Abstract
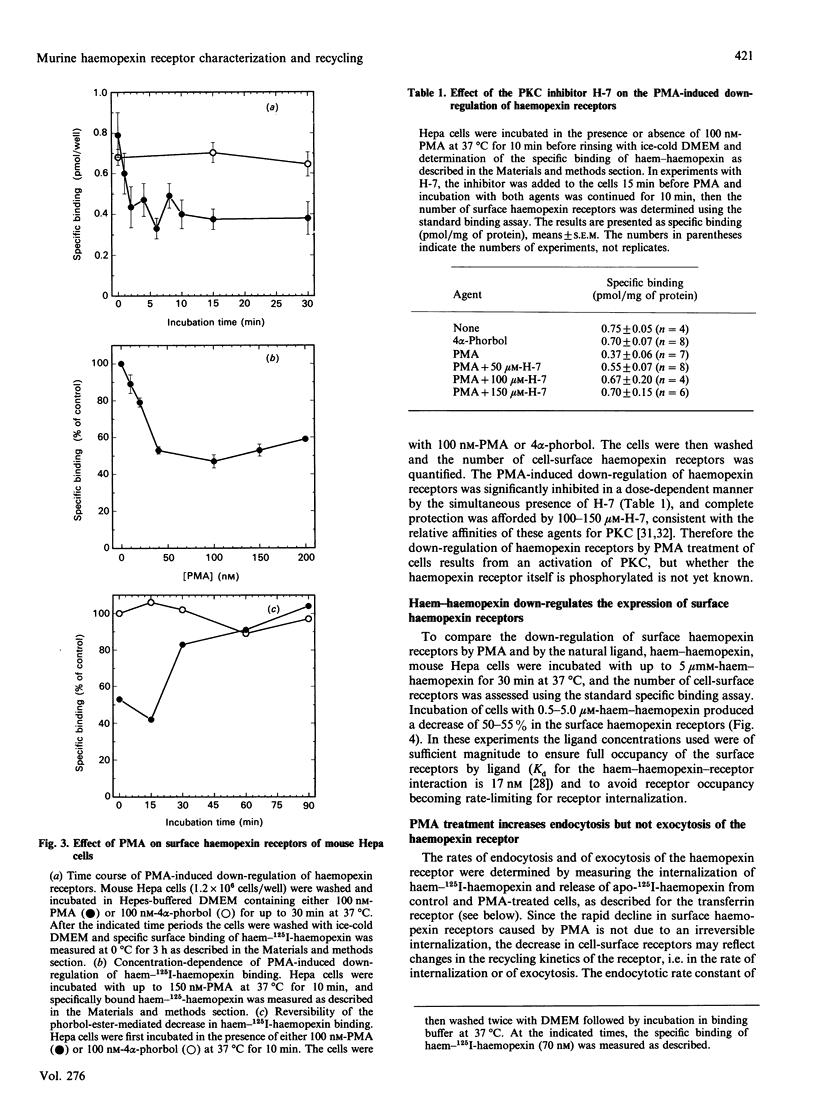
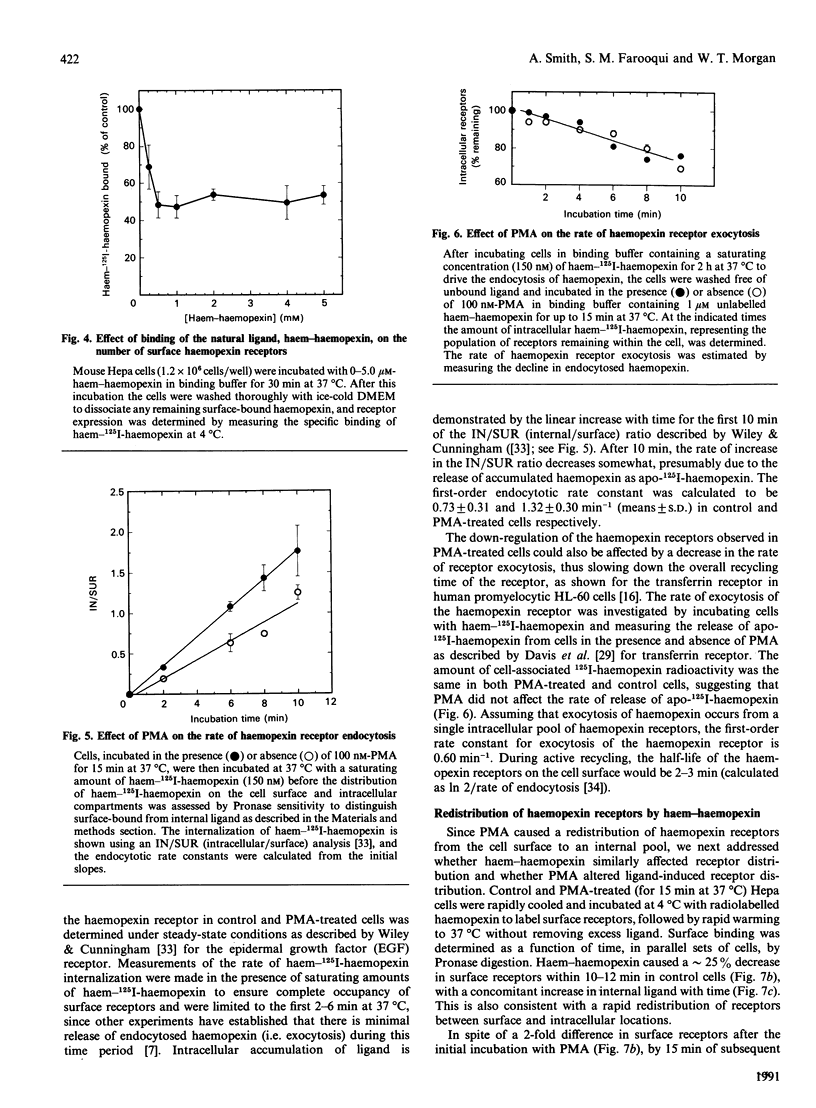
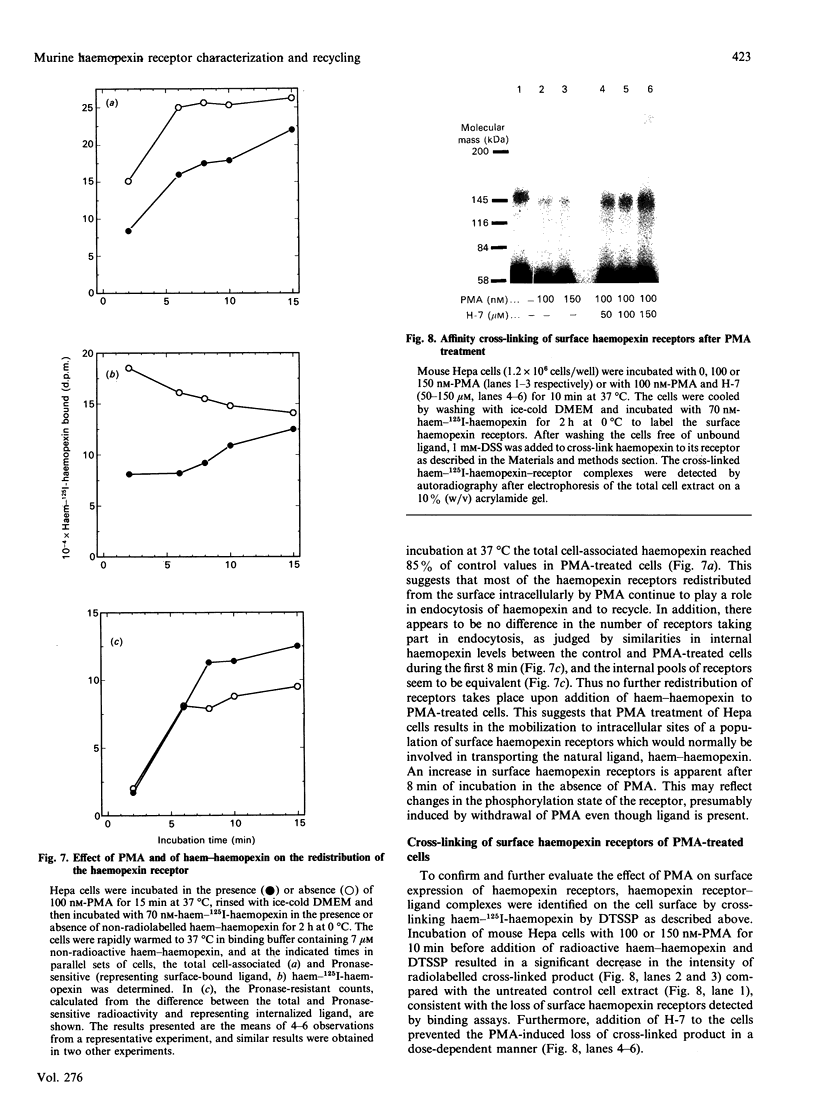
Haemopexin receptors from mouse hepatoma (Hepa) cells were affinity-labelled by cross-linking to haem-125I-haemopexin complexes using two homo-[disuccinimidyl suberate (DSS) and 3,3'-dithiobis(succinimidyl propionate) (DTSSP)] and one hetero-[sulphosuccinimidyl 4-(p-maleimidophenyl)butyrate (sulpho-SMPB)] bifunctional cross-linking agents. Analysis of the cross-linked products by SDS/PAGE in the absence of reducing agents revealed that 125I-haemopexin was cross-linked specifically to a protein of apparent molecular mass 85-90 kDa. Upon reduction, haemopexin remained cross-linked to a protein of 20 kDa, suggesting that the murine haemopexin receptor has a subunit structure. Two subunits were identified: alpha (p65) and beta (p20). Furthermore, because haemopexin was cross-linked by all three agents to p20, the shortest cross-linker arm being 1.1 nm (11 A), we propose that the haem-haemopexin-binding site resides on this subunit. In addition, a cysteine residue of p20 is located near the haemopexin-binding site, since haemopexin, which has no free thiol groups, is cross-linked to this subunit by the hetero-bifunctional agent sulpho-SMPB. Exposure of Hepa cells to the tumour-promoting phorbol ester 4 alpha-phorbol 12-myristate 13-acetate (PMA) causes a rapid redistribution of haemopexin receptors from the cell surface to the cell interior. Within 2-4 min of incubation with 100 nM-PMA, there was an approx. 50% decrease in cell-surface haemopexin receptors, as judged by ligand binding at 0 degrees C and affinity labelling of the receptor. This time- and dose-dependent down-regulation was fully reversible within 60-90 min after removal of PMA, and the affinity of the remaining receptors was unaltered by PMA. The specificity of PMA was demonstrated by comparison with the non-tumour-promoter 4 alpha-phorbol, which did not affect any of the parameters examined. The amine H-7, a specific inhibitor of protein kinase C, antagonised the receptor redistribution effect of PMA, suggesting that the down-regulation of haemopexin receptors on the cell surface was a consequence of protein kinase C activation. The PMA-induced decrease in surface haemopexin receptors was due to a 2-fold increase in the rate of internalization (from 0.73 min-1 to 1.32 min-1), whereas the rate of exocytosis (0.6 min-1) was unchanged. PMA treatment, like binding of the natural ligand, haem-haemopexin, results in a lower steady-state level of surface haemopexin receptors independent of receptor synthesis, and the receptors were not degraded but were recycled back to the cell surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Lodish H. F. The asialoglycoprotein receptor internalizes and recycles independently of the transferrin and insulin receptors. Cell. 1983 Jan;32(1):267–275. doi: 10.1016/0092-8674(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Conway T. P., Morgan W. T., Liem H. H., Muller-Eberhard U. Catabolism of photo-oxidized and desialylated hemopexin in the rabbit. J Biol Chem. 1975 Apr 25;250(8):3067–3073. [PubMed] [Google Scholar]
- Davis R. J., Faucher M., Racaniello L. K., Carruthers A., Czech M. P. Insulin-like growth factor I and epidermal growth factor regulate the expression of transferrin receptors at the cell surface by distinct mechanisms. J Biol Chem. 1987 Sep 25;262(27):13126–13134. [PubMed] [Google Scholar]
- Davis R. J., Meisner H. Regulation of transferrin receptor cycling by protein kinase C is independent of receptor phosphorylation at serine 24 in Swiss 3T3 fibroblasts. J Biol Chem. 1987 Nov 25;262(33):16041–16047. [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F., Binns G., Leung J. O. Primary structure of the rat liver asialoglycoprotein receptor. Structural evidence for multiple polypeptide species. J Biol Chem. 1984 Jan 25;259(2):770–778. [PubMed] [Google Scholar]
- Fallon R. J., Schwartz A. L. Regulation by phorbol esters of asialoglycoprotein and transferrin receptor distribution and ligand affinity in a hepatoma cell line. J Biol Chem. 1986 Nov 15;261(32):15081–15089. [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Fate of oxygen free radicals in extracellular fluids. Biochem Soc Trans. 1982 Apr;10(2):72–73. doi: 10.1042/bst0100072. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988 Dec 15;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hrkal Z., Muller-Eberhard U. Partial characterization of the heme-binding serum glycoproteins rabbit and human hemopexin. Biochemistry. 1971 May 11;10(10):1746–1750. doi: 10.1021/bi00786a002. [DOI] [PubMed] [Google Scholar]
- Hunt R. C. Endocytosis and control of expression of transferrin receptor. Dev Comp Immunol. 1986 Spring;10(2):273–277. doi: 10.1016/0145-305x(86)90012-1. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Kawamoto S., Hidaka H. Serotonin secretion from human platelets may be modified by Ca2+-activated, phospholipid-dependent myosin phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14321–14323. [PubMed] [Google Scholar]
- Johnston R. B., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978 Nov;37(13):2759–2764. [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutter D., Caldwell A. B., Morin M. J. Dissociation of protein kinase C activation from phorbol ester-induced maturation of HL-60 leukemia cells. J Biol Chem. 1985 May 25;260(10):5979–5984. [PubMed] [Google Scholar]
- Majuri R., Gräsbeck R. Isolation of the haemopexin-haem receptor from pig liver cells. FEBS Lett. 1986 Apr 7;199(1):80–84. doi: 10.1016/0014-5793(86)81227-3. [DOI] [PubMed] [Google Scholar]
- Marshall S., Green A., Olefsky J. M. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981 Nov 25;256(22):11464–11470. [PubMed] [Google Scholar]
- Marshall S. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J Biol Chem. 1985 Apr 10;260(7):4136–4144. [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Sahyoun N., Jacobs S., Wolf M., Cuatrecasas P. Mechanism of phorbol diester-induced regulation of surface transferrin receptor involves the action of activated protein kinase C and an intact cytoskeleton. J Biol Chem. 1985 Aug 5;260(16):9419–9426. [PubMed] [Google Scholar]
- May W. S., Tyler G. Phosphorylation of the surface transferrin receptor stimulates receptor internalization in HL60 leukemic cells. J Biol Chem. 1987 Dec 5;262(34):16710–16718. [PubMed] [Google Scholar]
- McGraw T. E., Dunn K. W., Maxfield F. R. Phorbol ester treatment increases the exocytic rate of the transferrin receptor recycling pathway independent of serine-24 phosphorylation. J Cell Biol. 1988 Apr;106(4):1061–1066. doi: 10.1083/jcb.106.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rothenberger S., Iacopetta B. J., Kühn L. C. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell. 1987 May 8;49(3):423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., Bement W. J., Gorman N., Liem H. H., Wolkoff A. W., Muller-Eberhard U. Effect of serum proteins on haem uptake and metabolism in primary cultures of liver cells. Biochem J. 1988 Nov 15;256(1):159–165. doi: 10.1042/bj2560159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Hunt R. C. Hemopexin joins transferrin as representative members of a distinct class of receptor-mediated endocytic transport systems. Eur J Cell Biol. 1990 Dec;53(2):234–245. [PubMed] [Google Scholar]
- Smith A. Intracellular distribution of haem after uptake by different receptors. Haem-haemopexin and haem-asialo-haemopexin. Biochem J. 1985 Nov 1;231(3):663–669. doi: 10.1042/bj2310663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Ledford B. E. Expression of the haemopexin-transport system in cultured mouse hepatoma cells. Links between haemopexin and iron metabolism. Biochem J. 1988 Dec 15;256(3):941–950. doi: 10.1042/bj2560941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J. 1979 Jul 15;182(1):47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated heme transport to the liver. Evidence for a heme-binding protein in liver plasma membranes. J Biol Chem. 1985 Jul 15;260(14):8325–8329. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated heme uptake by liver. Characterization of the interaction of heme-hemopexin with isolated rabbit liver plasma membranes. J Biol Chem. 1984 Oct 10;259(19):12049–12053. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated transport of heme into isolated rat hepatocytes. J Biol Chem. 1981 Nov 10;256(21):10902–10909. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Transport of heme by hemopexin to the liver: evidence for receptor-mediated uptake. Biochem Biophys Res Commun. 1978 Sep 14;84(1):151–157. doi: 10.1016/0006-291x(78)90276-0. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Kohno H., Naitoh Y., Tokunaga R. Isolation of the hemopexin receptor from human placenta. J Biol Chem. 1987 Jun 25;262(18):8668–8671. [PubMed] [Google Scholar]
- Taketani S., Kohno H., Tokunaga R. Cell surface receptor for hemopexin in human leukemia HL60 cells. Specific binding, affinity labeling, and fate of heme. J Biol Chem. 1987 Apr 5;262(10):4639–4643. [PubMed] [Google Scholar]
- Taketani S., Kohno H., Tokunaga R. Receptor-mediated heme uptake from hemopexin by human erythroleukemia K562 cells. Biochem Int. 1986 Aug;13(2):307–312. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Primary amines inhibit recycling of alpha 2M receptors in fibroblasts. Cell. 1980 May;20(1):37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- Zerial M., Suomalainen M., Zanetti-Schneider M., Schneider C., Garoff H. Phosphorylation of the human transferrin receptor by protein kinase C is not required for endocytosis and recycling in mouse 3T3 cells. EMBO J. 1987 Sep;6(9):2661–2667. doi: 10.1002/j.1460-2075.1987.tb02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]