Abstract
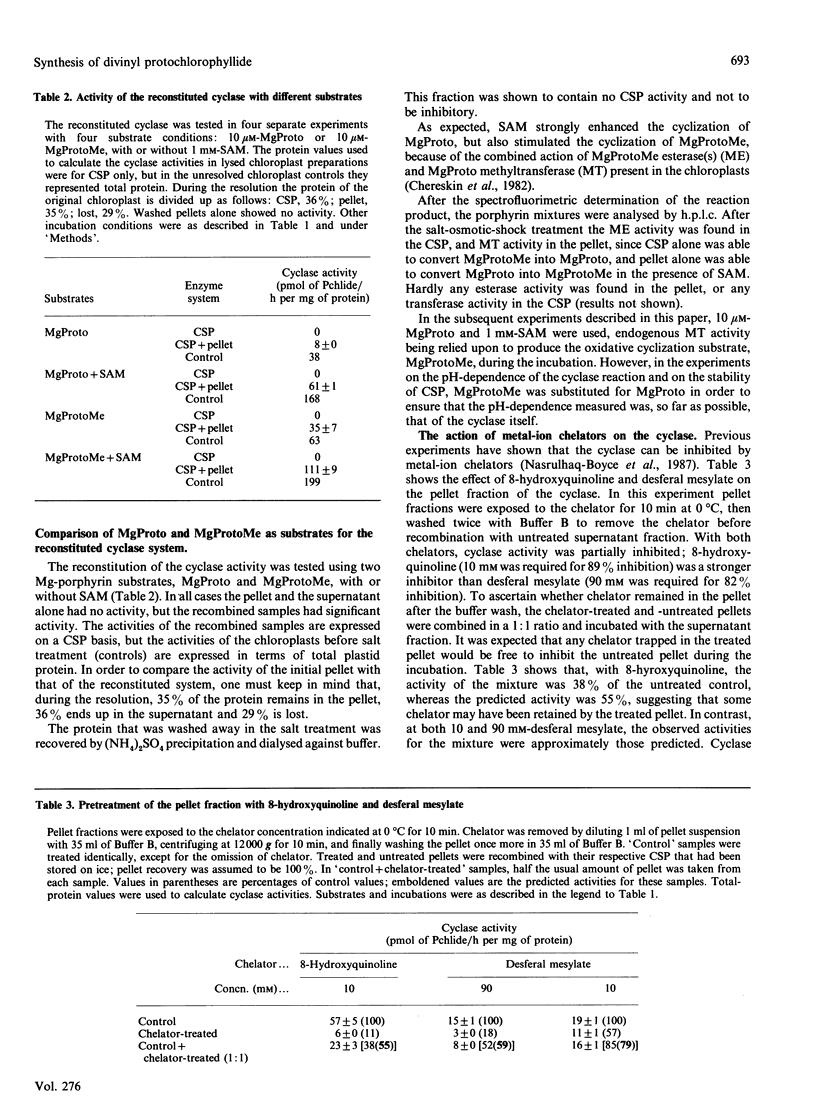
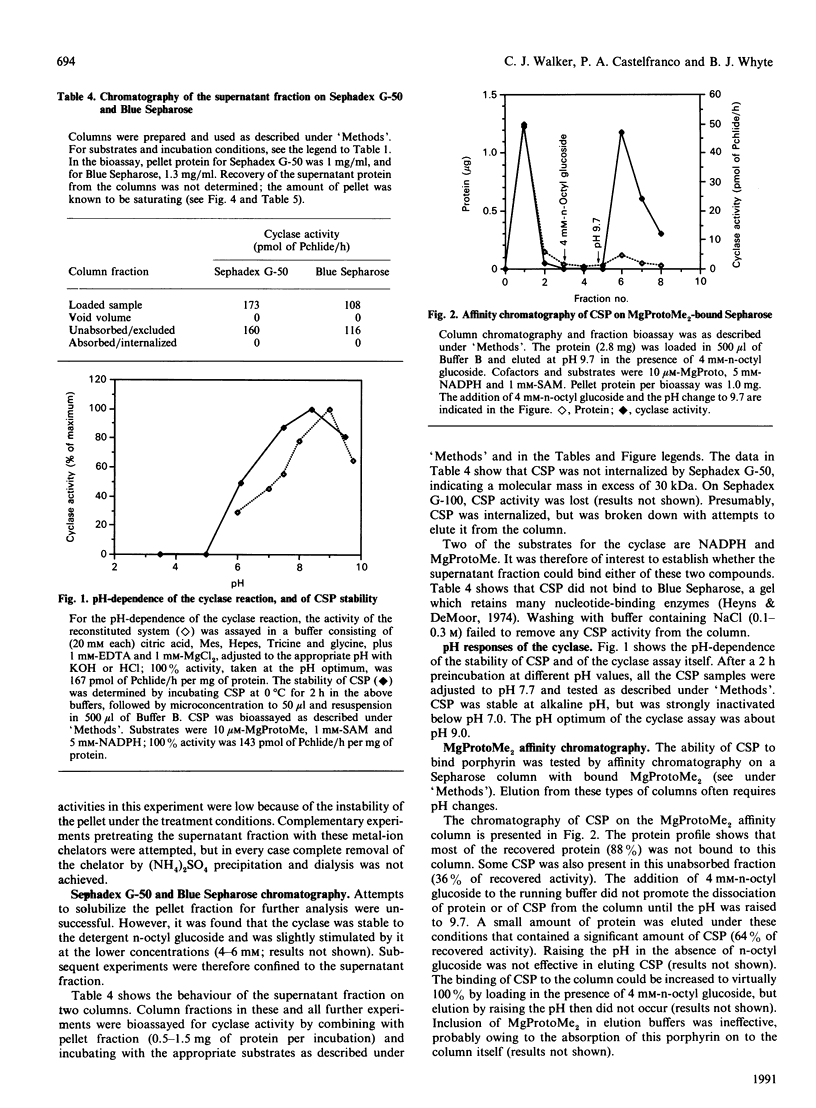
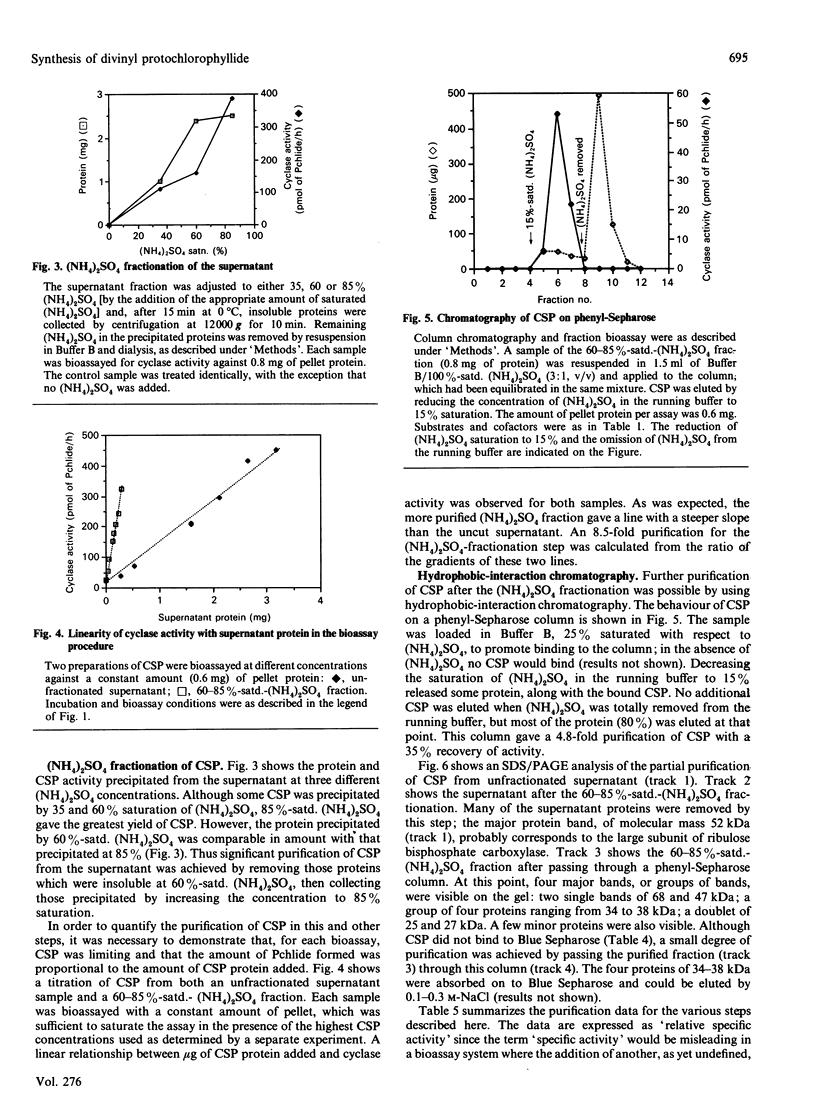
The resolution and reconstitution of the Mg-protoporphyrin IX monomethyl ester oxidative cyclase system into a supernatant and a pellet fraction was accomplished by a procedure involving salt treatment followed by osmotic shock. Recombination of pellet and supernatant fractions was required for cyclase activity. This restoration effect could be demonstrated using either Mg-protoporphyrin IX or Mg-protoporphyrin IX monomethyl ester as the cyclase substrate in the presence or absence of S-adenosylmethionine. Pretreatment of the pellet fraction with either 8-hydroxyquinoline or desferal mesylate inhibited cyclase activity, indicating that there is a heavy-metal-ion requirement in this fraction. The cyclase supernatant protein(s) was not internalized by Sephadex G-50 and did not bind to Blue Sepharose, suggesting that it has a molecular mass of over 30 kDa and that it does not bind the cofactor NADPH. The cyclase supernatant protein did bind to MgProtoMe2-bound Sepharose and could be eluted by raising the pH to 9.7 in the presence of 4 mM-n-octyl glucoside. The pH optimum of the cyclase was 9.0. About a 40-fold purification of the cyclase supernatant protein was achieved by a combination of (NH4)2SO4 fractionation and phenyl-Sepharose chromatography.
Full text
PDF



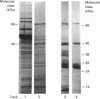
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. E., Marrs B. L. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7074–7078. doi: 10.1073/pnas.85.19.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A., Dallas J. L., Straub K. M. Mg-2,4-divinyl pheoporphyrin a5: the product of a reaction catalyzed in vitro by developing chloroplasts. Arch Biochem Biophys. 1983 Oct 1;226(1):10–18. doi: 10.1016/0003-9861(83)90266-7. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : II. OBSERVATIONS ON THE BIOSYNTHETIC PATHWAY IN ISOLATED ETIOCHLOROPLASTS. Plant Physiol. 1982 Jan;69(1):112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereskin B. M., Wong Y. S., Castelfranco P. A. In Vitro Synthesis of the Chlorophyll Isocyclic Ring : Transformation of Magnesium-Protoporphyrin IX and Magnesium-Protoporphyrin IX Monomethyl Ester into Magnesium-2,4-Divinyl Pheoporphyrin A(5). Plant Physiol. 1982 Oct;70(4):987–993. doi: 10.1104/pp.70.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Hanamoto C. M., Castelfranco P. A. Separation of Mg-Protoporphyrin IX and Mg-Protoporphyrin IX Monomethyl Ester Synthesized de novo by Developing Cucumber Etioplasts. Plant Physiol. 1982 Feb;69(2):421–423. doi: 10.1104/pp.69.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948 1949;Series 44:220–245. [PubMed] [Google Scholar]
- Griffiths W. T. Reconstitution of chlorophyllide formation by isolated etioplast membranes. Biochem J. 1978 Sep 15;174(3):681–692. doi: 10.1042/bj1740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns W., De Moor P. A 3(17)beta-hydroxysteroid dehydrogenase in raterythrocytes. Conversion of 5alpha-dihydrotestosterone into 5alpha-androstane-3beta,17beta-diol and purification of the enzyme by affinity chromatography. Biochim Biophys Acta. 1974 Jul 17;358(1):1–13. doi: 10.1016/0005-2744(74)90251-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nasrulhaq-Boyce A., Griffiths W. T., Jones O. T. The use of continuous assays to characterize the oxidative cyclase that synthesizes the chlorophyll isocyclic ring. Biochem J. 1987 Apr 1;243(1):23–29. doi: 10.1042/bj2430023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Mansfield K. E., Rezzano I. N., Hanamoto C. M., Smith K. M., Castelfranco P. A. The magnesium-protoporphyrin IX (oxidative) cyclase system. Studies on the mechanism and specificity of the reaction sequence. Biochem J. 1988 Oct 15;255(2):685–692. [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Mansfield K. E., Smith K. M., Castelfranco P. A. Incorporation of atmospheric oxygen into the carbonyl functionality of the protochlorophyllide isocyclic ring. Biochem J. 1989 Jan 15;257(2):599–602. doi: 10.1042/bj2570599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A., Goff D. A., Smith K. M. Intermediates in the formation of the chlorophyll isocyclic ring. Plant Physiol. 1985 Nov;79(3):725–729. doi: 10.1104/pp.79.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A. Properties of the Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase System. Plant Physiol. 1985 Nov;79(3):730–733. doi: 10.1104/pp.79.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A. Resolution and Reconstitution of Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase, the Enzyme System Responsible for the Formation of the Chlorophyll Isocyclic Ring. Plant Physiol. 1984 Jul;75(3):658–661. doi: 10.1104/pp.75.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]