190:39–73. [PubMed] [Google Scholar]
- 13.Gould K G, Scotney H, Brownlee G G. Characterization of two distinct major histocompatibility complex class I Kk-restricted T-cell epitopes within the influenza A/PR/8/34 virus hemagglutinin. J Virol. 1991;65:5401–5409. doi: 10.1128/jvi.65.10.5401-5409.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulder P J R, Lechner F, Klenerman P, Mclntosh K, Walker B D. Characterization of a novel respiratory syncytial virus-specific human cytotoxic T-lymphocyte epitope. J Virol. 2000;74:7694–7697. doi: 10.1128/jvi.74.16.7694-7697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas B, Becht H, Rott R. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J Gen Virol. 1986;67:235–241. doi: 10.1099/0022-1317-67-2-235. [DOI] [PubMed] [Google Scholar]
- 16.Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausmann J, Hallensleben W, De la Torre J C, Pagenstecher A, Zimmermann C, Pircher H, Staeheli P. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc Natl Acad Sci USA. 1999;96:9769–9774. doi: 10.1073/pnas.96.17.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 19.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 20.Irani D N, Griffin D E. Isolation of brain parenchymal lymphocytes for flow cytometric analysis. Application to acute viral encephalitis. J Immunol Methods. 1991;139:223–231. doi: 10.1016/0022-1759(91)90192-i. [DOI] [PubMed] [Google Scholar]
- 21.Kita H, Hiroishi K, Moriyama T, Okamoto H, Kaneko T, Ohnishi S, Yazaki Y, Imawari M. A minimal and optimal cytotoxic T cell epitope within hepatitis C virus nucleoprotein. J Gen Virol. 1995;76:3189–3193. doi: 10.1099/0022-1317-76-12-3189. [DOI] [PubMed] [Google Scholar]
- 22.Lechner F, Wong D K, Dunbar P R, Chapman R, Chung R T, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker B D. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 24.Ludwig H, Furuya K, Bode L, Klein N, Dürrwald R, Lee D S. Biology and neurobiology of Borna disease viruses (BDV), defined by antibodies, neutralizability and their pathogenic potential. Arch Virol. 1993;7(Suppl.):111–133. doi: 10.1007/978-3-7091-9300-6_10. [DOI] [PubMed] [Google Scholar]
- 25.Lukacher A E, Wilson C S. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol. 1998;160:1724–1734. [PubMed] [Google Scholar]
- 26.Mackett M. Manipulation of vaccinia virus vectors. In: Murray E J, editor. Methods in Molecular Biology. Vol. 7. Clifton, N.J: Humana Press; 1991. pp. 129–146. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 28.Mylin L M, Bonneau R H, Lippolis J D, Tevethia S S. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J Virol. 1995;69:6665–6677. doi: 10.1128/jvi.69.11.6665-6677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunpathological responses to persistent Borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 30.Nöske K, Bilzer T, Planz O, Stitz L. Virus-specific CD4+ T cells eliminate Borna disease virus from the brain via induction of cytotoxic CD8+ T cells. J Virol. 1998;72:4387–4395. doi: 10.1128/jvi.72.5.4387-4395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Ostler, T., K. Schamel, T. Hussell, P. Openshaw, J. Hausmann, and S. Ehl. An improved protocol for measuring cytotoxic T cell activity in anatomic compartments with low cell numbers. J. Immunol. Methods, in press. [DOI] [PubMed]
- 31.Parker K C, Bednarek M A, Coligan J E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 32.Pircher H, Moskophidis D, Rohrer U, Burki K, Hengartner H, Zinkernagel R M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 33.Planz O, Bilzer T, Stitz L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Planz O, Stitz L. Borna disease virus nucleoprotein (p40) is a major target for CD8+-T-cell-mediated immune response. J Virol. 1999;73:1715–1718. doi: 10.1128/jvi.73.2.1715-1718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rammensee H, Bachmann J, Emmerich N P, Bachor O A, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 36.Rammensee H G, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 37.Reddy V B, Tevethia S S, Tevethia M J, Weissman S M. Nonselective expression of simian virus 40 large tumor antigen fragments in mouse cells. Proc Natl Acad Sci USA. 1982;79:2064–2067. doi: 10.1073/pnas.79.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richt J A, Schmeel A, Frese K, Carbone K M, Narayan O, Rott R. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J Exp Med. 1994;179:1467–1473. doi: 10.1084/jem.179.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez F, Zhang J, Whitton J L. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 41.Salter R D, Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 1986;5:943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauder C, Wolfer D P, Lipp H, Staeheli P, Hausmann J. Learning deficits in mice with persistent Borna disease virus infection of the CNS associated with elevated chemokine expression. Behav Brain Res. 2001;120:189–201. doi: 10.1016/s0166-4328(00)00370-3. [DOI] [PubMed] [Google Scholar]
- 43.Selin L K, Welsh R M. Specificity and editing by apoptosis of virus-induced cytotoxic T lymphocytes. Curr Opin Immunol. 1994;6:553–559. doi: 10.1016/0952-7915(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 44.Sobbe M, Bilzer T, Gommel S, Nöske K, Planz O, Stitz L. Induction of degenerative brain lesions after adoptive transfer of brain lymphocytes from Borna disease virus-infected rats: presence of CD8+ T cells and perforin mRNA. J Virol. 1997;71:2400–2407. doi: 10.1128/jvi.71.3.2400-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staeheli P, Sauder C, Hausmann J, Ehrensperger F, Schwemmle M. Epidemiology of borna disease virus. J Gen Virol. 2000;81:2123–2135. doi: 10.1099/0022-1317-81-9-2123. [DOI] [PubMed] [Google Scholar]
- 46.Stitz L, Sobbe M, Bilzer T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J Virol. 1992;66:3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stitz L, Soeder D, Deschl U, Frese K, Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus-infected rats by cyclosporine A. J Immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 48.Townsend A R, Gotch F M, Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985;42:457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]