Abstract
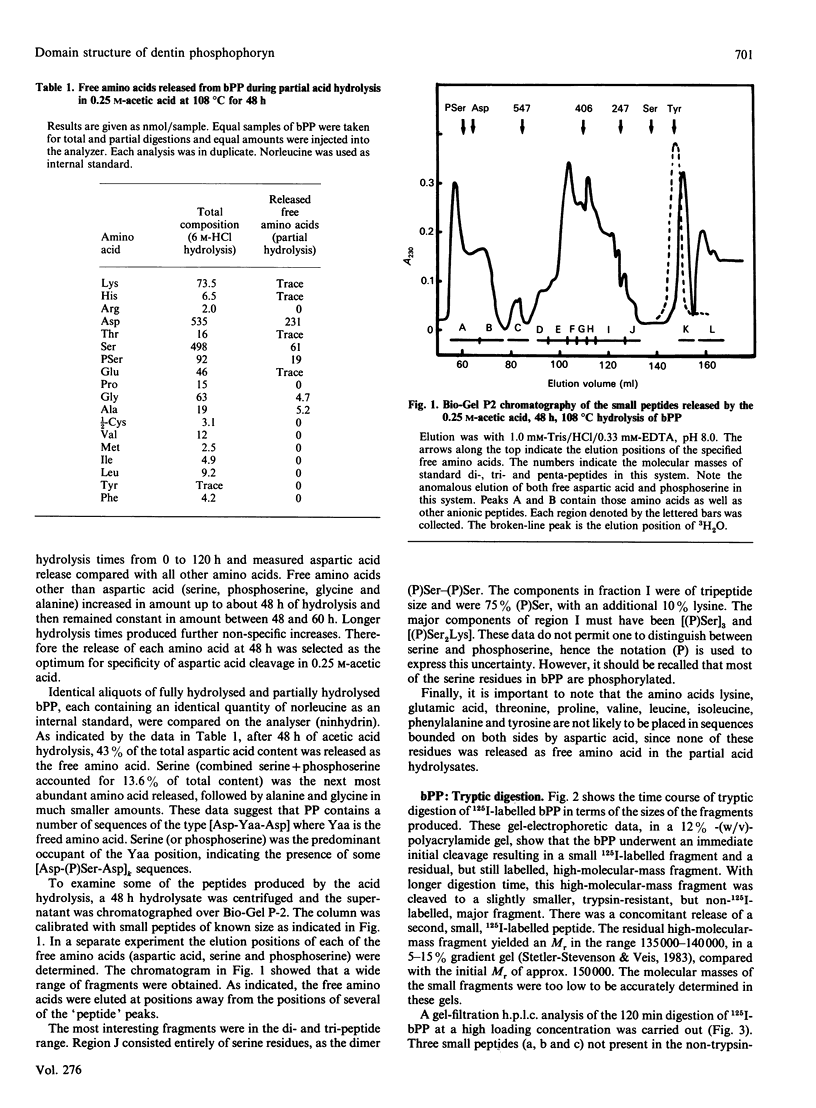
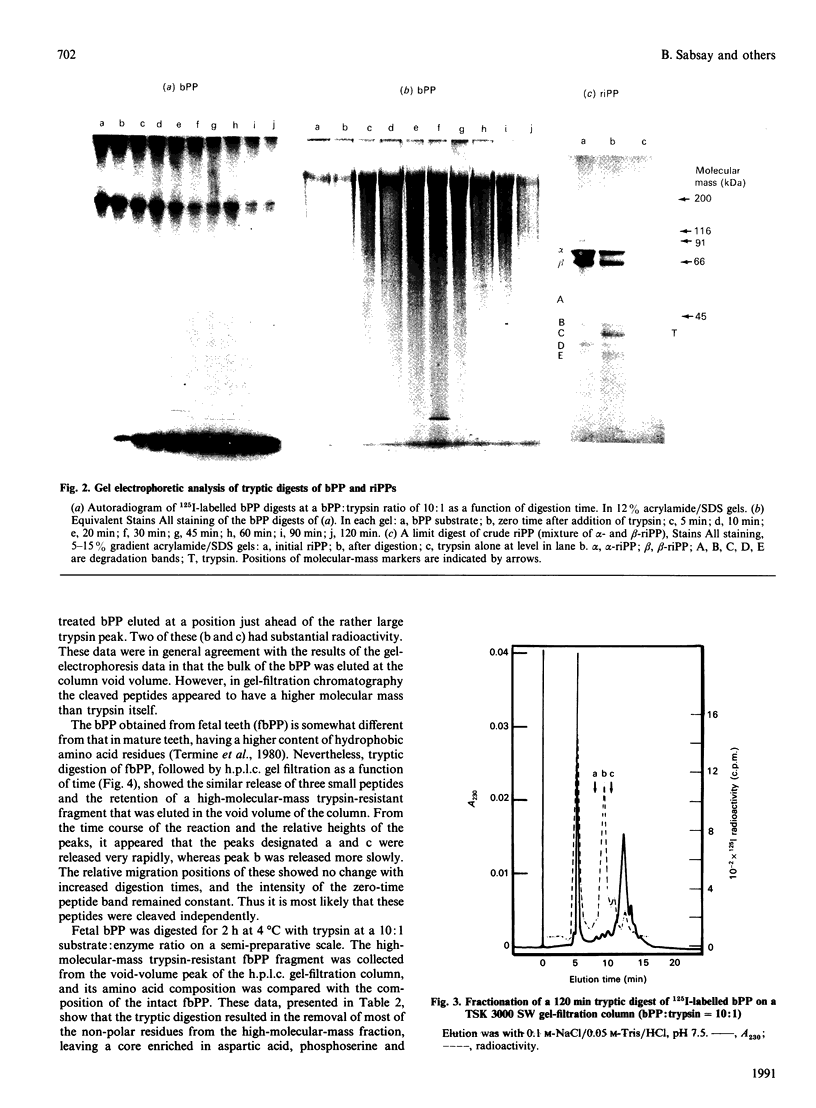
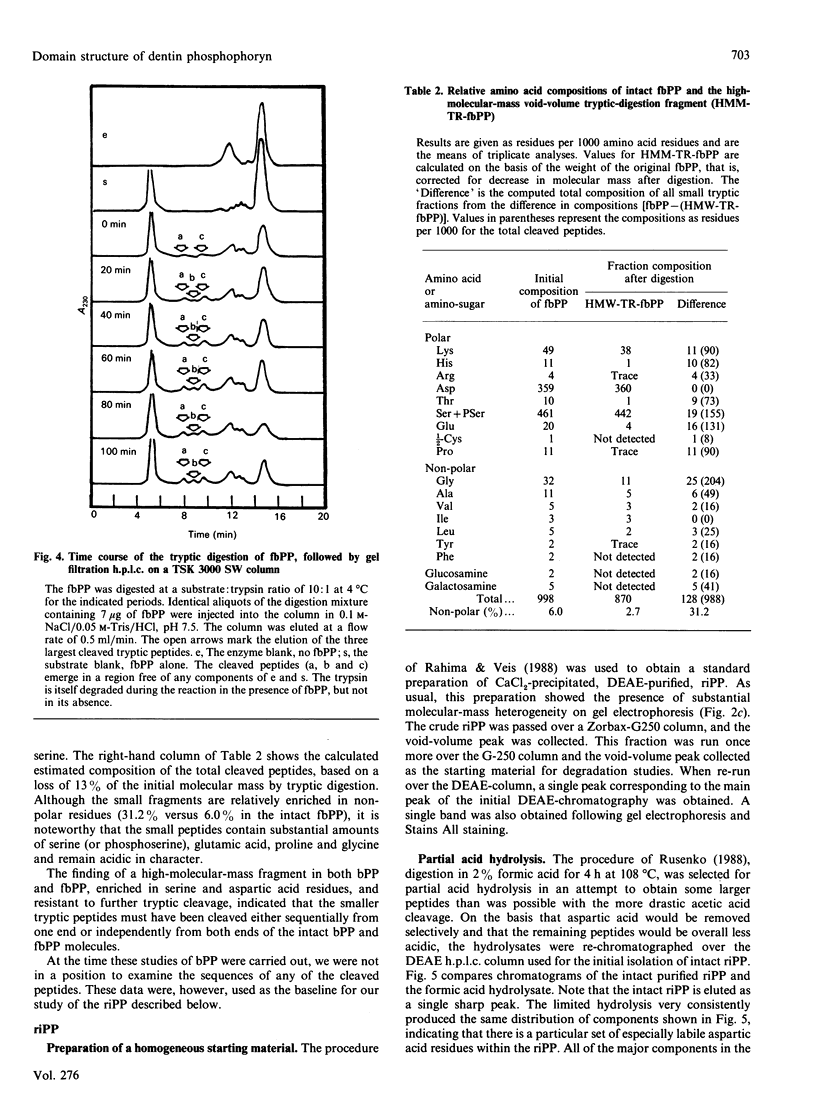
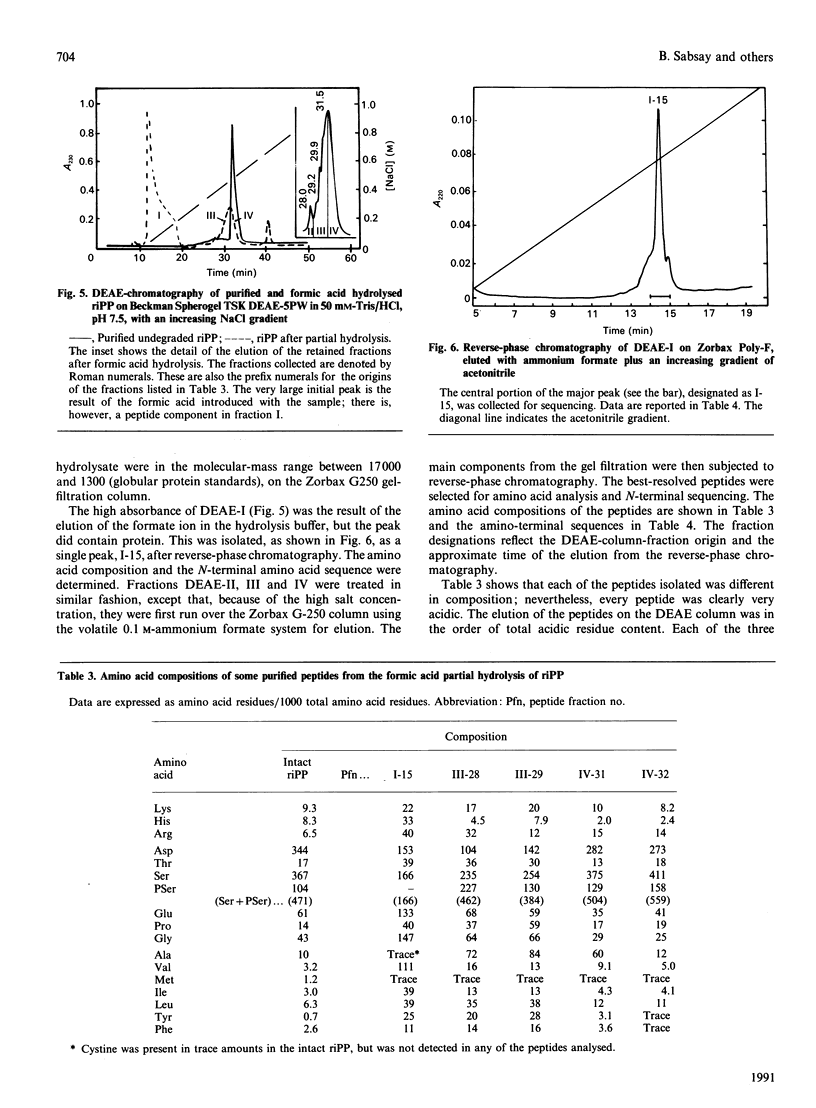
Phosphophoryn (PP) is a protein unique to the mineralized matrix of dentin. It also has a unique composition, with aspartic acid and phosphoserine comprising greater than 85% of all amino acid residues. Because of this unique composition and high content of phosphoserine, it has been difficult to apply direct peptide sequencing procedures effectively. However, to understand its function, and to prepare suitable probes for screening cDNA libraries, some sequence distribution information is required. To this end, using bovine (b) and rat incisor (ri) PPs, partial mild acid hydrolysis has been used to cleave at the aspartic acid residues and generate free amino acids and small peptides. The nature of the released amino acids and peptides has been determined. Peptides have also been generated by limited digestion with trypsin. Some of the peptides have been purified by h.p.l.c. techniques and sequenced. About 90% of the bPP and riPP were resistant to trypsin, and the large resistant fragment was sharply depleted of the non-aspartic acid and non-phosphoserine [(P)Ser] residues. All peptides isolated were acidic, but the remaining residues (other than aspartic acid and serine) appeared to be collected in regions flanking the trypsin-resistant core. These data show directly the presence of regions [Asp]n, [(P)Ser]m and [Asp-(P)Ser-Asp]k as prominent sequence features. A domain structure model is proposed.
Full text
PDF

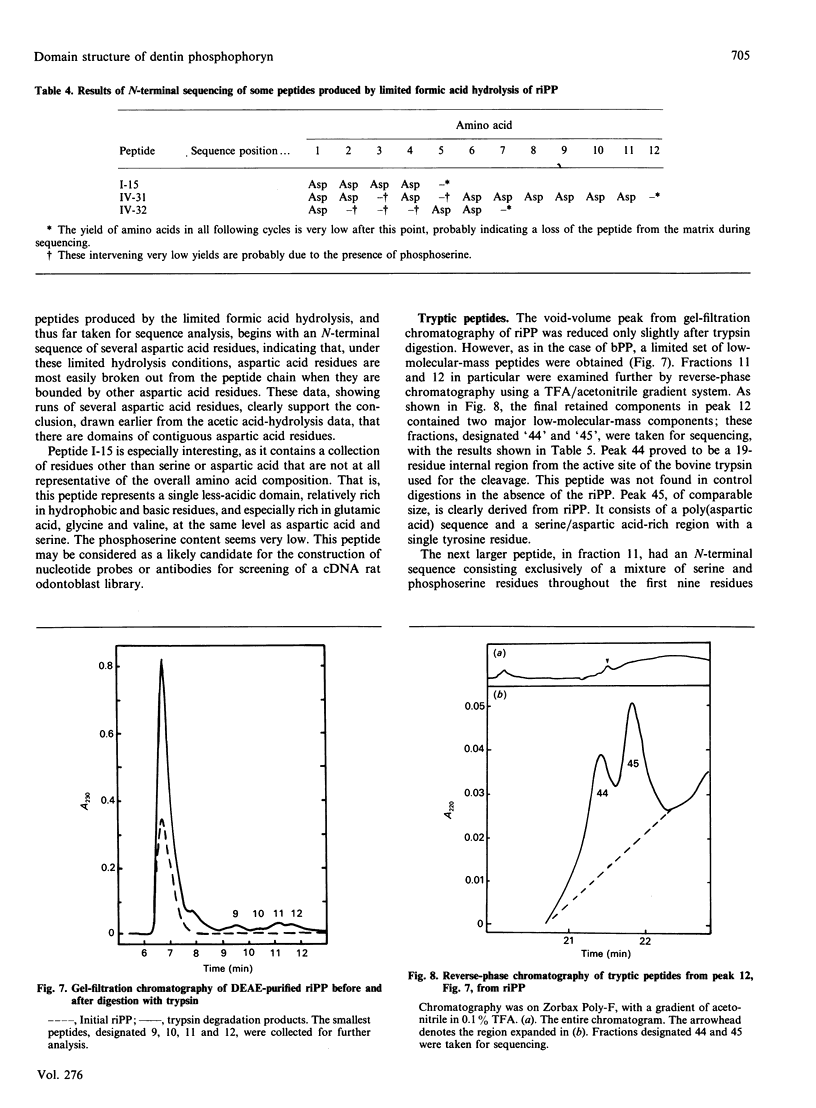
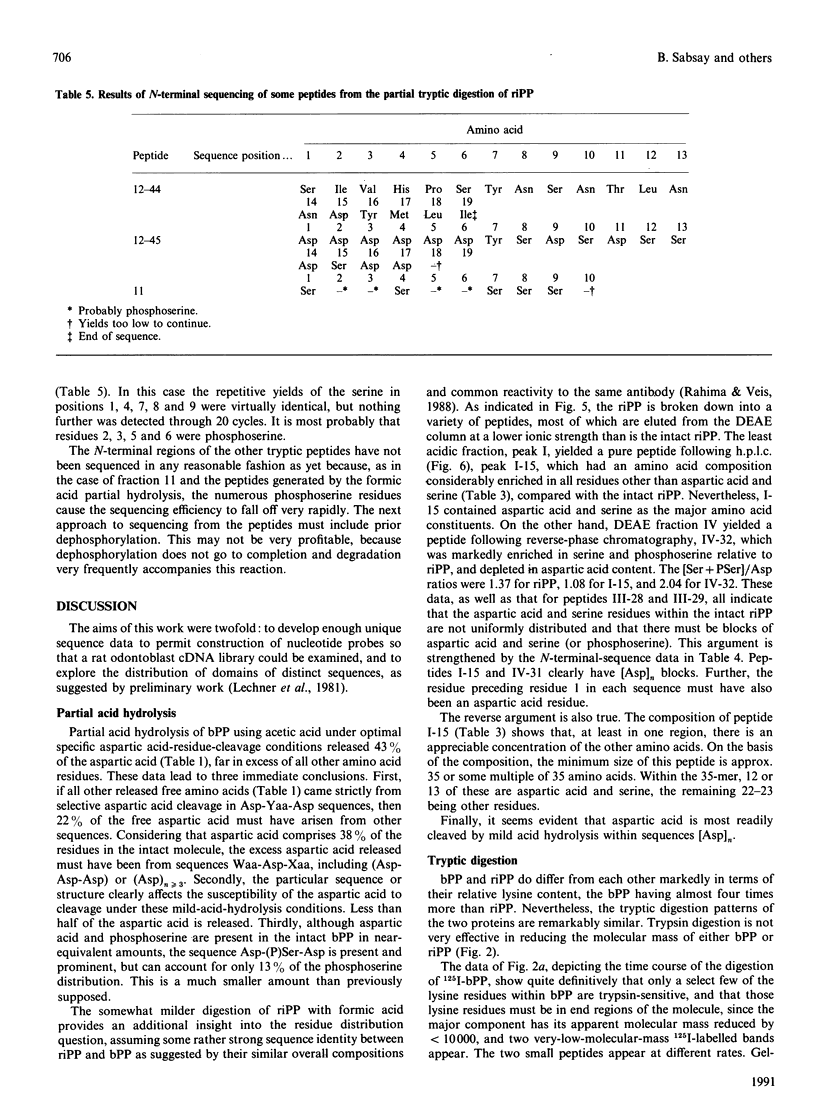

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. T., Bhown M., DiMuzio M. T., Cothran W. C., Linde A. Multiple forms of rat dentin phosphoproteins. Arch Biochem Biophys. 1983 Aug;225(1):178–186. doi: 10.1016/0003-9861(83)90021-8. [DOI] [PubMed] [Google Scholar]
- Carmichael D. J., Chovelon A., Pearson C. H. The composition of the insoluble collagenous matrix of bovine predentine. Calcif Tissue Res. 1975 Jun 18;17(4):263–271. doi: 10.1007/BF02546599. [DOI] [PubMed] [Google Scholar]
- Dimuzio M. T., Veis A. Phosphophoryns-major noncollagenous proteins of rat incisor dentin. Calcif Tissue Res. 1978 May 26;25(2):169–178. doi: 10.1007/BF02010765. [DOI] [PubMed] [Google Scholar]
- Inglis A. S. Cleavage at aspartic acid. Methods Enzymol. 1983;91:324–332. doi: 10.1016/s0076-6879(83)91030-3. [DOI] [PubMed] [Google Scholar]
- Jontell M., Linde A. Non-collagenous proteins of predentine from dentinogenically active bovine teeth. Biochem J. 1983 Sep 15;214(3):769–776. doi: 10.1042/bj2140769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippner R. D., Nawrot C. F. The distribution of aspartic acid residues in bovine dentine phosphoprotein. J Dent Res. 1977 Jul;56(7):873–873. doi: 10.1177/00220345770560072801. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Glonek T., Glimcher M. J. 31P nuclear magnetic resonance spectroscopic evidence for ternary complex formation of fetal dentin phosphoprotein with calcium and inorganic orthophosphate ions. Calcif Tissue Int. 1983 Sep;35(6):815–818. doi: 10.1007/BF02405129. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Veis A., Glonek T. Dentin phosphoprotein: an extracellular calcium-binding protein. Biochemistry. 1977 Jun 28;16(13):2971–2979. doi: 10.1021/bi00632a026. [DOI] [PubMed] [Google Scholar]
- MacDougall M., Zeichner-David M., Slavkin H. C. Production and characterization of antibodies against murine dentine phosphoprotein. Biochem J. 1985 Dec 1;232(2):493–500. doi: 10.1042/bj2320493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier G. D., Lechner J. H., Veis A. The dynamics of formation of a collagen-phosphophoryn conjugate in relation to the passage of the mineralization front in rat incisor dentin. J Biol Chem. 1983 Feb 10;258(3):1450–1455. [PubMed] [Google Scholar]
- Masters P. M. In vivo decomposition of phosphoserine and serine in noncollagenous protein from human dentin. Calcif Tissue Int. 1985 May;37(3):236–241. doi: 10.1007/BF02554869. [DOI] [PubMed] [Google Scholar]
- Nakamura O., Gohda E., Ozawa M., Senba I., Miyazaki H., Murakami T., Daikuhara Y. Immunohistochemical studies with a monoclonal antibody on the distribution of phosphophoryn in predentin and dentin. Calcif Tissue Int. 1985 Sep;37(5):491–500. doi: 10.1007/BF02557832. [DOI] [PubMed] [Google Scholar]
- Rahima M., Tsay T. G., Andujar M., Veis A. Localization of phosphophoryn in rat incisor dentin using immunocytochemical techniques. J Histochem Cytochem. 1988 Feb;36(2):153–157. doi: 10.1177/36.2.3335773. [DOI] [PubMed] [Google Scholar]
- Rahima M., Veis A. Two classes of dentin phosphophoryns, from a wide range of species, contain immunologically cross-reactive epitope regions. Calcif Tissue Int. 1988 Feb;42(2):104–112. doi: 10.1007/BF02556342. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., ALLISON H., GRICE M. Specificity of the cleavage of proteins by dilute acid. I. Release of aspartic acid from insulin, ribonuclease, and glucagon. Biochemistry. 1962 Jul;1:694–698. doi: 10.1021/bi00910a024. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Veis A. Bovine dentin phosphophoryn: calcium ion binding properties of a high molecular weight preparation. Calcif Tissue Int. 1987 Feb;40(2):97–102. doi: 10.1007/BF02555712. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Veis A. Bovine dentin phosphophoryn: composition and molecular weight. Biochemistry. 1983 Aug 30;22(18):4326–4335. doi: 10.1021/bi00287a025. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Veis A. Type I collagen shows a specific binding affinity for bovine dentin phosphophoryn. Calcif Tissue Int. 1986 Mar;38(3):135–141. doi: 10.1007/BF02556873. [DOI] [PubMed] [Google Scholar]
- Takagi Y., Fujisawa R., Sasaki S. Identification of dentin phosphophoryn localization by histochemical stainings. Connect Tissue Res. 1986;14(4):279–292. doi: 10.3109/03008208609017471. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Miyamoto M. S., Conn K. M. Properties of dissociatively extracted fetal tooth matrix proteins. II. Separation and purification of fetal bovine dentin phosphoprotein. J Biol Chem. 1980 Oct 25;255(20):9769–9772. [PubMed] [Google Scholar]
- Tsay T. G., Veis A. Preparation, detection, and characterization of an antibody to rat alpha-phosphophoryn. Biochemistry. 1985 Nov 5;24(23):6363–6369. doi: 10.1021/bi00344a007. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Radioautographic visualization of the deposition of a phosphoprotein at the mineralization front in the dentin of the rat incisor. J Cell Biol. 1973 Mar;56(3):838–845. doi: 10.1083/jcb.56.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M., de Bernard B., Jontell M., Linde A. Ca2+-binding studies of the phosphoprotein from rat-incisor dentine. Eur J Biochem. 1981 Jan;113(3):541–545. doi: 10.1111/j.1432-1033.1981.tb05096.x. [DOI] [PubMed] [Google Scholar]



