Abstract
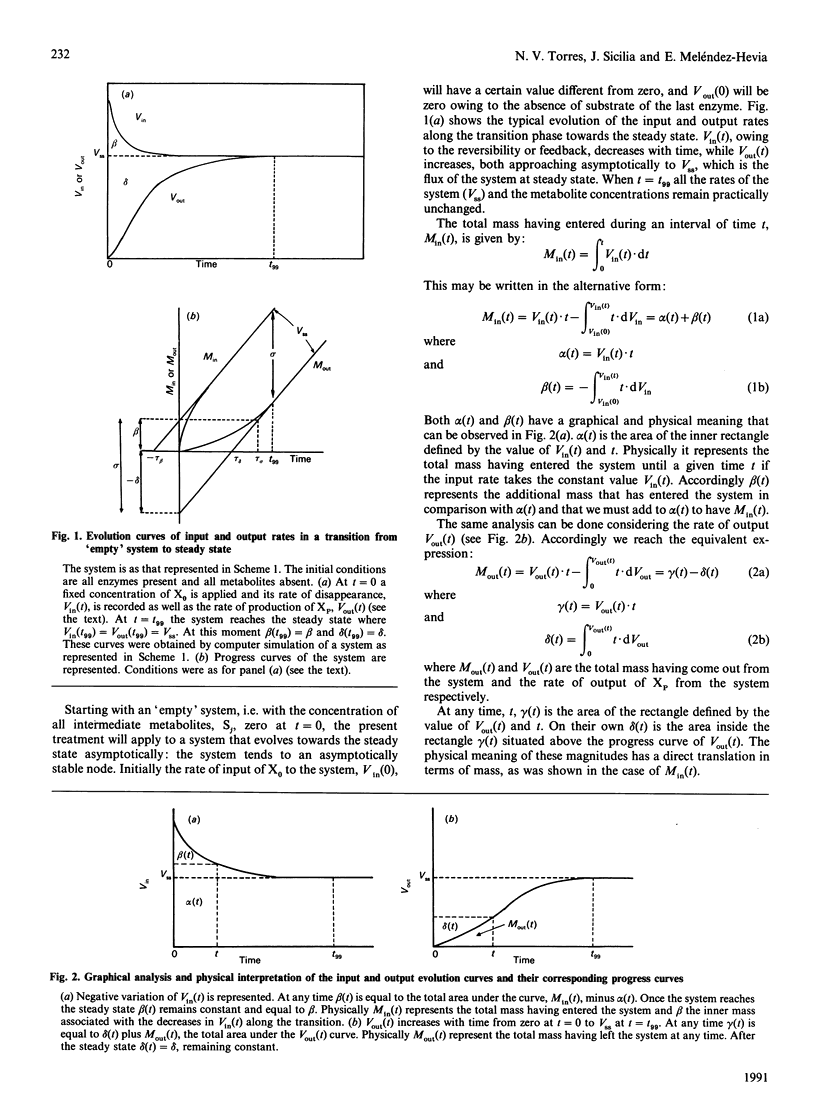
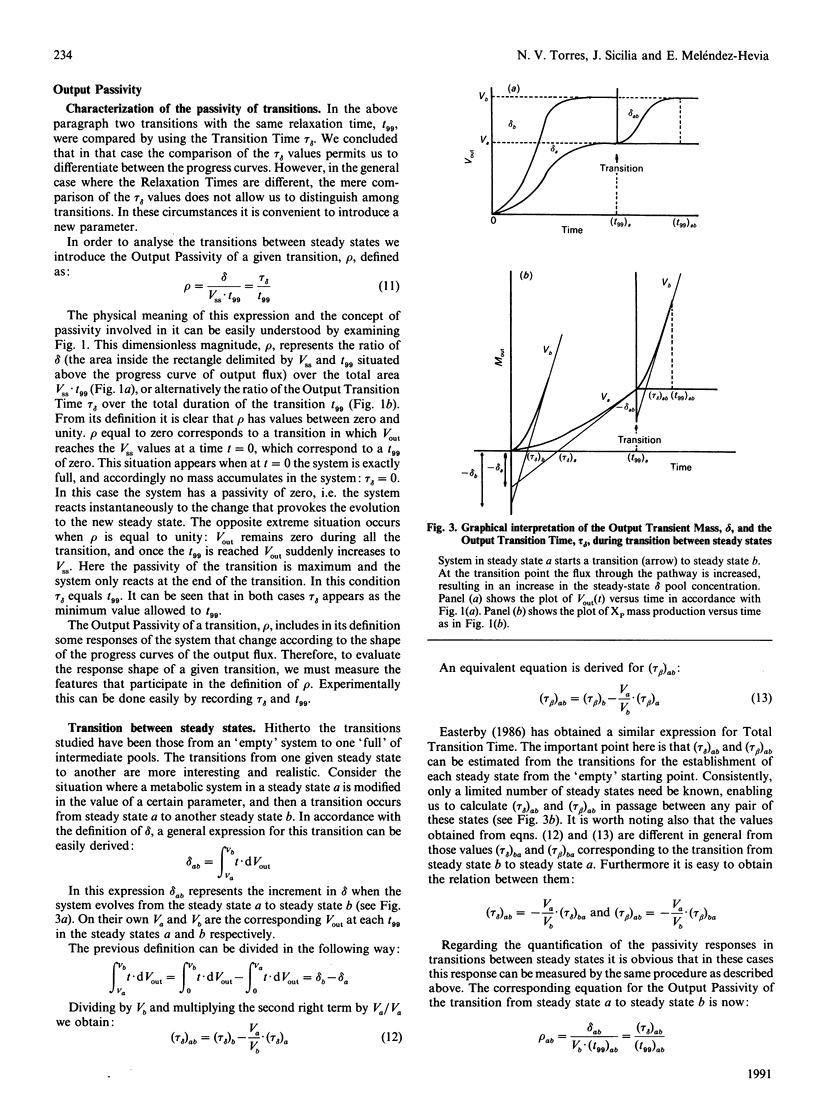
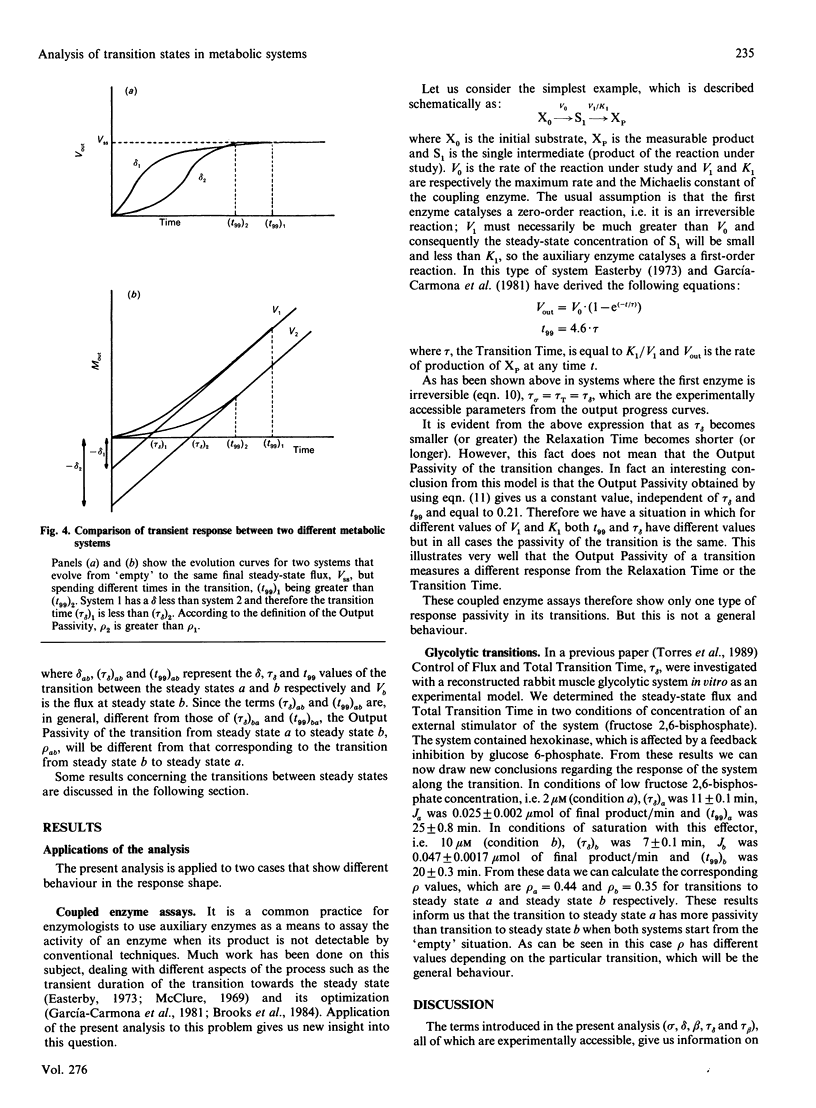
In this paper we study the transitions between steady states in metabolic systems. In order to deal with this task we divided the total metabolite concentration at steady state, sigma, into two new fractions, delta (the Output Transition Time) and tau beta (Input Transition Time), which are related with the course of output and input mass to the system respectively. We show the equivalence time between these terms and the Total Transition Time, tau T, previously defined [Easterby (1986) Biochem. J. 233, 871-875]. Next, we define a new magnitude, the Output Passivity of a transition, rho, which quantifies a new aspect of the transition phase that we call the passivity of the output progress curve. With these magnitudes, all of them being experimentally accessible, several features of the transient state can be measured. We apply the present analysis to (a) the case of coupled enzyme assays, which allows us to reach conclusions about the progress curves in these particular transitions and the equivalence between tau sigma and tau delta, and (b) some experimental results that allow us to discuss the biological significance of the Output Passivity in the transition between steady states in metabolic systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acerenza L., Sauro H. M., Kacser H. Control analysis of time-dependent metabolic systems. J Theor Biol. 1989 Apr 20;137(4):423–444. doi: 10.1016/s0022-5193(89)80038-4. [DOI] [PubMed] [Google Scholar]
- Barwell C. J., Hess B. The transient time of the hexokinase-pyruvate kinase-lacae dehydrogenase system in vitro. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1531–1536. doi: 10.1515/bchm2.1970.351.2.1531. [DOI] [PubMed] [Google Scholar]
- Brooks S. P., Espinola T., Suelter C. H. Theory and practical application of coupled enzyme reactions: one and two auxiliary enzymes. Can J Biochem Cell Biol. 1984 Oct;62(10):945–955. doi: 10.1139/o84-121. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. A generalized theory of the transition time for sequential enzyme reactions. Biochem J. 1981 Oct 1;199(1):155–161. doi: 10.1042/bj1990155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterby J. S. Coupled enzyme assays: a general expression for the transient. Biochim Biophys Acta. 1973 Feb 15;293(2):552–558. doi: 10.1016/0005-2744(73)90362-8. [DOI] [PubMed] [Google Scholar]
- Easterby J. S. The effect of feedback on pathway transient response. Biochem J. 1986 Feb 1;233(3):871–875. doi: 10.1042/bj2330871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterby J. S. The kinetics of consecutive enzyme reactions. The design of coupled assays and the temporal response of pathways. Biochem J. 1984 May 1;219(3):843–847. doi: 10.1042/bj2190843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carmona F., García-Cánovas F., Lozano J. A. Optimizing enzyme assays with one or two coupling enzymes. Anal Biochem. 1981 May 15;113(2):286–291. doi: 10.1016/0003-2697(81)90079-8. [DOI] [PubMed] [Google Scholar]
- Hess B., Wurster B. Transient time of the pyruvate kinase-lactate dehydrogenase system of rabbit muscle in vitro. FEBS Lett. 1970 Jul 29;9(2):73–77. doi: 10.1016/0014-5793(70)80316-7. [DOI] [PubMed] [Google Scholar]
- McClure W. R. A kinetic analysis of coupled enzyme assays. Biochemistry. 1969 Jul;8(7):2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- Meléndez-Hevia E., Torres N. V., Sicilia J., Kacser H. Control analysis of transition times in metabolic systems. Biochem J. 1990 Jan 1;265(1):195–202. doi: 10.1042/bj2650195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. The kinetics of coupled enzyme reactions. Applications to the assay of glucokinase, with glucose 6-phosphate dehydrogenase as coupling enzyme. Biochem J. 1974 Jul;141(1):205–209. doi: 10.1042/bj1410205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres N. V., Souto R., Meléndez-Hevia E. Study of the flux and transition time control coefficient profiles in a metabolic system in vitro and the effect of an external stimulator. Biochem J. 1989 Jun 15;260(3):763–769. doi: 10.1042/bj2600763. [DOI] [PMC free article] [PubMed] [Google Scholar]


