Abstract
The first steps in insulin action are binding of insulin to its receptor and activation of the insulin receptor kinase. As there is indirect evidence that further signal transduction might involve a guanine-nucleotide-binding protein (G-protein), we studied whether insulin modulates GTP binding to plasma membrane proteins of fat cells and skeletal muscle. We found that insulin rapidly increased (30 s) binding of guanosine 5'-[gamma-thio]triphosphate (GTP[S]) in a dose dependent manner (0.03-2.0 nM). This effect was not altered by pertussis toxin, but it was abolished by cholera toxin treatment of fat cells. Scatchard analysis of the binding data showed that the increased GTP[S] binding is due to a decrease in the Kd for GTP from 100 nM to 50 nM. Furthermore, binding of GTP to these plasma membranes inhibited both the binding of 125I-insulin to the insulin receptor and the stimulation of the insulin receptor kinase, suggesting a feedback interaction between the insulin-stimulated GTP-binding site and the insulin receptor. In order to identify this insulin-stimulated GTP-binding site, plasma membranes were labelled with the photoreactive GTP analogue [alpha-32P]GTP gamma-azidoanilide. We found that insulin selectively stimulated GTP binding to a 40 kDa protein. In conclusion, in plasma membranes of fat cells and skeletal muscle, the insulin receptor interacts with a 40 kDa GTP-binding site. We speculate that this 40 kDa GTP-binding site might be a G-protein which is involved in insulin signal transmission.
Full text
PDF

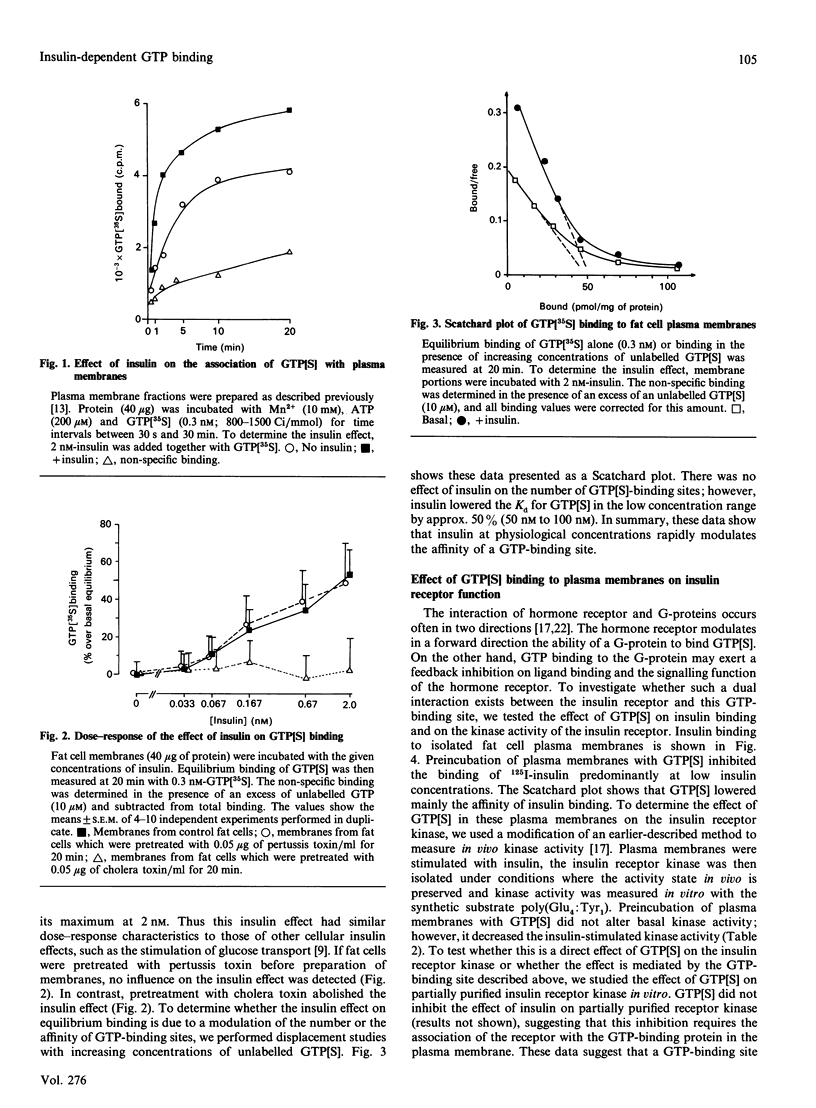
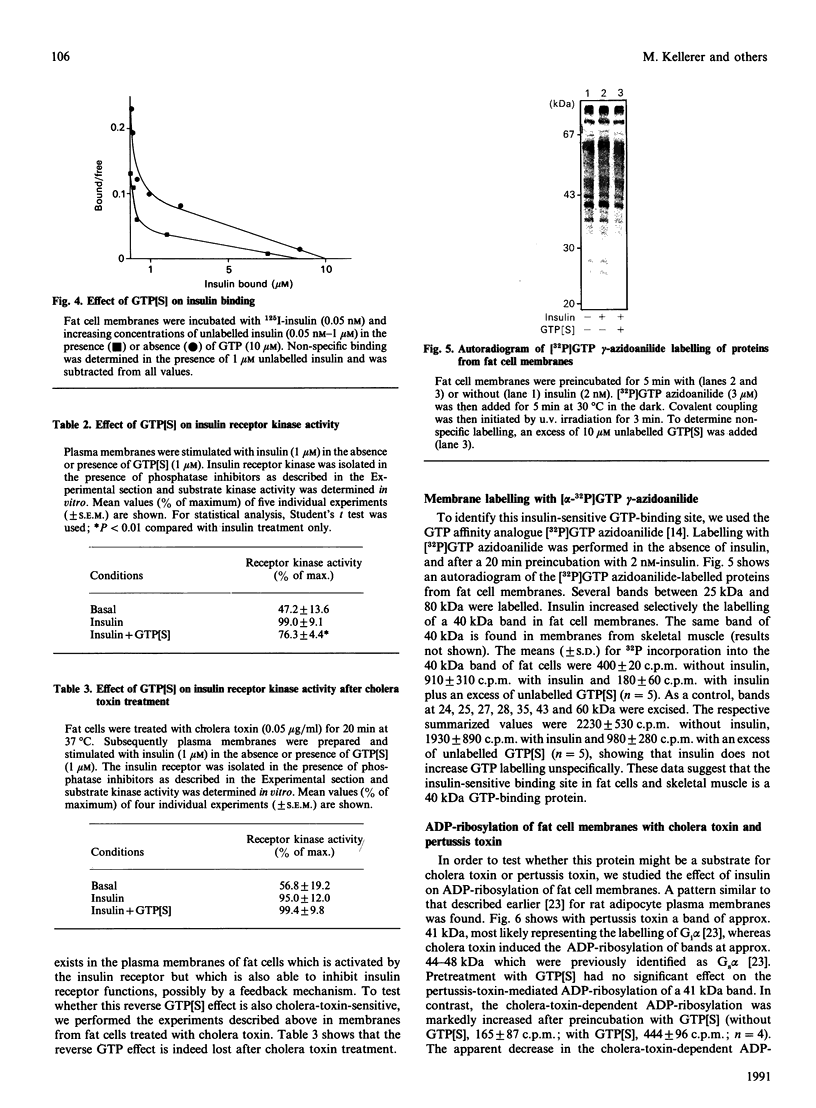


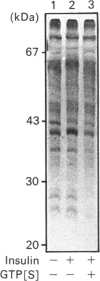
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray P., Carter A., Simons C., Guo V., Puckett C., Kamholz J., Spiegel A., Nirenberg M. Human cDNA clones for four species of G alpha s signal transduction protein. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8893–8897. doi: 10.1073/pnas.83.23.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Evans T., Brown M. L., Fraser E. D., Northup J. K. Purification of the major GTP-binding proteins from human placental membranes. J Biol Chem. 1986 May 25;261(15):7052–7059. [PubMed] [Google Scholar]
- Fox J. A., Soliz N. M., Saltiel A. R. Purification of a phosphatidylinositol-glycan-specific phospholipase C from liver plasma membranes: a possible target of insulin action. Proc Natl Acad Sci U S A. 1987 May;84(9):2663–2667. doi: 10.1073/pnas.84.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Baukal A., Catt K. J. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem. 1974 Jan 25;249(2):664–666. [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Insulin exerts actions through a distinct species of guanine nucleotide regulatory protein: inhibition of adenylate cyclase. Biochem J. 1983 Aug 15;214(2):547–552. doi: 10.1042/bj2140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Wong S., Martin B. R., Houslay M. D. Insulin inhibits the cholera-toxin-catalysed ribosylation of a Mr-25000 protein in rat liver plasma membranes. Biochem J. 1985 Jun 15;228(3):593–603. doi: 10.1042/bj2280593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle P. M., Phillips W. J. Thyrotropin-releasing hormone stimulates GTP hydrolysis by membranes from GH4C1 rat pituitary tumor cells. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6183–6187. doi: 10.1073/pnas.81.19.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsch K. D., Rosenthal W., Spicher K., Binder T., Gausepohl H., Frank R., Schultz G., Joost H. G. Adipocyte plasma membranes contain two Gi subtypes but are devoid of Go. FEBS Lett. 1988 Sep 26;238(1):191–196. doi: 10.1016/0014-5793(88)80254-0. [DOI] [PubMed] [Google Scholar]
- Häring H. U., Kasuga M., Kahn C. R. Insulin receptor phosphorylation in intact adipocytes and in a cell-free system. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1538–1545. doi: 10.1016/s0006-291x(82)80082-x. [DOI] [PubMed] [Google Scholar]
- Häring H. U., White M. F., Machicao F., Ermel B., Schleicher E., Obermaier B. Insulin rapidly stimulates phosphorylation of a 46-kDa membrane protein on tyrosine residues as well as phosphorylation of several soluble proteins in intact fat cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):113–117. doi: 10.1073/pnas.84.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H., Kirsch D., Obermaier B., Ermel B., Machicao F. Tumor-promoting phorbol esters increase the Km of the ATP-binding site of the insulin receptor kinase from rat adipocytes. J Biol Chem. 1986 Mar 15;261(8):3869–3875. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Koepfer-Hobelsberger B., Wieland O. H. Insulin activates phospholipase C in fat cells: similarity with the activation of pyruvate dehydrogenase. Mol Cell Endocrinol. 1984 Jun;36(1-2):123–129. doi: 10.1016/0303-7207(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Rajaram R., Lakonishok M., Benovic J. L., Cerione R. A. Insulin-dependent phosphorylation of GTP-binding proteins in phospholipid vesicles. J Biol Chem. 1988 Sep 5;263(25):12333–12341. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Siddle K., Houslay M. D., Hall A. Interaction of the human insulin receptor with the ras oncogene product p21. FEBS Lett. 1987 Jun 15;217(2):253–259. doi: 10.1016/0014-5793(87)80673-7. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., Mühlbacher C., Mushack J., Rattenhuber E., Fehlmann M., Haring H. U. Regulation of glucose carrier activity by AlCl3 and phospholipase C in fat-cells. Biochem J. 1988 Dec 1;256(2):515–520. doi: 10.1042/bj2560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Kahn C. R. Insulin inhibits pertussis toxin-catalyzed ADP-ribosylation of G-proteins. Evidence for a novel interaction between insulin receptors and G-proteins. J Biol Chem. 1988 Oct 25;263(30):15546–15552. [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Christian A. L., Schulz I. Photoaffinity labeling with GTP-gamma-azidoanilide of a cholera toxin-sensitive 40 kDa protein from pancreatic acinar cells. Biochem Biophys Res Commun. 1988 Sep 15;155(2):1051–1059. doi: 10.1016/s0006-291x(88)80603-x. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Zick Y., Sagi-Eisenberg R., Pines M., Gierschik P., Spiegel A. M. Multisite phosphorylation of the alpha subunit of transducin by the insulin receptor kinase and protein kinase C. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]