Abstract
Growth of quail chondrocytes in the presence of retinoic acid (RA) results in the suppression of the differentiated phenotype. RA-treated chondrocytes recover their differentiated phenotype if they are cultured for an additional 15 days in the absence of RA. A few days after removal from RA, treated chondrocytes acquire the polygonal morphology characteristic of chondrocytes growing as attached cells; they also gradually resume collagen II expression and synthesize cultures. The levels of collagen X mRNA decrease during the second week of culture in the absence of RA. Finally, at the end of 15 days, the absolute levels of collagen II and collagen X mRNAs are very similar in control and recovering chondrocytes.
Full text
PDF
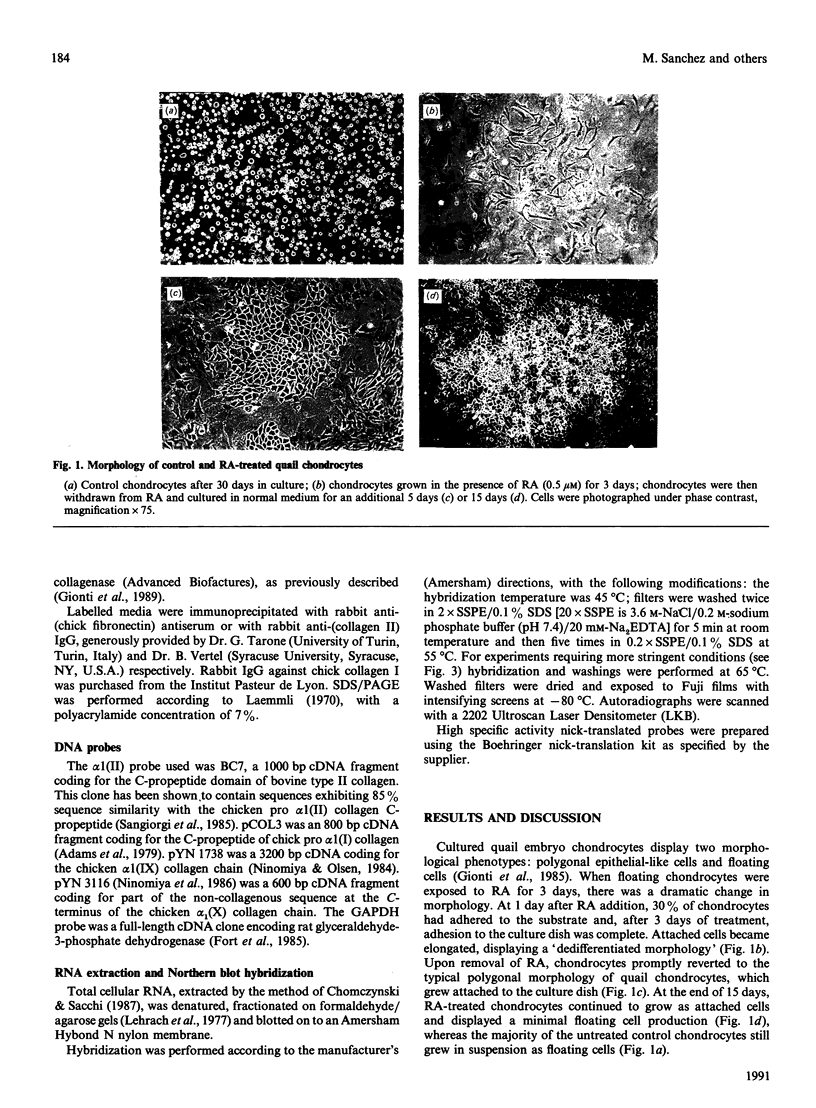
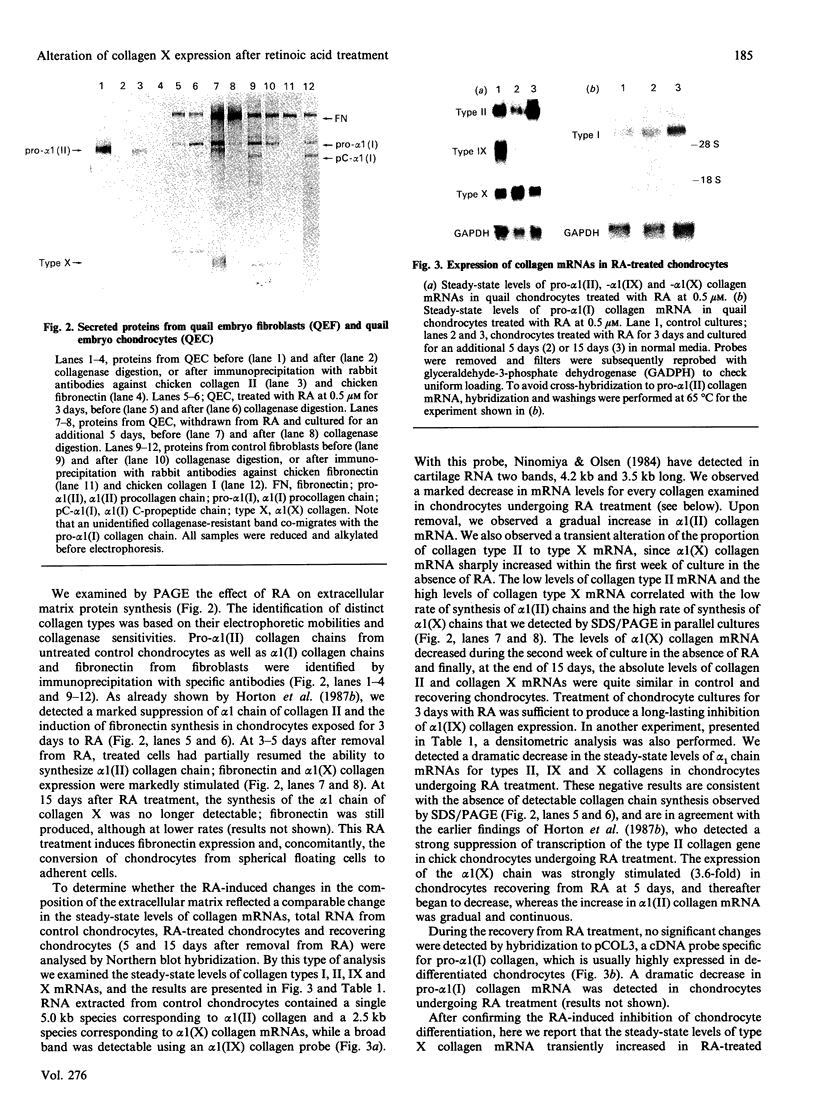


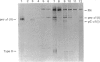
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Brown P. D., Padilla S. R. Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol. 1988 Jan;106(1):161–170. doi: 10.1083/jcb.106.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R. Modulation of the rabbit chondrocyte phenotype by retinoic acid terminates type II collagen synthesis without inducing type I collagen: the modulated phenotype differs from that produced by subculture. Dev Biol. 1986 Nov;118(1):296–305. doi: 10.1016/0012-1606(86)90096-5. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Capasso O., Gionti E., Pontarelli G., Ambesi-Impiombato F. S., Nitsch L., Tajana G., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. I. Characterization of the chondrocyte-specific phenotypes. Exp Cell Res. 1982 Nov;142(1):197–206. doi: 10.1016/0014-4827(82)90423-2. [DOI] [PubMed] [Google Scholar]
- Castagnola P., Torella G., Cancedda R. Type X collagen synthesis by cultured chondrocytes derived from the permanent cartilaginous region of chick embryo sternum. Dev Biol. 1987 Oct;123(2):332–337. doi: 10.1016/0012-1606(87)90391-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Cortese R., Nilsson M., Lundvall J., Båvik C. O., Eriksson U., Peterson P. A., Sundelin J. Cloning and sequencing of a full length cDNA corresponding to human cellular retinol-binding protein. Biochem Biophys Res Commun. 1985 Jul 16;130(1):431–439. doi: 10.1016/0006-291x(85)90435-8. [DOI] [PubMed] [Google Scholar]
- Daniel J. C., Pauli B. U., Kuettner K. E. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. III. Effects of ascorbate. J Cell Biol. 1984 Dec;99(6):1960–1969. doi: 10.1083/jcb.99.6.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Beaumont B. W., Flint M. H. Synthesis of a low molecular weight collagen by chondrocytes from the presumptive calcification region of the embryonic chick sterna: the influence of culture with collagen gels. J Cell Biol. 1984 Jul;99(1 Pt 1):208–216. doi: 10.1083/jcb.99.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Schor S. L., Grant M. E. Effects of matrix macromolecules on chondrocyte gene expression: synthesis of a low molecular weight collagen species by cells cultured within collagen gels. J Cell Biol. 1982 Jun;93(3):767–774. doi: 10.1083/jcb.93.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Gionti E., Capasso O., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. Transformation by rous sarcoma virus induces a switch in the collagen type synthesis and enhances fibronectin expression. J Biol Chem. 1983 Jun 10;258(11):7190–7194. [PubMed] [Google Scholar]
- Gionti E., Jullien P., Pontarelli G., Sanchez M. A continuous line of chicken embryo cells derived from a chondrocyte culture infected with RSV. Cell Differ Dev. 1989 Sep;27(3):215–223. doi: 10.1016/0922-3371(89)90701-6. [DOI] [PubMed] [Google Scholar]
- Gionti E., Pontarelli G., Cancedda R. Avian myelocytomatosis virus immortalizes differentiated quail chondrocytes. Proc Natl Acad Sci U S A. 1985 May;82(9):2756–2760. doi: 10.1073/pnas.82.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton W. E., Yamada Y., Hassell J. R. Retinoic acid rapidly reduces cartilage matrix synthesis by altering gene transcription in chondrocytes. Dev Biol. 1987 Oct;123(2):508–516. doi: 10.1016/0012-1606(87)90409-x. [DOI] [PubMed] [Google Scholar]
- Horton W., Hassell J. R. Independence of cell shape and loss of cartilage matrix production during retinoic acid treatment of cultured chondrocytes. Dev Biol. 1986 Jun;115(2):392–397. doi: 10.1016/0012-1606(86)90258-7. [DOI] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide H., Aono H. Retinoic acid promotes proliferation and chondrogenesis in the distal mesodermal cells of chick limb bud. Dev Biol. 1988 Dec;130(2):767–773. doi: 10.1016/0012-1606(88)90365-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- LuValle P., Hayashi M., Olsen B. R. Transcriptional regulation of type X collagen during chondrocyte maturation. Dev Biol. 1989 Jun;133(2):613–616. doi: 10.1016/0012-1606(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Gordon M., van der Rest M., Schmid T., Linsenmayer T., Olsen B. R. The developmentally regulated type X collagen gene contains a long open reading frame without introns. J Biol Chem. 1986 Apr 15;261(11):5041–5050. [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Yoshimura M., Muto M., Chi J., Roth S., Kaji A. Transformation of chicken chondrocytes by Rous sarcoma virus. Cancer Res. 1977 Mar;37(3):712–717. [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Paulsen D. F., Langille R. M., Dress V., Solursh M. Selective stimulation of in vitro limb-bud chondrogenesis by retinoic acid. Differentiation. 1988 Dec;39(2):123–130. doi: 10.1111/j.1432-0436.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Reginato A. M., Lash J. W., Jimenez S. A. Biosynthetic expression of type X collagen in embryonic chick sternum cartilage during development. J Biol Chem. 1986 Feb 25;261(6):2897–2904. [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Ramirez F. Analysis of cDNA and genomic clones coding for the pro alpha 1 chain of calf type II collagen. Nucleic Acids Res. 1985 Apr 25;13(8):2815–2826. doi: 10.1093/nar/13.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. Developmental acquisition of type X collagen in the embryonic chick tibiotarsus. Dev Biol. 1985 Feb;107(2):373–381. doi: 10.1016/0012-1606(85)90319-7. [DOI] [PubMed] [Google Scholar]
- Shubeita H. E., Sambrook J. F., McCormick A. M. Molecular cloning and analysis of functional cDNA and genomic clones encoding bovine cellular retinoic acid-binding protein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5645–5649. doi: 10.1073/pnas.84.16.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solursh M., Jensen K. L., Singley C. T., Linsenmayer T. F., Reiter R. S. Two distinct regulatory steps in cartilage differentiation. Dev Biol. 1982 Dec;94(2):311–325. doi: 10.1016/0012-1606(82)90350-5. [DOI] [PubMed] [Google Scholar]
- Summerbell D., Maden M. Retinoic acid, a developmental signalling molecule. Trends Neurosci. 1990 Apr;13(4):142–147. doi: 10.1016/0166-2236(90)90006-v. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Takano T., Suzuki F. Restoration by cyclic AMP of the differentiated phenotype of chondrocytes from de-differentiated cells pretreated with retinoids. Mol Cell Biochem. 1982 Feb 19;42(3):145–153. doi: 10.1007/BF00238508. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982 Apr 8;296(5857):564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Reddi A. H. Synthesis and localization of fibronectin during collagenous matrix-mesenchymal cell interaction and differentiation of cartilage and bone in vivo. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2074–2078. doi: 10.1073/pnas.77.4.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui N., Benya P. D., Nimni M. E. Coordinate regulation of type IX and type II collagen synthesis during growth of chick chondrocytes in retinoic acid or 5-bromo-2'-deoxyuridine. J Biol Chem. 1986 Jun 15;261(17):7997–8001. [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]