Abstract
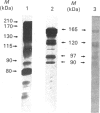
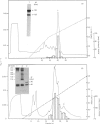
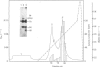
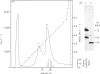
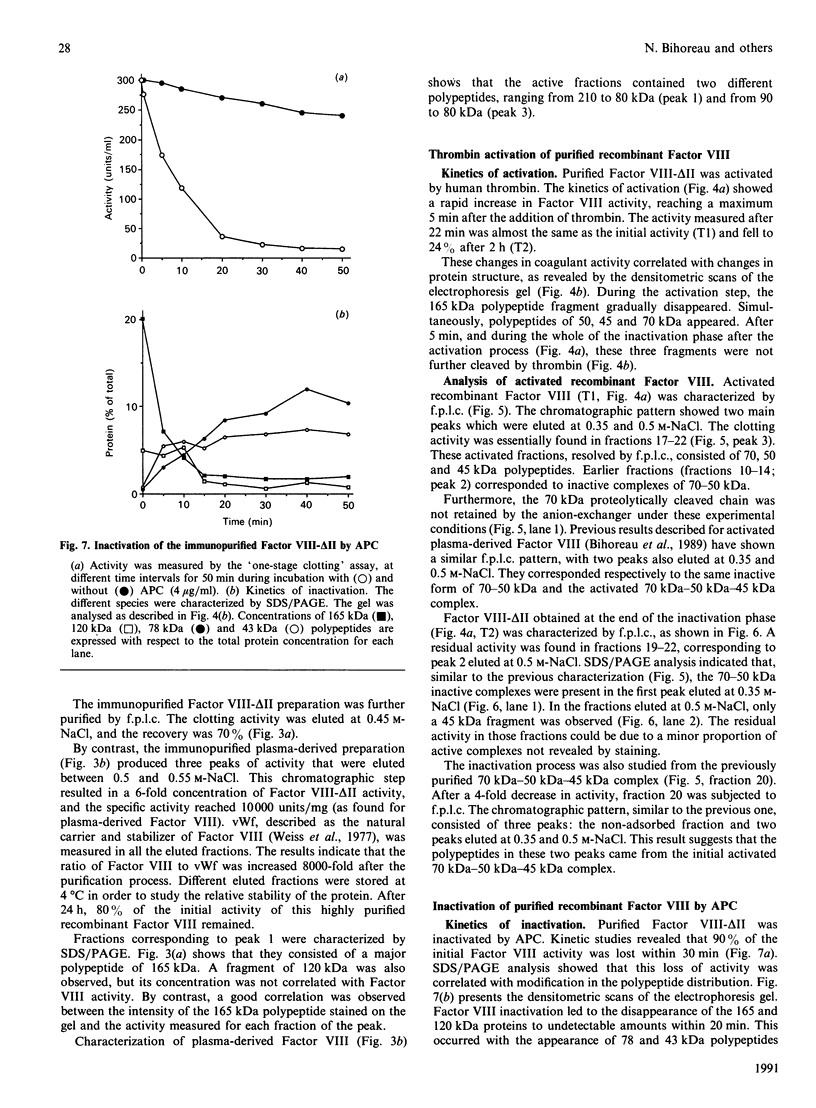
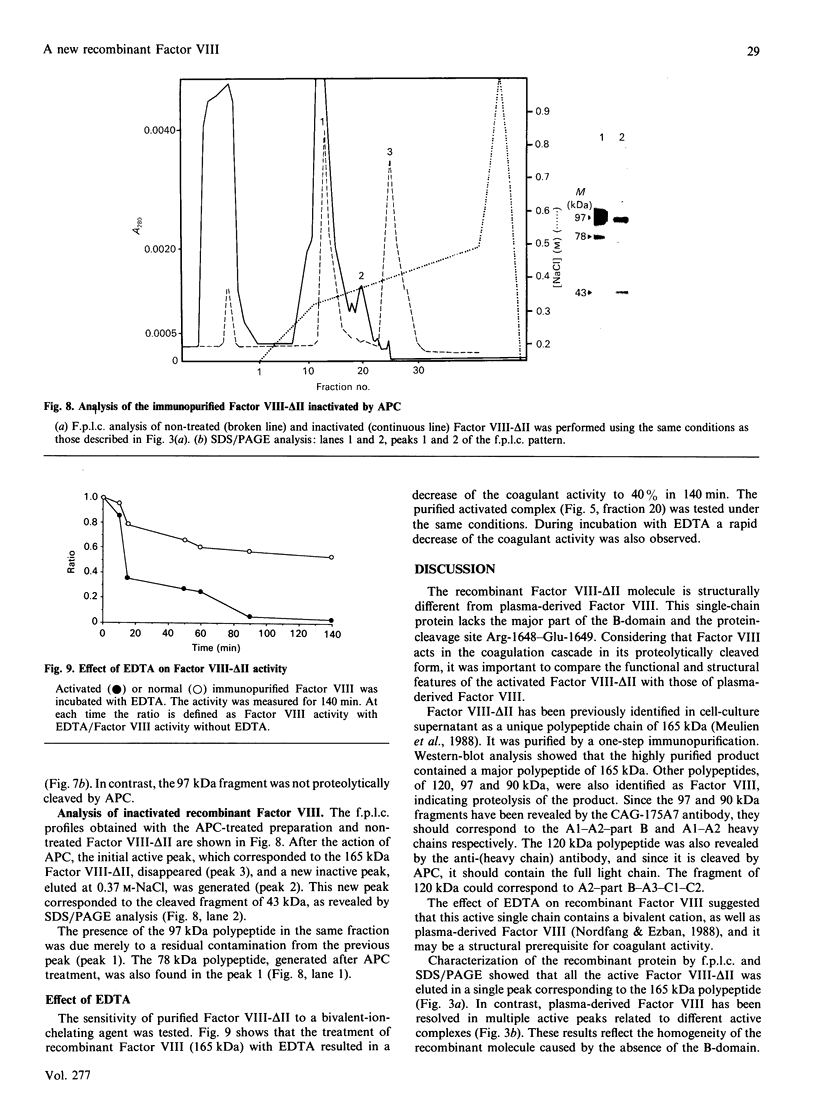
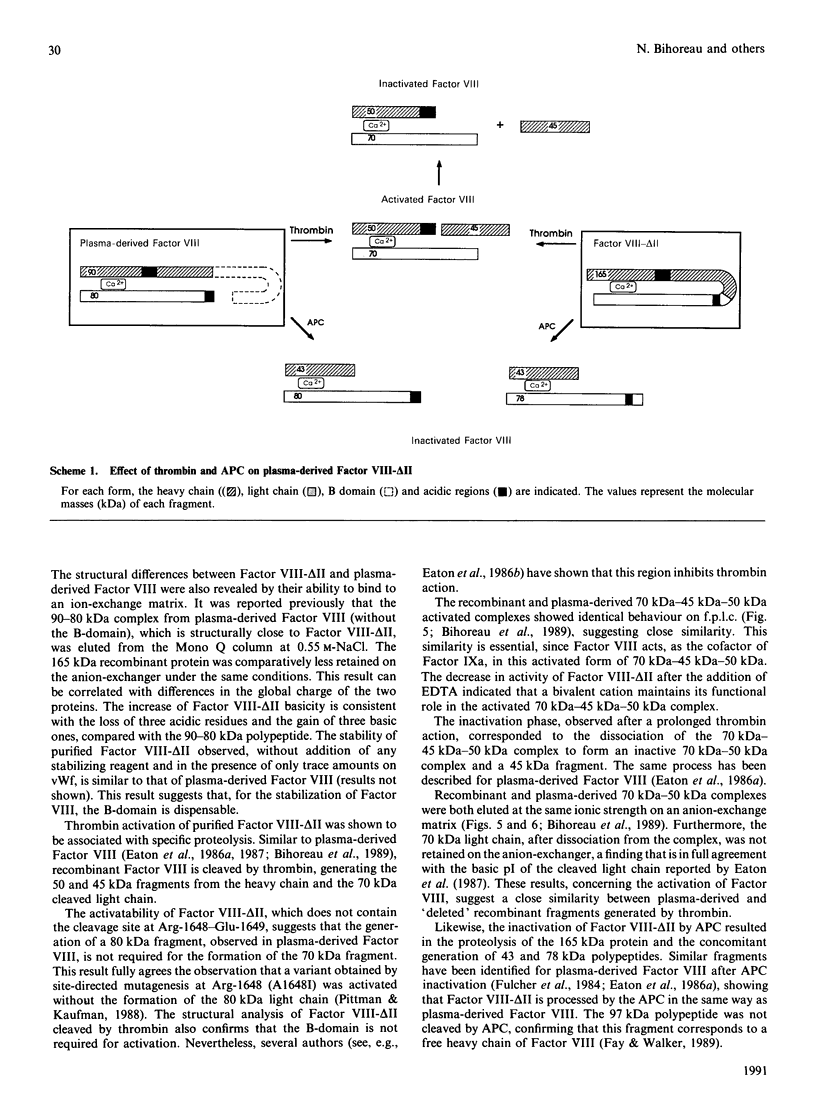
A recombinant Factor VIII (Factor VIII-delta II) consists of a unique polypeptide chain of 165 kDa deleted from the major part of the B-domain and from the cleavage site at Arg-1648-Glu-1649 found in plasma-derived Factor VIII. It was expressed in mammalian cells in serum-free medium containing von Willebrand factor and purified by a one-step immunopurification. The recombinant Factor VIII was characterized as a single active peak when subjected to f.p.l.c., in contrast with the plasma-derived molecule. Its coagulant activity was decreased in the presence of EDTA, suggesting that a bivalent ion is required, as for plasma-derived Factor VIII. The activation by thrombin and the inactivation by activated protein C were studied and the resulting molecular forms were analysed by f.p.l.c. and SDS/PAGE. The results clearly demonstrate that, despite the structural differences between plasma-derived and recombinant Factor VIII, activation and inactivation of Factor VIII-delta II generate proteolysed complexes similar to that described for plasma-derived Factor VIII. Thus this deleted recombinant Factor VIII, which is processed similarly to plasma-derived Factor VIII, should be normally integrated in the regulation system of Factor X activation in the blood-coagulation cascade.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Forsman N., Huang K., Larsen K., Lundin A., Pavlu B., Sandberg H., Sewerin K., Smart J. Isolation and characterization of human factor VIII: molecular forms in commercial factor VIII concentrate, cryoprecipitate, and plasma. Proc Natl Acad Sci U S A. 1986 May;83(9):2979–2983. doi: 10.1073/pnas.83.9.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihoreau N., Sauger A., Yon J. M., Van de Pol H. Isolation and characterization of different activated forms of factor VIII, the human antihemophilic A factor. Eur J Biochem. 1989 Oct 20;185(1):111–118. doi: 10.1111/j.1432-1033.1989.tb15089.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Pachl C., Quiroga M., Rosenberg S., Haigwood N., Nordfang O., Ezban M. The functional domains of coagulation factor VIII:C. J Biol Chem. 1986 Sep 25;261(27):12574–12578. [PubMed] [Google Scholar]
- Cooper H. A., Griggs T. R., Wagner R. H. Factor VIII recombination after dissociation by CaCl12. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2326–2329. doi: 10.1073/pnas.70.8.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croissant M. P., van de Pol H., Lee H. H., Allain J. P. Characterization of four monoclonal antibodies to factor VIII coagulant protein and their use in immunopurification of factor VIII. Thromb Haemost. 1986 Dec 15;56(3):271–276. [PubMed] [Google Scholar]
- Eaton D. L., Hass P. E., Riddle L., Mather J., Wiebe M., Gregory T., Vehar G. A. Characterization of recombinant human factor VIII. J Biol Chem. 1987 Mar 5;262(7):3285–3290. [PubMed] [Google Scholar]
- Eaton D. L., Wood W. I., Eaton D., Hass P. E., Hollingshead P., Wion K., Mather J., Lawn R. M., Vehar G. A., Gorman C. Construction and characterization of an active factor VIII variant lacking the central one-third of the molecule. Biochemistry. 1986 Dec 30;25(26):8343–8347. doi: 10.1021/bi00374a001. [DOI] [PubMed] [Google Scholar]
- Eaton D., Rodriguez H., Vehar G. A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986 Jan 28;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- Fass D. N., Knutson G. J., Katzmann J. A. Monoclonal antibodies to porcine factor VIII coagulant and their use in the isolation of active coagulant protein. Blood. 1982 Mar;59(3):594–600. [PubMed] [Google Scholar]
- Fay P. J., Anderson M. T., Chavin S. I., Marder V. J. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim Biophys Acta. 1986 Jun 23;871(3):268–278. doi: 10.1016/0167-4838(86)90208-6. [DOI] [PubMed] [Google Scholar]
- Fay P. J. Subunit structure of thrombin-activated human factor VIIIa. Biochim Biophys Acta. 1988 Jan 29;952(2):181–190. doi: 10.1016/0167-4838(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Walker F. J. Inactivation of human factor VIII by activated protein C: evidence that the factor VIII light chain contains the activated protein C binding site. Biochim Biophys Acta. 1989 Feb 2;994(2):142–148. doi: 10.1016/0167-4838(89)90153-2. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Gardiner J. E., Griffin J. H., Zimmerman T. S. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984 Feb;63(2):486–489. [PubMed] [Google Scholar]
- Fulcher C. A., Roberts J. R., Zimmerman T. S. Thrombin proteolysis of purified factor viii procoagulant protein: correlation of activation with generation of a specific polypeptide. Blood. 1983 Apr;61(4):807–811. [PubMed] [Google Scholar]
- Hoyer L. W. The factor VIII complex: structure and function. Prog Clin Biol Res. 1981;72:1–26. [PubMed] [Google Scholar]
- Kane W. H., Davie E. W. Blood coagulation factors V and VIII: structural and functional similarities and their relationship to hemorrhagic and thrombotic disorders. Blood. 1988 Mar;71(3):539–555. [PubMed] [Google Scholar]
- Kaufman R. J. Genetic engineering of factor VIII. Nature. 1989 Nov 9;342(6246):207–208. doi: 10.1038/342207a0. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Dorner A. J. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988 May 5;263(13):6352–6362. [PubMed] [Google Scholar]
- LANGDELL R. D., WAGNER R. H., BRINKHOUS K. M. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953 Apr;41(4):637–647. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leyte A., Verbeet M. P., Brodniewicz-Proba T., Van Mourik J. A., Mertens K. The interaction between human blood-coagulation factor VIII and von Willebrand factor. Characterization of a high-affinity binding site on factor VIII. Biochem J. 1989 Feb 1;257(3):679–683. doi: 10.1042/bj2570679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulien P., Faure T., Mischler F., Harrer H., Ulrich P., Bouderbala B., Dott K., Sainte Marie M., Mazurier C., Wiesel M. L. A new recombinant procoagulant protein derived from the cDNA encoding human factor VIII. Protein Eng. 1988 Oct;2(4):301–306. doi: 10.1093/protein/2.4.301. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nordfang O., Ezban M. Generation of active coagulation factor VIII from isolated subunits. J Biol Chem. 1988 Jan 25;263(3):1115–1118. [PubMed] [Google Scholar]
- Pittman D. D., Kaufman R. J. Proteolytic requirements for thrombin activation of anti-hemophilic factor (factor VIII). Proc Natl Acad Sci U S A. 1988 Apr;85(8):2429–2433. doi: 10.1073/pnas.85.8.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblat F., O'Brien D. P., O'Brien F. J., Goodall A. H., Tuddenham E. G. Purification of human factor VIII:C and its characterization by Western blotting using monoclonal antibodies. Biochemistry. 1985 Jul 30;24(16):4294–4300. doi: 10.1021/bi00337a007. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Pittman D. D., Orr E. C., Murtha P., Wasley L. C., Kaufman R. J. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5939–5942. doi: 10.1073/pnas.83.16.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T. H., Duckert F. Dissociation of factor VIII procoagulant antigen VIII:CAg and factor VIII related antigen VIIIR:Ag by EDTA - influence of divalent cation on the binding of VIII:CAg and VIIIR:Ag. Thromb Haemost. 1983 Aug 30;50(2):547–551. [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Sussman I. I., Hoyer L. W. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand's disease. J Clin Invest. 1977 Aug;60(2):390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]