Abstract
Regulatory T cell (Treg) impairment is implicated in the pathogenesis of chronic inflammatory diseases, but relatively little is known about the therapeutic potential of Treg restoration. Here we present clinical evidence for the Treg-selective interleukin-2 receptor agonist rezpegaldesleukin (REZPEG) in two randomized, double-blind, placebo-controlled Phase 1b trials in patients with moderate-to-severe atopic dermatitis (AD) (NCT04081350) or chronic plaque psoriasis (PsO) (NCT04119557). Key inclusion criteria for AD included an Eczema Area and Severity Index (EASI) score ≥ 16 and a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) ≥ 3, and for PsO included a Psoriasis Area and Severity Index (PASI) score of ≥ 12 and a static Physician’s Global Assessment (sPGA) score of ≥ 3. REZPEG is safe and well-tolerated and demonstrates consistent pharmacokinetics in participants receiving subcutaneous doses of 10 to 12 µg/kg or 24 µg/kg once every 2 weeks for 12 weeks, meeting the primary and secondary objectives, respectively. AD patients receiving the higher dose demonstrate an 83% improvement in EASI score after 12 weeks of treatment. EASI improvement of ≥ 75% (EASI-75) and vIGA-AD responses are maintained for 36 weeks after treatment discontinuation in 71% and 80% of week 12 responders, respectively. These exploratory clinical improvements are accompanied by sustained increases in CD25bright Tregs. REZPEG thus represents a homeostatic approach to cutaneous disease therapy and holds clinical potential in providing long-term, treatment-free disease control.
Subject terms: Skin diseases, Randomized controlled trials, Interleukins, Regulatory T cells
Impaired interleukin-2 production and regulatory T cell dysfunction is implicated in multiple autoimmune diseases. Here, in two phase lb trials, the authors show that rezpegaldesleukin, a Treg-selective pegylated IL-2, is safe and improves symptoms in patients with atopic dermatitis or psoriasis.
Introduction
Inflammatory cutaneous conditions represent the largest class of chronic skin diseases and cause major health burdens and deterioration of quality of life in association with various comorbidities1–5. Among the most common T cell-driven inflammatory skin disorders, atopic dermatitis (AD) and psoriasis (PsO) collectively affect up to 10% of adults globally6,7. AD is classically viewed as a predominantly T helper type 2 (Th2)-skewed inflammatory disease, with PsO displaying a Th1/Th17 dominance8. Recently developed biologic therapies successfully target the specific immune pathways dysregulated in each disease: interleukin (IL)−4 or IL-13 in AD9,10 and tumor necrosis factor alpha (TNFα), IL-17 or IL-12/IL-23 antagonists in PsO11–13, with Janus kinase (JAK) inhibitors also approved for both indications14,15. While these immunosuppressive drugs represent undisputed advancements in treatment, challenges remain, including unpredictable or incomplete responses, adverse events, and chronic relapse16.
Regulatory T cells (Tregs) hold a pivotal role in orchestrating immune homeostasis through their ability to modulate the activity of Th1, Th2, and Th17 effector subsets17,18, but are impaired in both PsO and AD19–21. Interleukin-2 (IL-2) plays a role in controlling the proliferation and survival of Tregs22, with low dose IL-2 shown to partially rescue Treg function and provide clinical benefit in autoimmune diseases23–25. However, low-dose IL-2 has limited therapeutic practicality, with shortcomings including a narrow therapeutic window and short half-life, requiring more frequent dosing that could result in conventional T cell (Tcon) induction26. Rezpegaldesleukin (REZPEG) is an IL-2 receptor (IL-2R) pathway agonist designed to stimulate the expansion and function of Tregs. It utilizes the approved recombinant human IL-2 (rhIL-2) aldesleukin sequence with stable, covalently attached polyethylene glycol (PEG) moieties which extends the half-life and confers a selectivity for Treg stimulation over Tcons compared with rhIL-227,28. We have previously shown that REZPEG effectively expands Treg populations in preclinical animal models as well as in healthy volunteers and patients with systemic lupus erythematosus (SLE)27,28. Six-week treatment with REZPEG led to a rapid, dose-dependent improvement in the Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity (CLASI-A) score, encouraging the application of REZPEG to other T cell-driven cutaneous diseases.
Here, hypothesizing that REZPEG may provide benefit in inflammatory skin diseases by therapeutic restoration of the Treg compartment, we apply preclinical experimental models and report the results from two randomized, double-blind, placebo-controlled Phase 1b studies in patients with moderate-to-severe PsO (NCT04119557) or AD (NCT04081350). REZPEG is safe and well tolerated and demonstrates consistent pharmacokinetics in both patient populations. Patients receiving REZPEG in either disease setting demonstrate clinical improvement as assessed by exploratory physician-assessed and patient-reported disease outcomes at the end of treatment at week 12 that are maintained for an additional 36 weeks. REZPEG holds great promise for cutaneous disease therapy through its novel mechanism of Treg restoration.
Results
REZPEG induces antigen-specific immune tolerance in mouse skin
Keyhole limpet hemocyanin (KLH) delayed-type hypersensitivity (DTH) in mice is a classic preclinical model of cell-mediated immunity and was used to establish a basis for REZPEG-mediated activity in inflammatory skin diseases. Pilot pharmacological studies showed that REZPEG doses between 0.003 and 0.3 mg/kg administered subcutaneously (SC) reduced DTH response as measured by ear thickness 24 to 96 hours after KLH challenge in a dose-dependent manner compared to vehicle (Supplementary Fig. 1a).
A follow-on mouse DTH study using sensitization and challenge with multiple antigens was conducted to evaluate the antigen-specific effects of REZPEG during and after administration (Supplementary Fig. 1b). In the first period, mice were sensitized and challenged with KLH in the presence or absence of REZPEG. As established in the pilot studies, ongoing treatment with 0.1 mg/kg REZPEG potently suppressed ear inflammation (Supplementary Fig. 1c). One month later, the same mice were sensitized with a new antigen, Ovalbumin (OVA), without any additional REZPEG treatment and challenged five days later with the new antigen OVA and the original antigen KLH (Supplementary Fig. 1b, Second Period). Mice that were treated with 0.1 mg/kg REZPEG in the primary efficacy period demonstrated reduced DTH ear thickness compared to vehicle when re-challenged with KLH but not with OVA (Supplementary Fig. 1d). The antigen-specific protection against the DTH reaction in REZPEG animals was detectable within 24 hours and was durable through 168 hours after KLH re-challenge, suggesting that REZPEG induces long-lived antigen-specific immune tolerance that lasts well beyond the end of dose administration.
Patient disposition, baseline demographics, and disease characteristics
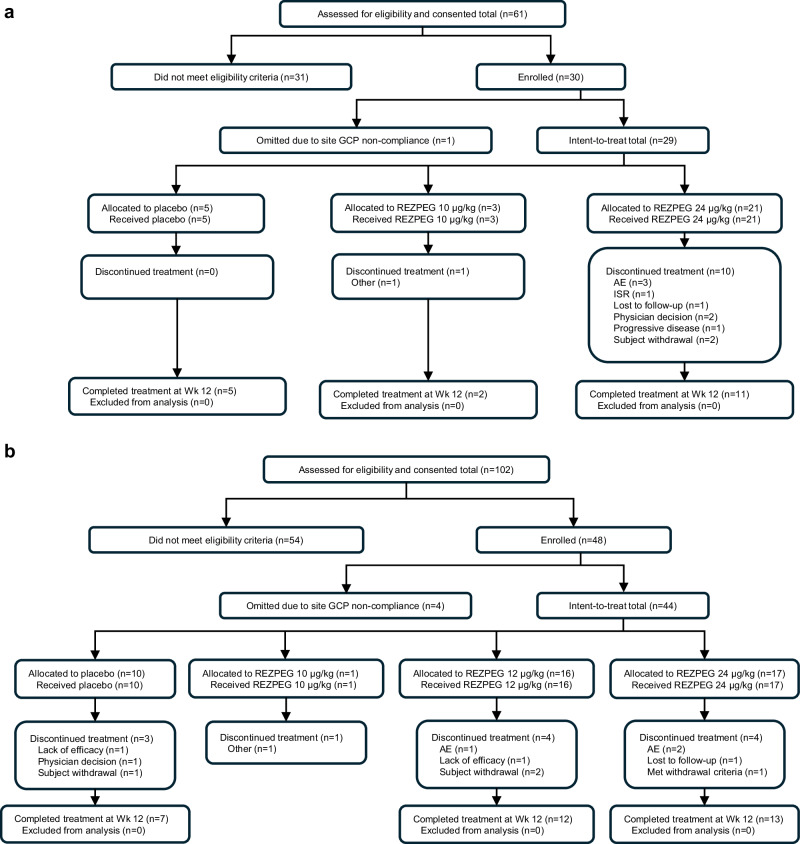
Thirty patients diagnosed with chronic plaque PsO and 48 patients with moderate-to-severe AD were enrolled in two multi-center, double-blind, placebo-controlled phase 1b randomized clinical trials (Fig. 1, CONSORT diagrams, and Supplementary Data 1 and Supplementary Data 2 for protocols). Subcutaneous doses of 10 µg/kg and 24 µg/kg were selected for these studies based on previous phase 1 studies of REZPEG in healthy adult volunteers that demonstrated this dose range to be safe and selectively induce Tregs but not Tcons28. Enrollment was paused due to the COVID-19 pandemic and resumed only at the higher dose level for PsO, limiting analyses due to fewer placebo patients enrolled than originally planned. In the lower dose AD cohort, enrollment resumed at 12 µg/kg. Data from the 10 µg/kg cohorts are included only for the demographic and safety analyses due to the small sample sizes for these groups (n = 3 and n = 1 for PsO and AD, respectively). In each study, baseline demographics were similar across cohorts (Table 1). REZPEG or placebo was administered SC every 2 weeks through week 12, with post-treatment follow-up visits continuing through week 19, and extended visits for week 19 responders continuing through week 48. The study designs and patient completion information for PsO and AD are summarized in Supplementary Figs. 2 and 3, respectively.
Fig. 1. CONSORT diagrams.
a CONSORT diagram for Phase 1b trial of REZPEG in PsO patients. b CONSORT diagram for Phase 1b trial of REZPEG in AD patients. AD atopic dermatitis, AE adverse event, GCP Good Clinical Practice, ISR injection site reaction, PsO psoriasis.
Table 1.
Demographic and clinical characteristics of the patient populations at baseline
| Atopic Dermatitis | Psoriasis | ||||||
|---|---|---|---|---|---|---|---|
| Pooled | 10 μg/kg | 12 μg/kg | 24 μg/kg | Pooled | 10 μg/kg | 24 μg/kg | |
| Placebo | REZPEG | REZPEG | REZPEG | Placebo | REZPEG | REZPEG | |
| n = 10 | n = 1 | n = 16 | n = 17 | n = 5 | n = 3 | n = 21 | |
| Age, mean (SD), years | 42.5 (19.8) | 27.0 (N/A) | 47.9 (17.5) | 37.5 (16.4) | 42.6 (15.1) | 47.0 (11.3) | 47.5 (13.4) |
| Sex, n (%) | |||||||
| Female | 6 (60.0) | 1 (100.0) | 11 (68.8) | 7 (41.2) | 2 (40.0) | 0 | 9 (42.9) |
| Male | 4 (40.0) | 0 | 5 (31.3) | 10 (58.8) | 3 (60.0) | 3 (100.0) | 12 (57.1) |
| Race, n (%) | |||||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) |
| Asian | 1 (10.0) | 0 | 2 (12.5) | 0 | 0 | 0 | 3 (14.3) |
| Black or African American | 3 (30.0) | 0 | 3 (18.8) | 3 (17.6) | 0 | 0 | 2 (9.5) |
| White | 6 (60.0) | 1 (100.0) | 11 (68.8) | 14 (82.4) | 5 (100.0) | 3 (100.0) | 14 (66.7) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) |
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 0 | 0 | 3 (18.8) | 7 (41.2) | 0 | 0 | 2 (9.5) |
| Not Hispanic or Latino | 10 (100.0) | 1 (100.0) | 13 (81.3) | 10 (58.8) | 5 (100.0) | 3 (100.0) | 19 (90.5) |
| Weight, mean (SD), kg | 81.1 (22.5) | 74.8 (N/A) | 81.3 (18.5) | 81.6 (20.6) | 83.6 (12.2) | 89.3 (18.5) | 87.9 (18.6) |
| BMI, mean (SD), kg/m2 | 28.2 (7.5) | 25.0 (N/A) | 29.4 (5.4) | 28.7 (5.5) | 29.8 (4.9) | 26.8 (5.2) | 31.2 (6.2) |
| EASI, mean (SD) | 23.7 (7.1) | 27.1 (N/A) | 23.5 (11.2) | 21.9 (5.1) | --- | --- | --- |
| vIGA-AD, n (%) | |||||||
| Moderate | 5 (50.0) | 1 (100.0) | 9 (56.3) | 11 (64.7) | --- | --- | --- |
| Severe | 5 (50.0) | 0 | 7 (43.8) | 6 (35.3) | --- | --- | --- |
| POEM, mean (SD) | 21.2 (5.7) | 25.0 (N/A) | 20.0 (5.2) | 19.6 (7.0) | --- | --- | --- |
| DLQI, mean (SD) | 13.0 (5.9) | 9.0 (N/A) | 12.4 (6.7) | 11.3 (7.2) | --- | --- | --- |
| BSA, mean (SD), m2 | 39.0 (21.6) | 31.0 (N/A) | 33.8 (20.1) | 33.5 (15.8) | 39.6 (21.0) | 22.0 (5.3) | 27.7 (17.4) |
| Itch NRS, mean (SD) | 8.5 (1.3) | 8.0 (N/A) | 7.8 (2.1) | 7.4 (2.5) | 6.2 (3.0) | 8.3 (1.2) | 7.9 (1.9) |
| PASI, mean (SD) | --- | --- | --- | --- | 27.1 (11.9) | 17.6 (2.9) | 19.2 (6.6) |
| PGA, mean (SD) | --- | --- | --- | --- | 4.2 (1.3) | 3.3 (0.6) | 4.5 (0.9) |
| Target lesion TSS, mean (SD) | --- | --- | --- | --- | 7.6 (1.5) | 6.3 (0.6) | 7.6 (1.1) |
BMI body mass index, BSA body surface area, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, NRS numeric rating scale, PASI Psoriasis Area and Severity Index, PGA Patient's Global Assessment of Disease Severity, POEM Patient-Oriented Eczema Measure, SD standard deviation, sPGA static Physician's Global Assessment, TSS Total Sign Score, vIGA-AD validated Investigator Global Assessment for Atopic Dermatitis.
Primary endpoints addressing safety and tolerability
Treatment-emergent adverse events (TEAEs) were reported by 17 (70.8%) PsO patients and 23 (67.7%) AD patients. All TEAEs were of mild or moderate severity except one involving PsO exacerbation in the 24 µg/kg group (Table 2). There were no serious adverse events (SAEs) related to REZPEG administration in either study. In the AD placebo group, 2 patients experienced SAEs, with cellulitis and tenosynovitis in one of these patients judged to be related to treatment, and a staphylococcal infection in the other patient unrelated to treatment. Both patients recovered, the study treatment (placebo) was not withdrawn, and the participants continued in the study. No deaths occurred in either trial. The incidence of TEAEs was similar between AD groups, reported in 8 (80%) patients receiving placebo, 10 (62.5%) receiving 12 µg/kg REZPEG, and 13 (76.5%) receiving 24 µg/kg REZPEG, and was similar in PsO (14 [66.7%] patients in the 24 µg/kg REZPEG group) (Table 2). Injection site reactions (ISRs) were the most common REZPEG-related findings reported in both trials (Table 2) with erythema being the most common feature that was self-resolving. No clinically meaningful changes in laboratory values, vital signs, or electrocardiogram were reported during either study.
Table 2.
Summary of treatment-emergent adverse events
| Atopic Dermatitis | Psoriasis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pooled | 10 μg/kg | 12 μg/kg | 24 μg/kg | Overall | Pooled | 10 μg/kg | 24 μg/kg | Overall | |
| Placebo | REZPEG | REZPEG | REZPEG | REZPEG | Placebo | REZPEG | REZPEG | REZPEG | |
| Adverse Event Category, n (%) | n = 10 | n = 1 | n = 16 | n = 17 | n = 34 | n = 5 | n = 3 | n = 21 | n = 24 |
| Any TEAE | 8 (80.0) | 0 | 10 (62.5) | 13 (76.5) | 23 (67.6) | 0 | 3 (100) | 14 (66.7) | 17 (70.8) |
| Severe TEAEs | 3 (30.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8) | 1 (4.2) |
| SAEs | 2 (20.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAEs related to treatment | 3 (30.0) | 0 | 2 (12.5) | 5 (29.4) | 7 (20.6) | 0 | 1 (33.3) | 10 (47.6) | 11 (45.8) |
| TEAEs leading to discontinuation of study | 0 | 0 | 1 (6.3) | 3 (17.6) | 4 (11.8) | 0 | 0 | 4 (19.0) | 4 (16.7) |
| All deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse events in at least 5% of patients in the overall REZPEG groupa | |||||||||
| Infections and infestations | 2 (20.0) | 0 | 7 (43.8) | 7 (41.2) | 14 (41.2) | 0 | 1 (33.3) | 6 (28.6) | 7 (29.2) |
| Coronavirus infection | 0 | 0 | 2 (12.5) | 2 (11.8) | 4 (11.8) | N/A | N/A | N/A | N/A |
| Folliculitis | 0 | 0 | 2 (12.5) | 0 | 2 (5.9) | N/A | N/A | N/A | N/A |
| Sinusitis | 0 | 0 | 2 (12.5) | 0 | 2 (5.9) | N/A | N/A | N/A | N/A |
| Upper respiratory tract infection | N/A | N/A | N/A | N/A | N/A | 0 | 1 (33.3) | 1 (4.8) | 2 (8.3) |
| Urinary tract infection | 0 | 0 | 0 | 2 (11.8) | 2 (5.9) | N/A | N/A | N/A | N/A |
| Gastrointestinal disorders | 3 (30.0) | 0 | 1 (6.3) | 3 (17.6) | 4 (11.8) | 0 | 1 (33.3) | 1 (4.8) | 2 (8.3) |
| Nausea | 0 | 0 | 1 (6.3) | 1 (5.9) | 2 (5.9) | N/A | N/A | N/A | N/A |
| General disorders and administration site conditions | 1 (10.0) | 0 | 2 (12.5) | 2 (11.8) | 4 (11.8) | 0 | 0 | 4 (19.0) | 4 (16.7) |
| Pain | 0 | 0 | 1 (6.3) | 1 (5.9) | 2 (5.9) | N/A | N/A | N/A | N/A |
| Pyrexia | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 3 (14.3) | 3 (12.5) |
| Musculoskeletal and connective tissue disorders | N/A | N/A | N/A | N/A | N/A | 0 | 1 (33.3) | 2 (9.5) | 3 (12.5) |
| Arthralgia | N/A | N/A | N/A | N/A | N/A | 0 | 1 (33.3) | 1 (4.8) | 2 (8.3) |
| Investigations | 0 | 0 | 0 | 4 (23.5) | 4 (11.8) | 0 | 0 | 2 (9.5) | 2 (8.3) |
| Eosinophil count increased | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 2 (9.5) | 2 (8.3) |
| Nervous system disorders | 0 | 0 | 2 (12.5) | 2 (11.8) | 4 (11.8) | N/A | N/A | N/A | N/A |
| Headache | 0 | 0 | 2(12.5) | 0 | 2 (5.9) | N/A | N/A | N/A | N/A |
| Blood and lymphatic system disorders | 0 | 0 | 1 (6.3) | 1 (5.9) | 2 (5.9) | 0 | 0 | 2 (9.5) | 2 (8.3) |
| Lymphadenopathy | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 2 (9.5) | 2 (8.3) |
| Eye disorders | 0 | 0 | 2 (12.5) | 0 | 2 (5.9) | N/A | N/A | N/A | N/A |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 1 (6.3) | 1 (5.9) | 2 (5.9) | 0 | 0 | 2 (9.5) | 2 (8.3) |
| Skin and subcutaneous tissue disorders | 1 (10.0) | 0 | 1 (6.3) | 1 (5.9) | 2 (5.9) | 0 | 0 | 3 (14.3) | 3 (12.5) |
| Injection site reactionsb | 1 (10.0) | 1 (100.0) | 12 (75.0) | 10 (58.8) | 23 (67.6) | 0 | 3 (100) | 15 (71.4) | 18 (75.0) |
Adverse events were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 23.0 (AD) or version 22.0 (PsO). For each system organ class and preferred term, patients were included only once. Data are presented from the adjusted safety population. aN/A indicates < 5% of patients in the overall REZPEG group which includes 0. bNumber of patients with at least one injection site occurrence. AD atopic dermatitis, PsO psoriasis SAE serious adverse event, TEAE treatment-emergent adverse event.
Secondary endpoints addressing pharmacokinetics
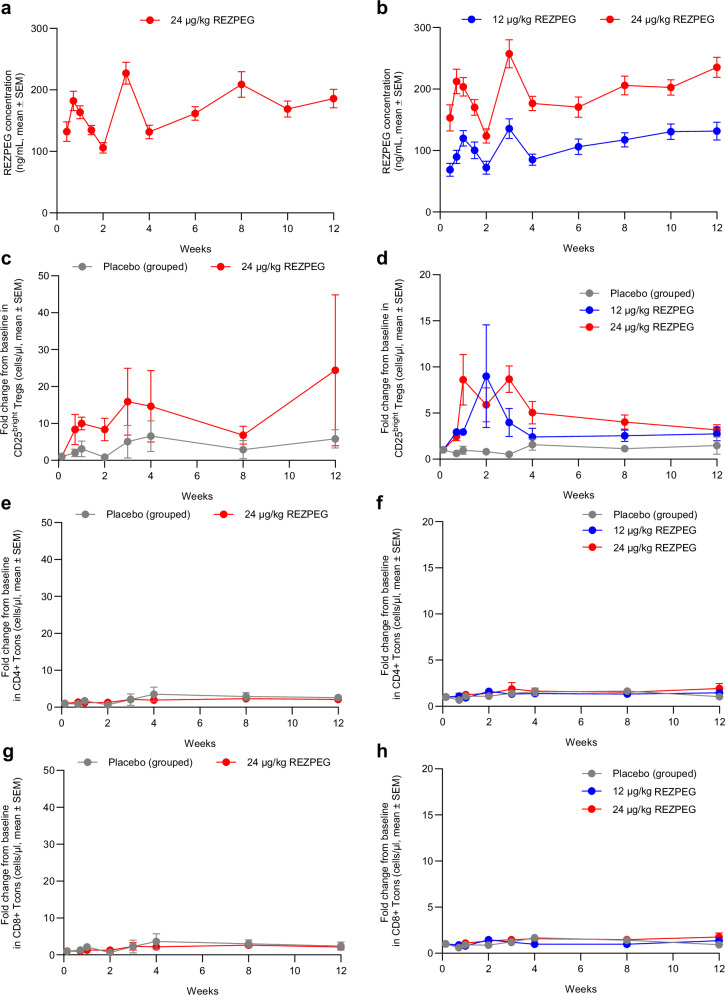
After first administration in either PsO or AD patients, REZPEG was slowly absorbed into the systemic circulation with the median time to reach maximum concentration (Tmax) achieved 5 to 8 days after dosing, followed by a decline in plasma concentrations (Fig. 2a, b). REZPEG exposure increased in an approximately dose-proportional manner (Fig. 2b). The estimated geometric mean maximum concentration (Cmax) and area under the curve from dosing to the time of the last measured concentration (AUClast) estimates were dose-proportional in AD patients and were similar at the 24 µg/kg dose for both AD and PsO patients (Supplementary Table 1).
Fig. 2. REZPEG-induced pharmacokinetics and pharmacodynamics.
REZPEG concentration in a, PsO and b, AD patients, shown as mean ± SEM. CD25bright Tregs measured in c, PsO and d, AD patients. CD4 + T cells in e, PsO and f, AD patients. CD8 + T cells in g, PsO and h, AD patients. All measurements performed using peripheral blood samples from patients treated with placebo (grey circles), 12 μg/kg (blue circles) or 24 μg/kg (red circles) REZPEG administered once every 2 weeks for 12 weeks. Pharmacodynamic data shown as mean fold change from baseline ± SEM. Number of samples at each time point provided in Supplementary Tables 6 (PsO) and 7 (AD). AD atopic dermatitis; PsO psoriasis; SEM standard error of the mean; Tcons conventional T cells; Tregs regulatory T cells. Source data are provided as a Source Data file.
Exploratory endpoints addressing pharmacodynamics
A dose-dependent increase in total and CD25bright Tregs (measured either as absolute number or proportion of CD4 + T cells) was observed in REZPEG-treated cohorts (Fig. 2c, d, Supplementary Fig. 4a-d & Supplementary Fig. 5a-d). During the induction period, REZPEG-treated PsO patients demonstrated a respective maximum 3.1- and 15.9-fold mean change from baseline in the number of total Tregs and CD25bright Tregs with no increases in CD4+ or CD8+ Tcons compared to placebo (Fig. 2e, g, Supplementary Fig. 5e, g). Similarly, REZPEG-treated AD patients demonstrated a maximum 1.9- and 4.0-, and 2.7- and 8.7- mean fold change from baseline in total Tregs and CD25bright Tregs, for the 12 and 24 µg/kg dose levels, respectively, with no increases in CD4+ or CD8+ Tcons compared to placebo (Fig. 2f, h, Supplementary Fig. 5f, h). A gradual increase in the overall numbers of CD56+ natural killer (NK) cells was observed that was dose-dependent in AD (Supplementary Fig. 6a, b). In both PsO and AD patient peripheral blood NK cell subsets, the CD56bright subpopulation of NK cells gradually increased as the CD56dim subpopulation decreased over the 12-week induction period (Supplementary Fig. 6c-f).
Exploratory endpoints addressing PsO efficacy
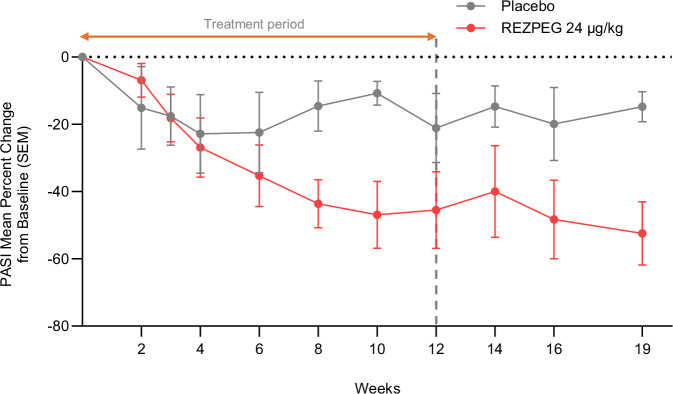
Patients treated with REZPEG demonstrated clinical improvements in the mean percent change from baseline in the psoriasis area and severity index (PASI) score relative to placebo (Fig. 3). At the end of the treatment period at week 12, the REZPEG-treated patients experienced a 44.5% least-squares (LS) mean improvement from baseline in PASI score compared to a 26.2% improvement in the placebo group, with greater improvement indicated by a greater reduction (negative value) in PASI score. Patients in the REZPEG group continued to see benefit during the off-treatment period, with an LS mean 51.4% PASI score improvement from baseline at week 19 compared to 19.9% in the placebo group. A greater LS mean percent change from baseline in the target lesion Total Sign Score and body surface area (BSA) involvement was also observed in PsO patients receiving REZPEG (−51.0% and −29.4%, respectively) compared to placebo (−1.1% and −8.6%, respectively) (Supplementary Table 2). Improvements from baseline in patient-reported outcomes including itch numerical rating scale (NRS) and Patient’s Global Assessment of Disease Severity were numerically greater in the REZPEG group compared to placebo (Supplementary Table 2).
Fig. 3. REZPEG efficacy in PsO patients.
Reduction in PASI scores from baseline in patients with PsO after 12 weeks of 24 μg/kg REZPEG treatment (red circles) compared to placebo (grey circles) administered once every 2 weeks for 12 weeks. Data shown as mean percent change from baseline ± SEM. Number of subjects at each time point shown in Supplementary Table 8. PASI, psoriasis area and severity index; PsO, psoriasis; SEM, standard error of the mean. Source data are provided as a Source Data file.
Exploratory endpoints addressing AD efficacy during the induction period
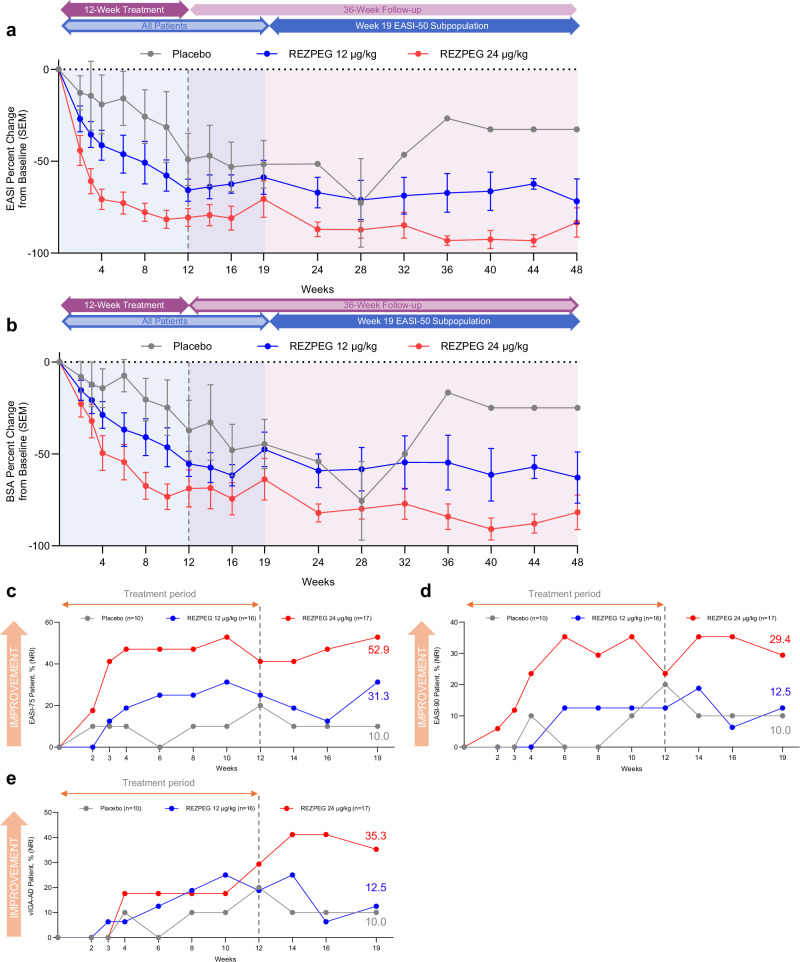
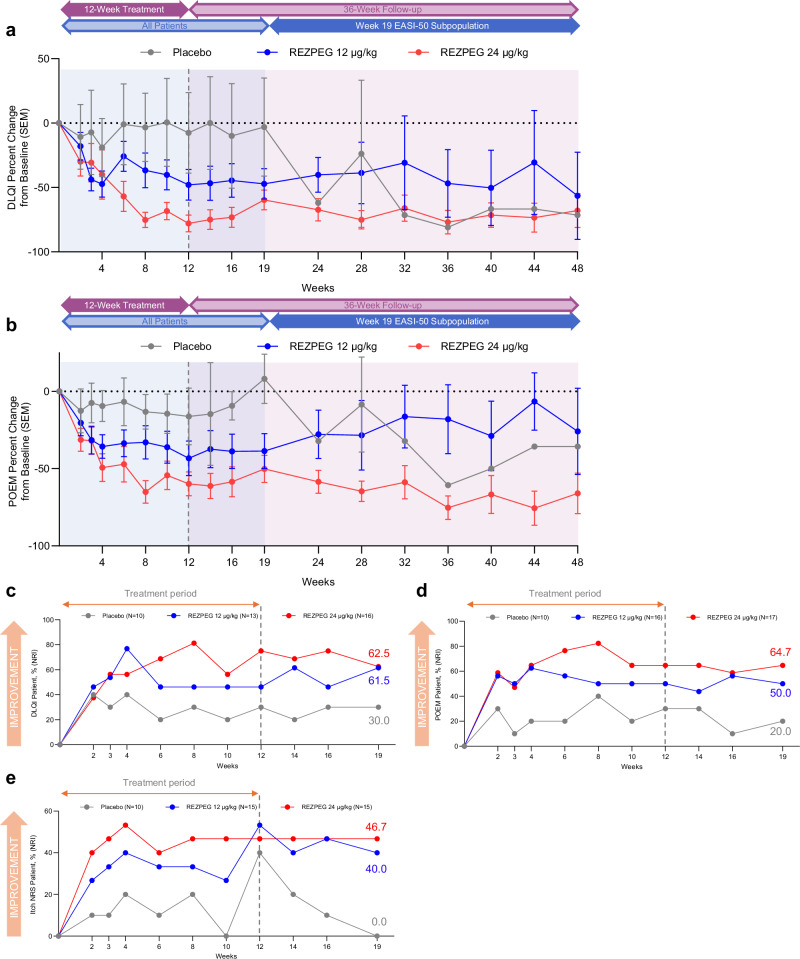
Dose-dependent improvements in measures of overall disease severity and quality of life, as measured by Eczema Area and Severity Index (EASI) score, BSA involvement, Dermatology Life Quality Index (DLQI) and Patient-Oriented Eczema Measure (POEM) scores, were observed as early as 2-4 weeks after treatment initiation (within the first and second dose administrations) and continued through the induction period (Figs. 4a, b, and 6a, b). At week 12, the LS mean percent change in EASI score from baseline was significantly greater for patients in the 24 µg/kg group (-83.0%, p = 0.002) than those receiving placebo (-47.2%) (Table 3, Fig. 4a), with greater improvement indicated by a greater reduction (negative value) in scores. Treatment with 24 µg/kg REZPEG resulted in significant decreases in skin involvement as measured by LS mean percent change from baseline in affected BSA (-73.1%, p = 0.019) versus the placebo group (-36.4%) (Table 3, Fig. 4b). Compared to placebo, a greater proportion of those in the 24 µg/kg group achieved a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 0 or 1 with a 2-point or more improvement from baseline to week 12 (Table 3). REZPEG groups also had a higher proportion of patients reporting a 4-point or more improvement in POEM scores (24 µg/kg, 64.7%; 12 µg/kg, 50.0%) or DLQI scores (24 µg/kg, 75.0%; 12 µg/kg, 46.2%) versus placebo (30.0%) at week 12. Similar 24 µg/kg REZPEG treatment differences compared to placebo were also seen at week 12 in the LS mean percent change from baseline in vIGA-AD (-26.3, p = 0.053), itch NRS (-33.4, p = 0.041), POEM (-35.4, p = 0.057) and DLQI (-65.1, p = 0.008) scores (Table 3).
Fig. 4. AD investigator-assessed efficacy outcomes.
a EASI score mean % change from baseline ± SEM. b BSA score mean % change from baseline ± SEM. c, Proportion of EASI-75 patients whose EASI score decreased by at least 75% relative to baseline. d Proportion of EASI-90 patients whose EASI score decreased by at least 90% relative to baseline. e Proportion of vIGA-AD responders, patients with a post-baseline vIGA-AD score of 0 or 1 and a ≥ 2-point improvement from baseline. Red, REZPEG 24 µg/kg; blue, REZPEG 12 µg/kg; grey, placebo. For continuous endpoints using observed data (a and b), number of subjects at each time point shown in Supplementary Table 9. All responder data shown as % of adjusted ITT populations. BSA, body surface area; EASI, Eczema Area and Severity Index; ITT, intention to treat; NRI, non-responder imputation; SEM, standard error of the mean, vIGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis. Source data are provided as a Source Data file.
Fig. 6. AD patient-reported outcomes.
a DLQI mean % change from baseline ± SEM. b POEM mean % change from baseline ± SEM. c, Proportion with post-baseline DLQI score reduced by ≥ 4 points among patients with baseline score ≥ 4. d Proportion with post-baseline POEM score reduced by ≥ 4 points among patients with baseline score ≥ 4. e Proportion with post-baseline itch NRS scale reduced by ≥ 4 points among patients with baseline score ≥ 4. Red, REZPEG 24 µg/kg; blue, REZPEG 12 µg/kg; grey, placebo. For continuous endpoints using observed data (a and b), number of subjects at each time point shown in Supplementary Table 9. All responder data shown as % of adjusted ITT populations. DLQI, Dermatology Life Quality Index; ITT, intention to treat; NRI, non-responder imputation; NRS, numeric rating scale; POEM Patient-Oriented Eczema Measure; SEM standard error of the mean. Source data are provided as a Source Data file.
Table 3.
AD exploratory efficacy outcomes at week 12
| Pooled | 12 μg/kg | 24 μg/kg | ||
|---|---|---|---|---|
| n | Placebo | REZPEG | REZPEG | |
| EASI % change from baseline, (SEM) | 7, 12, 12 | -47.2 (8.72) | -65.1 (6.57) | −83.0 (6.38) |
| Treatment difference (95% CI) | --- | -17.9 (-40.13, 4.38) | -35.8 (-57.78, -13.76) | |
| p-value | 0.112 | 0.002 | ||
| BSA % change from baseline, (SEM) | 7, 12, 12 | -36.4 (12.0) | -52.8 (8.90) | -73.1 (8.66) |
| Treatment difference (95% CI) | --- | -16.4 (-46.85, 13.98) | -36.7 (-66.84, -6.59) | |
| p-value | 0.279 | 0.019 | ||
| vIGA-AD % change from baseline, (SEM) | 7, 12, 12 | -29.7 (10.6) | -40.9 (7.80) | -56.0 (7.69) |
| Treatment difference (95% CI) | --- | -11.2 (-56.76, -25.02) | -26.3 (-71.63, -40.31) | |
| p-value | 0.400 | 0.053 | ||
| Itch NRS % change from baseline, (SEM) | 7, 12, 12 | -19.2 (12.5) | -52.0 (9.31) | -52.5 (9.25) |
| Treatment difference (95% CI) | --- | -32.8 (-71.06, -32.91) | -33.4 (-71.47, -33.56) | |
| p-value | 0.045 | 0.041 | ||
| POEM % change from baseline, (SEM) | 7, 12, 12 | -15.9 (14.5) | -44.2 (10.8) | -51.3 (10.5) |
| Treatment difference (95% CI) | --- | -28.4 (-65.31, 8.56) | -35.4 (-72.02, 1.17) | |
| p-value | 0.127 | 0.057 | ||
| DLQI % change from baseline, (SEM) | 7, 12, 12 | -10.9 (18.3) | -46.8 (13.4) | -76.1 (13.3) |
| Treatment difference (95% CI) | --- | -35.8 (-82.22, 10.53) | -65.1 (-111.41, -18.84) | |
| p-value | 0.125 | 0.008 | ||
| EASI-50, n (%) | 10, 16, 17 | 3 (30.0) | 11 (68.8) | 12 (70.6) |
| Treatment difference | --- | 38.8 | 40.6 | |
| EASI-75, n (%) | 10, 16, 17 | 2 (20.0) | 4 (25.0) | 7 (41.2) |
| Treatment difference | --- | 5.00 | 21.2 | |
| EASI-90, n (%) | 10, 16, 17 | 2 (20.0) | 2 (12.5) | 4 (23.5) |
| Treatment difference | --- | -7.50 | 3.50 | |
| vIGA-ADa, n (%) | 10, 16, 17 | 2 (20.0) | 3 (18.8) | 5 (29.4) |
| Treatment difference | --- | -1.20 | 9.40 | |
| Itch NRSb, n (%) | 10, 15, 15 | 4 (40.0) | 8 (53.3) | 7 (46.7) |
| Treatment difference | --- | 13.3 | 6.70 | |
| POEMc, n (%) | 10, 16, 17 | 3 (30.0) | 8 (50.0) | 11 (64.7) |
| Treatment difference | --- | 20.0 | 34.7 | |
| DLQId, n (%) | 10, 13, 16 | 3 (30.0) | 6 (46.2) | 12 (75.0) |
| Treatment difference | --- | 16.2 | 45.0 |
A mixed model for repeated measures (MMRM) was used to generate least-squares (LS) means for percent change from baseline in continuous efficacy variables with baseline score as the covariate, treatment arm and protocol-defined visit time and their interaction as the fixed factors. Visit time is also used as repeated measure to account for within-subject variability. Differences and their p-values were derived for treatment vs placebo based on LS means. Missing data were imputed with NRI for categorical endpoints. 10 ug/kg REZPEG group data were not included due to small sample size. BSA; body surface area; CI, confidence interval; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EASI-50, patients with a 50% decrease in EASI score; EASI-75, patients with a 75% decrease in EASI score; EASI-90, patients with a 90% decrease in EASI score; LS, least-squares; MMRM, Mixed Model for Repeated Measures; NRI, non-responder imputation; NRS, numeric rating scale; PBO, placebo; POEM, Patient-Oriented Eczema Measure; SEM, standard error of the mean; vIGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis. aPatients with post-baseline vIGA-AD scores of 0 or 1, with ≥ 2-point improvement from baseline. bProportion with post-baseline itch scale reduced by ≥ 4 points among patients with baseline score ≥ 4. cProportion with post-baseline POEM score reduced by ≥ 4 points among patients with baseline score ≥ 4. dProportion with post-baseline DLQI score reduced by ≥ 4 points among patients with baseline score ≥ 4.
Exploratory endpoints addressing AD post-treatment efficacy outcomes
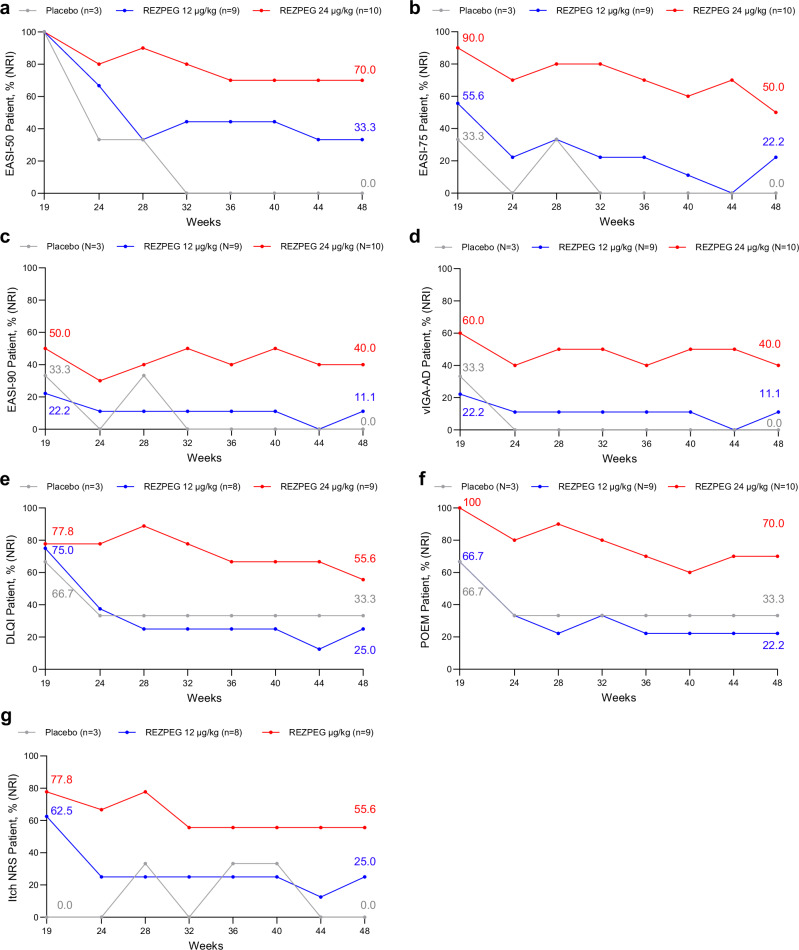
Improvements in disease severity as measured by EASI score and BSA involvement observed at the end of the 12-week induction period were maintained in both REZPEG groups through week 19 (Fig. 4a, b). To evaluate sustained responses beyond the initial follow-up period, patients in all cohorts meeting the established minimal clinically important difference (MCID) of 50% improvement from baseline in EASI score (EASI-50)29 at week 19 were followed through an extended efficacy period from week 19 through week 48 or until EASI-25 response was no longer met. For these long-term responders, disease improvements in EASI score were largely sustained through week 48. Of the week 19 EASI-50 responders, the proportion who maintained their EASI-50 responses through week 48 were 7/10 (70%) and 3/9 (33.3%) for the 24 µg/kg and 12 µg/kg groups, respectively, compared to 0/3 (0%) in the placebo group (Fig. 5a).
Fig. 5. AD off-treatment extended efficacy outcomes through week 48.
Proportion of week 19 EASI-50 responders maintaining a, EASI-50, decrease in EASI score by at least 50% relative to baseline; b, EASI-75, decrease in EASI score by at least 75% relative to baseline; c, EASI-90, decrease in EASI score by at least 90% relative to baseline; d, vIGA-AD score of 0 or 1 and a ≥ 2-point improvement from baseline; e DLQI score reduction by ≥ 4 points among patients with baseline score ≥ 4. f POEM score reduction by ≥ 4 points among patients with baseline score ≥ 4. g itch NRS score reduction by ≥ 4 points among patients with baseline score ≥ 4. Red, REZPEG 24 µg/kg; blue, REZPEG 12 µg/kg; grey, placebo. DLQI, Dermatology Life Quality Index; NRI, non-responder imputation; NRS, numeric rating scale; POEM, Patient-Oriented Eczema Measure; vIGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis. Source data are provided as a Source Data file.
Similarly, EASI-75 response rates were maintained during the off-drug follow-up from weeks 12 to 19, with the proportion of responders in the REZPEG groups (24 µg/kg, 52.9%; 12 µg/kg, 31.3%) remaining higher than in the placebo group (10.0%) at week 19 (Fig. 4c) and sustained through week 48 (Fig. 5b). Week 19 responders achieving a 90% improvement in EASI score (EASI-90) responders were numerically greater in the 24 µg/kg REZPEG group (29.4%) compared to placebo (10.0%) (Fig. 4d), and of the 5/10 (50%) of patients entering the extended follow-up period with EASI-90, 4 patients (80%) maintained their EASI-90 responses at week 48 (Fig. 5c). The proportion of vIGA-AD responders at week 19 was also higher in the 24 µg/kg REZPEG group (35.3%) versus placebo (10.0%, Fig. 4e), with similar rates maintained through week 48 (Fig. 5d). Altogether, across the key investigator-assessed efficacy endpoints in the 24 µg/kg REZPEG group, durable off-treatment responses were observed in EASI-75 (71.4%), EASI-90 (100%), and vIGA-AD (80%) (Supplementary Table 3). In stark contrast, patients in the placebo group experienced higher off-treatment relapse rates in the extended efficacy period compared to REZPEG treatment groups, with the loss of measurable benefit in all efficacy outcomes well before week 48 (Fig. 5).
Improvements observed in patient-reported disease outcomes through the 12-week induction period were also maintained during the initial follow-up period from weeks 12 to 19 and continued through week 48. After discontinuing treatment, DLQI and POEM percent change from baseline were sustained in both REZPEG treatment groups (Fig. 6a, b, respectively). DLQI response rates remained numerically higher in the 24 µg/kg REZPEG group (62.5%) compared to placebo (30.0%) at week 19 (Fig. 6c) and were durable in long-term responders through week 48 (Fig. 5e). The percentage of POEM responders also remained high at week 19 after discontinuing treatment at week 12, with the proportion of patients with a 4-or-more-point reduction in POEM score from baseline greater in the 24 µg/kg REZPEG group (64.7%) compared to placebo (20.0%, Fig. 6d). All patients in the 24 µg/kg REZPEG group entering the long-term follow-up period at week 19 were POEM responders, with responses sustained in 70% of these patients through week 48 (Fig. 5f). REZPEG treatment groups also showed sustained improvements in itch NRS response rates at week 19 (24 µg/kg, 46.7%; 12 µg/kg, 40.0%) compared to placebo (0.0%) (Fig. 6e), with response rates maintained through 48 weeks (Fig. 5g). Notably, for patients in the 24 µg/kg REZPEG group who at week 12 achieved an itch NRS (n = 7), POEM (n = 11), and DLQI (n = 12) response, 71.4%, 63.6%, and 41.7%, respectively, maintained their responses through 48 weeks (Supplementary Table 3).
Serum proteomic biomarkers
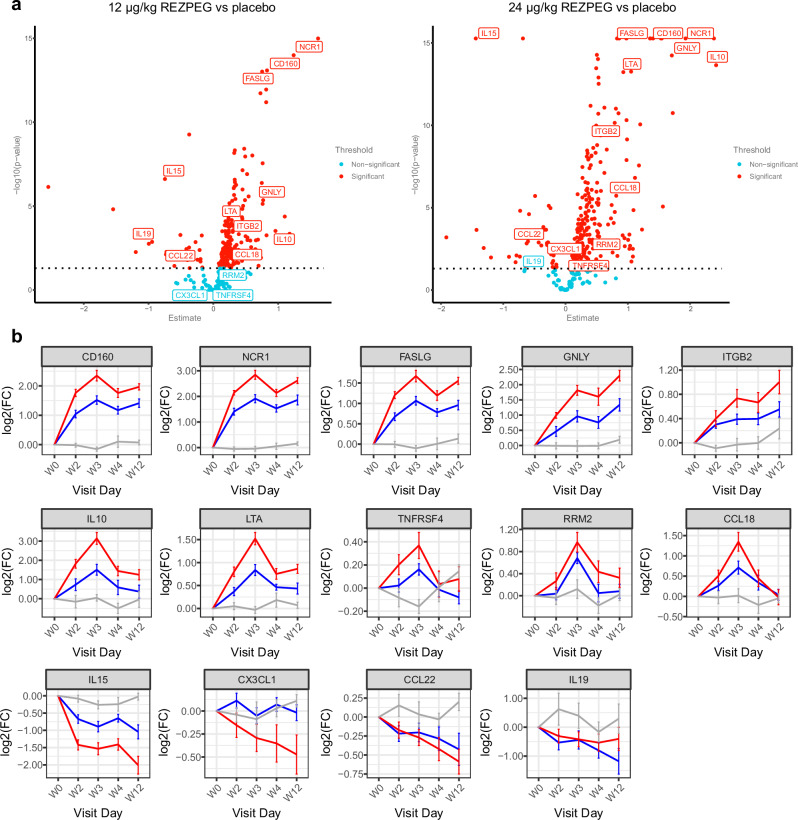
A proteomics analysis was conducted to investigate REZPEG’s biomolecular mechanisms beyond Treg cellular pharmacodynamics. The Olink proteomics platform was used to measure 1461 soluble serum proteins from AD patients treated with REZPEG or placebo at baseline and throughout the induction period on weeks 2, 3, 4, and 12 (Supplementary Table 4). Results were analyzed using a longitudinal linear mixed effects model to identify proteins that were differentially detected in treatment versus placebo groups as a function of REZPEG dose and time on treatment. Volcano plots summarize the 328 proteins that were significantly elevated or decreased in response to REZPEG treatment relative to baseline (Fig. 7a), with a selection of proteins relating to AD and immunoregulatory processes further identified in Fig. 7b. The expression profiles of these biomarkers exhibited dose- and time-dependency of REZPEG administration over the 12-week induction period (Fig. 7b). To further refine their functional associations, REZPEG-modulated proteins were assessed for pathway enrichment using the Reactome knowledgebase30. The top 15 statistically significant pathways induced by REZPEG included immunoregulatory processes (interleukin, TNF superfamily, and chemokine signaling) and cellular migration/adhesion networks (integrins, extracellular matrix organization, and cell surface interactions) (Supplementary Fig. 7, Supplementary Table 5).
Fig. 7. Serum proteomic biomarker analysis.
a Dose-dependent volcano plots of the serum proteins showing the -log10(p-value) vs fold-change in protein expression in response to REZPEG treatment compared to placebo. Data were fitted with a linear mixed model with multiple testing correction using Benjamini-Hochberg. Statistical comparisons were made between treatment and placebo using the Tukey method. Proteins with a statistically significant treatment-based change (threshold p < 0.05) are indicated by red circles; those with non-significant changes are indicated by teal circles. b Example line charts of differentially-detected serum proteins over the 12-week REZPEG treatment induction period shown as Log2 fold change (FC) in expression from baseline ± SEM. Red, REZPEG 24 µg/kg; blue, REZPEG 12 µg/kg; grey, placebo. Number of samples at each time point provided in Supplementary Table 10. CCL, CC motif chemokine ligand; CD160, cluster of differentiation 160; CX3CL1, C-X3-C motif chemokine ligand 1 (fractalkine); FASLG, Fas ligand; GNLY, granulysin; IL, interleukin; ITGB2, integrin beta 2 (CD18); Log2(FC), Log2 fold change; LTA, lymphotoxin alpha; NCR1, natural cytotoxicity triggering receptor 1 (NKp46); RRM2, ribonucleotide reductase regulatory subunit M2; SEM, standard error of the mean; TNFRSF4, tumor necrosis factor receptor superfamily member 4 (CD134, OX40). Source data are provided as a Source Data file.
Discussion
Tregs are key to the maintenance of immunological tolerance and their deficiency has been implicated in the pathogenesis of many chronic inflammatory diseases. Agents able to induce Treg proliferation and activation have only recently been identified as clinically relevant therapies31,32. We have previously shown that the novel IL-2R pathway agonist REZPEG causes a marked and selective dose-dependent increase in CD25bright Tregs accompanied by improvements in CLASI-A score in SLE patients28. Here, we hypothesized that REZPEG could restore immunological homeostasis for the treatment of inflammatory cutaneous pathologies. Our observations in a mouse model of DTH support this rationale, demonstrating the induction of antigen-specific immune tolerance that persists for months after the completion of REZPEG treatment. Furthermore, the results presented here from the Phase 1b trials in PsO and AD patients demonstrate the capacity of REZPEG to safely and dose-dependently increase Tregs and rapidly improve measurable exploratory disease outcomes that are largely durable for at least 36 weeks after ceasing treatment, consistent with the durability of response observed in the mouse DTH studies. To our knowledge, this is the first report of a randomized clinical trial evaluating an engineered Treg-selective rhIL-2 for the treatment of patients with moderate-to-severe PsO or AD.
The safety profile of REZPEG during the observational period reported here is consistent with prior studies of REZPEG28 and other clinical trials for low-dose rhIL-223,24. The overall incidence of TEAEs was similar between treatment arms in both studies. No REZPEG-treated patients reported any SAEs and there were no fatal TEAEs. Importantly, no patients experienced clinical manifestations of cytokine release syndrome or other adverse events seen with higher doses of rhIL-2 aldesleukin28, which poses risks of capillary leak syndrome, inflammatory disease, cardiac disorders, and hematologic toxicity33. In contrast to reports of a greater incidence of conjunctivitis after the use of IL-13 and/or IL-4 inhibitors34–36, conjunctivitis was not observed as a side effect of REZPEG treatment in AD.
REZPEG demonstrated dose-proportional pharmacokinetics and a terminal half-life consistent with previous studies28. REZPEG administration also resulted in a dose-dependent pharmacodynamic profile, with similar increases in CD25bright Tregs observed in PsO and AD, and exceeding those observed in a recent rhIL-2 study in PsO37. CD25bright Tregs are recognized as a Treg subpopulation with high activity and immunosuppressive capacity38. In both AD and PsO, Treg stimulation was sustained through multiple administrations of REZPEG without significant increases in Tcons at either dose level. The increases in Tregs but not Tcons in the current studies in patients with PsO and AD are consistent with prior studies of REZPEG in healthy volunteers and patients with SLE28. These results add to the growing body of evidence supporting REZPEG’s reliable Treg pharmacological profile across healthy and diseased immune systems.
NK cell dysregulation is a functionally relevant feature of inflammatory skin disorders contributing to the disease process, with reduced NK cell numbers and function described in patients with PsO and AD39,40. Long-term treatment with dupilumab was shown to reverse AD-associated NK cell deficiency correlated with clinical response41,42, suggesting a possible therapeutic role for NK cells40. In the current study, REZPEG treatment expanded the number of NK cells in some patients at the higher doses tested, suggesting that REZPEG may also contribute to rebalancing immunity through NK cell restoration. Within the NK cell population, REZPEG decreased the CD56dim subset of NK cells while simultaneously increasing that of CD56bright, the latter shown to have an important immunoregulatory role through the elimination of highly proliferative, activated autoreactive CD4+ Tcons that have escaped Treg suppression43,44.
While Tregs are the primary cellular target of REZPEG, proteomic analysis of serial serum samples performed separately from clinical trial endpoint assessments revealed additional insights into its potential disease-modifying mechanism of action and may also shed light on new target identification strategies for drug discovery. Consistent with its biological activity as an IL-2R agonist and observed therapeutic effects, REZPEG modulated multiple immunoregulatory pathways, including those involving Treg function, immunosuppressive cytokines such as IL-10, ectodomain shedding of immunomodulatory proteins, antigen recognition and major histocompatibility complex (MHC) binding, anti-microbial pathways, cell adhesion and migration, and antagonism of Th2 polarization. We also observed modulation of known targets for AD therapy, including tumor necrosis factor receptor superfamily member 4 (TNFRSF4/OX40 receptor)45 and C-C motif chemokine ligand 22 (CCL-22)46 that binds to the C-C motif chemokine receptor 4 (CCR4)47. In addition, we observed REZPEG-induced reductions in serum proteins known to be elevated in patients with AD, including IL-1548, C-X3-C motif chemokine ligand 1 (CX3CL1/fraktalkine)49, and IL-1950. REZPEG dramatically decreased serum levels of C-X-C motif chemokine ligand 16 (CXCL16), which could contribute to its therapeutic effect in PsO, where elevated levels are known to recruit pathogenic CD8 + T cells to the site of skin lesions51. The ability of REZPEG to directly induce Treg expansion, as well as its dose-dependent reduction of IL-15 supports an interesting hypothesis linking its potential effects on tissue-resident memory T cell populations52,53 to the durability of efficacy observed in the antigen challenge mouse model and in patients with AD or PsO. Work to explore the correlation between Tregs, these serum proteins, and treatment response to REZPEG during the induction period and beyond is ongoing and will be the subject of future reports.
REZPEG demonstrated clinical benefit in both PsO and AD, with responses exhibiting rapid onset especially in AD. Efficacy in PsO was observed but was less robust than that seen for AD patients. This may be due to the small sample size of the PsO trial or may reflect other mechanistic differences between PsO and AD immunology. Efficacy in AD was dose-dependent and demonstrated consistency across physician-assessed and patient-reported outcomes. The magnitude of the clinical effect at the end of the induction period was notable in these patient populations and demonstrated off-treatment durability, most remarkably in AD patients. Patients receiving REZPEG at the higher dose maintained disease control for 36 weeks after discontinuing treatment and without additional systemic therapy. Relapse is a common feature of AD, with many patients requiring long-term treatments for adequate disease control. The durable responses reported suggest the possibility for infrequent maintenance dosing regimens with REZPEG, such as once every 12 weeks, to more completely prevent recrudescence of disease activity54. Durable therapies that provide benefit after treatment discontinuation remain an unmet need in AD. Studies have demonstrated that up to 79% of patients who discontinue dupilumab or JAK inhibitors lose disease control and require rescue treatement55–58.
Supported by the serum biomarker analysis and the observed efficacy of REZPEG in improving CLASI-A in SLE, PASI in PsO, and EASI in AD, a potential mechanism of action is proposed whereby REZPEG administration induces Treg expansion with the ability to engage multiple immunoregulatory mechanisms to facilitate immune homeostasis by attenuating Th1, Th2, and Th17 responses (Supplementary Fig. 8). REZPEG is uniquely poised to address a diversity of immunopathologies through a central pathway of IL-2R-driven Treg rescue. This contrasts with the majority of available pharmacological agents with narrowly targeted biological activity demonstrated in limited disease settings.
Limitations of the studies included the small numbers of patients, which is a challenge inherent to all phase 1 clinical trials. Accordingly, the studies were not powered for analyses of a number of secondary efficacy endpoints or to directly compare different dose regimens. Additionally, with AD suggested to be more prevalent in adolescents than in adults, the demographics of the study population may not be reflective of the general population. In both studies, but especially in PsO, there were also fewer patients after week 19, as the long-term follow-up period through 48 weeks included only those with sustained PASI-50 or EASI-50 responses.
In conclusion, treatment with REZPEG for 12 weeks caused durable dose-dependent improvements in physician-assessed disease activity and patient-reported outcomes in patients with moderate-to-severe PsO and AD. Together, the results presented here validate a role for Tregs in chronic inflammatory skin diseases. IL-2R-induced Treg proliferation and activation holds clinical potential as a key target in the AD treatment paradigm, supporting the advancement of REZPEG into a Phase 2b study in AD (NCT06136741).
Methods
Mouse model of DTH
All animal studies were conducted in compliance with India’s Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, 2005) and per the recommendations of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The study design and methodology for studies LS-2016-924 and LS2017-900A were reviewed and permission was granted to perform the experiments by the Institutional Animal Ethics Committee of Nektar Therapeutics (India) Private Limited (IAEC/NKTI). BALB/c mice aged 6–8 weeks (N = 8 to 10 per group) were obtained through Taconic Biosciences (BALB/cAnNTac, stock number BALB-MPF-F). Female mice were used for these studies due to practicalities in animal husbandry; in all REZPEG nonclinical studies, there were no significant sex-based differences reported in toxicology, toxicokinetics, pharmacokinetics, or pharmacodynamics. Experimental and control animals were co-housed in a specific pathogen-free (SPF) facility and randomized by mean body weight on the day of the experiment. Four animals were group-housed in individually ventilated polysulfone cages. The facility had an automatically controlled light cycle of 12 hours light (0700–1900) and 12 hours dark (1900 to 0700), controlled temperature of 22 ± 3 °C, and relative humidity between 30 and 70%.
The murine keyhole limpet hemocyanin (KLH)-delayed-type hypersensitivity (DTH) model was based on Engstrom et al.59. Mice receiving SC REZPEG (0.003-0.3 mg/kg in the pilot study and 0.1 mg/kg in the subsequent multi-antigen challenge study) or vehicle on days 0, 3, and 6 were sensitized with KLH (100 µg in 100 µL) prepared with Complete Freund’s Adjuvant (CFA) and Incomplete Freund’s Adjuvant (IFA) in a 1:1:1 v/v proportion administered SC between the shoulder blades on day 0. Mice were challenged with KLH (10 µg in 5 µL intradermally [ID] into ear pinna) on day 5 with ear thickness measured by Mitutoyo Thickness gauge immediately prior to challenge and 24, 48, 72, and 96 hours postchallenge. On day 30 in the multi-antigen study, animals that had been previously sensitized/challenged with KLH and treated with 0.1 mg/kg REZPEG were sensitized with Ovalbumin (OVA, 100 µg in 100 µL) prepared with CFA in a 1:1 v/v proportion administered SC between the shoulder blades. No further REZPEG treatment was given. Mice were challenged with OVA (non-specific antigen, 50 µg in 5 µL ID into ear pinna) or re-challenged with KLH (specific antigen, 10 µg in 5 µL ID into opposite ear pinna) with ear thickness measured as above.
PsO and AD study designs
Two multi-center, double-blind, placebo-controlled, phase 1b randomized clinical trials (PsO, NCT04119557; AD, NCT04081350) were designed to evaluate the safety and tolerability of multiple SC doses of REZPEG (NKTR-358, LY3471851) in patients with chronic plaque PsO or moderate-to-severe AD. The PsO study was conducted at 12 study sites within the United States between November 26, 2019 and July 21, 2021, and the AD study was conducted at 20 study sites within the US between November 18, 2019 and June 16, 2022. The studies were conducted in accordance with ethical principles of the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation (ICH) Good Clinical Practice (GCP) Guidelines, and applicable local laws. Approvals for human subjects research and use of human material were obtained from the appropriate Institutional Review Boards (IRBs) at each study center. Protocols J1P-MC-KFAC (PsO) (Supplementary Data 1) and J1P-MC-KFAD (AD) (Supplementary Data 2) and amendments were reviewed and approved by Advarra, serving as the Central Ethics Review Board (PsO) and the IRB (AD), before the studies were initiated/amended. All participants provided written informed consent prior to participating in the trials. Compensation was provided to patients for participation in these studies. Both studies consisted of a 4-week screening period followed by a 12-week intervention period and a 7-week initial post-treatment follow-up period through week 19. Sustained responses were evaluated in PASI-50 (PsO) or EASI-50 (AD) responders during an extended follow-up period from week 19 through week 48 or until PASI-25 or EASI-25 response was no longer met. Overviews of the study designs are provided in Supplementary Fig. 2 (PsO) and Supplementary Fig. 3 (AD). The protocols are included as Supplementary Data 1 and Supplementary Data 2. The list of investigators is provided in the Acknowledgments.
PsO patients
Eligible patients included males and females of ages 18 through 70 with a diagnosis of moderate-to-severe chronic plaque PsO based on an investigator-confirmed diagnosis of chronic PsO vulgaris for at least 6 months prior to baseline. Eligible patients were also required to meet the following criteria: Plaque PsO involving ≥ 10% of body surface area in the affected skin other than the face and scalp at screening and baseline; static Physician’s Global Assessment (sPGA) score of ≥ 3 at screening at baseline; PASI score of ≥ 12 at screening and baseline; candidate for systemic therapy or phototherapy; and at least 2 similar and evaluable lesions that represent overall disease severity, located in 2 different body regions, preferably not exposed to sun, having at least a lesion size of 12 cm2 at baseline, with 1 lesion with a target lesion Total Sign Score (TSS) of ≥ 5, and the second lesion with a target lesion TSS of ≥ 6. The main criteria for exclusion from the study included a clinically significant flare of PsO during the 12 weeks before baseline, history of drug-induced PsO or para-PsO, unstable forms of PsO, and history of any non-PsO disease that required treatment with oral or parenteral corticosteroids for more than 2 weeks within the 24 weeks prior to signing the informed consent form. The full inclusion and exclusion criteria are provided in the PsO study protocol, included as Supplementary Data 1.
AD patients
Eligible patients included males and females of ages 18 through 70 with a diagnosis of moderate-to-severe AD (according to the American Academy of Dermatology Consensus Criteria) for 12 months or more before the screening visit60. Eligibility was confirmed for patients meeting all of the following criteria: an Eczema Area and Severity Index (EASI) score of 16 or higher, a validated Investigator’s Global Assessment scale for AD (vIGA-AD) score of 3 or higher (scale of 0-4), BSA involvement of 10% or greater at baseline, and a history of inadequate response or intolerance to treatment with topical medications. Patients experiencing or having a history of other concomitant skin conditions that would interfere with evaluations of the effect of study drug on AD were excluded. The full inclusion and exclusion criteria are provided in the AD study protocol, included as Supplementary Data 2.
PsO treatment and procedures
Patients were enrolled into 1 of 2 treatment cohorts. At baseline (day 1), eligible patients within each cohort were randomized in a 4:1 ratio to REZPEG or placebo (0.9% sodium chloride). Assignment to treatment groups was determined by a computer-generated random sequence using an interactive web-response system (IWRS). The investigator was blinded to block sizes. Cohort 1 started with a dose of 10 µg/kg every two weeks in Q4 2019 but was paused due to the SARS-CoV-2 pandemic in March 2020. Cohort 2 enrollment was started in June 2020 with a dose of 24 µg/kg every two weeks, and after a review of the data by an independent Assessment Committee, it was decided that enrollment in Cohort 1 would not resume and the study was unblinded. As a consequence, analyses were limited due to fewer placebo patients enrolled than originally planned. Data from the 10 µg/kg cohort are included only for the demographic and safety analyses due to the small sample size (n = 3). These dosages are supported by phase 1 studies (single ascending dose; NCT04133116, and multiple ascending dose; NCT03556007) in healthy volunteers and patients with SLE28. Treatment with concomitant medications for PsO and additional topical treatments was permitted during the study. A summary of study visits is provided in Supplementary Fig. 2a.
AD treatment and procedures
The trial protocol required patients to wash out from topical therapies for at least 2 weeks and/or systemic therapies for at least 6 months prior to baseline and throughout the study. Patients were enrolled into 1 of 2 treatment cohorts. At baseline (day 1), eligible patients within each cohort were randomized in a 4:1 ratio to REZPEG or placebo (0.9% sodium chloride). Assignment to treatment groups was determined by a computer-generated random sequence using IWRS. The investigator was blinded to block sizes. Cohort 1 started with a dose of 10 µg/kg in Q4 2019 but was paused due to the SARS-CoV-2 pandemic in March 2020. Cohort 2 enrollment was started June 2020 with a dose of 24 µg/kg, and after a review of the data by an Independent Assessment Committee, with the investigators and study team remaining blinded, Cohort 1 enrollment was restarted at a dose of 12 µg/kg. Data from the 10 µg/kg cohort are included only for the demographic and safety analyses due to the small sample size (n = 1). These dosages are supported by phase 1 studies (single ascending dose; NCT04133116, and multiple ascending dose; NCT03556007) in healthy volunteers and patients with SLE28. A summary of study visits is provided in Supplementary Fig. 3a.
AD prohibited concomitant medications and procedures
The concomitant use of the following therapies was prohibited during the entire study, with study treatment discontinued in patients requiring these treatments: topical treatments including topical corticosteroids (TCS), topical immune modulators (for example, tacrolimus or pimecrolimus) or PDE-4 inhibitors (for example, crisaborole) except when given as rescue therapy as described below; systemic corticosteroids including oral or parenteral corticosteroids (intramuscular, intra-articular or IV); synthetic (oral) immunomodulators including JAK inhibitors, cyclosporine, methotrexate, mycophenolate mofetil, or azathioprine; immunonomodulating monoclonal antibodies that are biological modifiers (for example, dupilumab, ustekinumab, omalizumab); leukotriene inhibitors, phototherapy, bleach baths, allergen immunotherapy or other investigational therapy.
AD rescue treatments
Rescue therapy with triamcinolone 0.1% cream, hydrocortisone 2.5% ointment, or topical calcineurin inhibitors was permitted for patients at any time after day 21 at the discretion of the investigator. A summary of rescue therapies administered for the indication of AD is included in Supplementary Table 11. No patients in the REZPEG arms required rescue therapy for AD during the induction period. Rescued patients remained included in the efficacy responder analysis.
PsO and AD outcomes
In both studies, the primary objective was to evaluate the safety and tolerability of multiple SC doses of REZPEG. The secondary objective was to characterize the pharmacokinetics (PK) of REZPEG, with additional exploratory objectives assessing injection site reactions and the effects of REZPEG on pharmacodynamics (PD) and measures of physician-assessed and patient-reported disease outcomes.
PsO and AD assessments
Demographics and baseline characteristics, including age, race, ethnicity, weight, height, body mass index, and sex at birth were summarized descriptively. Safety and tolerability were assessed by monitoring adverse events (AEs), treatment-emergent AEs (TEAEs), serious AEs, vital signs, physical examination, electrocardiograms, serum chemistry, hematology, and urinalysis evaluations. AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.0 (PsO) or 23.0 (AD). TEAEs were defined as AEs occurring on or after receiving the first dose of study drug.
PsO exploratory efficacy assessments
Physician-reported efficacy outcomes included the Psoriasis Area and Severity Index (PASI), which scores the severity of disease on a scale from 0 to 72 (where a score of 72 indicates extreme disease severity) by combining assessments of the extent of body surface involvement in the head, trunk, arms, and legs together with the severity of desquamation, erythema, and lesion induration/infiltration (thickness) in each region; the target lesion Total Sign Score (TSS)61, the sum of erythema, scaling, and lesion elevation scores, each on a 4-point scale (0 to 3, with 0 indicating a grade of clear and 3 indicating a grade of severe to very severe); and the static Physician’s Global Assessment (sPGA), with overall lesions graded for induration, erythema, and scaling (range 0 to 4), defined as the sum of 3 scores divided by 3 and rounded to the nearest whole number for analysis of responder rates. An sPGA (0,1) response was defined as a post-baseline sPGA score of 0 or 1. Patient-reported outcomes and quality-of-life measures included the itch numerical rating scale (NRS), an 11-point, horizontal scale anchored at 0 and 10, with 0 representing no itch and 10 representing worst itch imaginable to describe the worst level of itching in the past 24 hours62,63, and the Patient’s Global Assessment of Disease Severity (PGA) ranking the severity of PsO on a 0 (clear; no PsO) to 5 (severe; worst their PsO has ever been) NRS. Per protocol, efficacy data, including PASI were to be collected in follow-up visits during the study after patients had stopped treatment. No follow-up efficacy data were collected for patients who discontinued treatment as they discontinued the study at the same time.
AD exploratory efficacy assessments
Physician-reported efficacy outcomes included the Eczema Area and Severity Index (EASI) to assess the extent of disease at 4 body regions (head and neck, trunk, upper and lower extremities) and measuring 4 clinical signs with a maximum score of 7264; the percentage BSA involvement of AD on a scale of 0% (no involvement) to 100% (full involvement)65; and the validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) based on a numeric scale from 0 (clear skin) to 4 (severe disease). Clinical endpoints based on EASI included the percentage reduction in EASI score by at least 50% (EASI-50), 75% (EASI-75), or 90% (EASI-90) relative to baseline. The efficacy endpoint based on vIGA-AD 0/1 identified the percentage of patients reaching the status of 0 (clear) or 1 (almost clear) and a minimum 2-grade improvement66. Patient-reported efficacy outcomes/quality-of-life (QoL) measures included the Dermatology Life Quality Index (DLQI), with scores ranging from 0 to 30, with higher scores indicating greater impairment of QoL, and a 4-point change from baseline considered to be the minimal clinically important difference threshold67,68; the Patient-Oriented Eczema Measure (POEM), with scores ranging from 0 to 28 with higher total scores indicating greater disease severity69; and the itch NRS, with a score of 0 representing no itch and 10 representing worst itch imaginable within the past 24 hours62,63. The key exploratory disease activity assessments were performed on day 85 (week 12), prior to the final dose of the study drug. Injection site assessments were performed at the end of each visit through week 14. Injection site reactions were scored using the injection site assessment and pain visual analog scale (VAS) tools. Per protocol, efficacy data were to be collected in follow-up visits during the study after patients stopped treatment. Efficacy data for two additional visits were collected for 2 patients (1 patient each in the 12 µg/kg and 24 µg/kg REZPEG groups) who discontinued treatment early and are included in the analyses presented.
Pharmacokinetics and pharmacodynamics
REZPEG was measured in human plasma samples with a validated sandwich enzyme-linked immunosorbent assay (ELISA). Capture of REZPEG was achieved by coating with custom product-specific (Eli Lilly) and commercial (MabTech) mouse anti-human IL-2 antibodies. Detection was achieved by using a biotin-conjugated anti-PEG antibody (Abcam) as secondary, followed with streptavidin-conjugated horseradish peroxidase and color produced with 3,3’,5,5’-tetramethylbenzidine (TMB). The lower limit of quantitation (LLOQ) was 1.0 ng/mL. Accuracy and precision were ± 25% over the quantitative range and ± 30% at the LLOQ. PK parameters were calculated by standard noncompartmental analysis methods. PK data were analyzed with Phoenix WinNonlin Version 8.1.
For flow cytometry of peripheral whole blood samples, fluorescent antibodies against CD45, CD3, CD4, CD8, CD25, FoxP3, CD56, and Ki67 were obtained from BD Biosciences (San Jose, CA). Absolute cell counts were determined by inclusion of AccuCheck Counting Beads from ThermoFisher (Waltham, MA). Flow cytometry samples were analyzed using a FACSCanto II (Becton Dickinson) and instrument settings were set with machine software in conjunction with calibration beads. Data were acquired using BD FACSDiva software (San Jose, CA) and processed using De Novo FCS Express Flow Clinical Edition (Pasadena, CA). After gating on lymphocytes, positive populations were identified based on fluorescence minus one control. Total Tregs were defined as CD45 + CD3 + CD4 + CD25+FoxP3 + ; CD25bright Tregs were defined as CD45 + CD3 + CD4 + CD25 + +FoxP3 + ; CD4 + T cells as CD45 + CD3 + CD4 + ; CD8 + T cells as CD45 + CD3 + CD8 + ; and total NK cells as CD45 + CD3-CD56 + . NK cells were further phenotyped from the CD3-CD56+ population as CD56bright and CD56dim NK cells and defined as a percentage of total NK cells. Immunophenotyping results were enumerated as absolute cell counts (cells/μL) or percent relative (%) values for each phenotype. To ensure comparability across longitudinal samples, the baseline total Treg gate was set to approximately 5% (4–6%) of CD4 + T cells for each individual, and this gate position was maintained across the individual’s timepoints. For CD25bright Tregs, the baseline gate was set at approximately 0.5% (0.4–0.6%) of CD4 + T cells for each individual. An example of the gating strategy for the CD25+ Treg, Tcons, and NK cell populations are shown in Supplementary Fig. 9. Antibodies used for pharmacokinetic and pharmacodynamic assessments are provided in Supplementary Table 12.
AD biomarker analyses
In addition to clinical trial objectives, four Olink Explore panels were used for proteomic analyses: Explore 384 Cardiometabolic, Explore 384 Inflammation, Explore 384 Neurology, and Explore 384 Oncology. Analyses were performed using R version 4.3.1. Olink data was analyzed using the OlinkAnalyze R package v3.5.1. Olink protein levels are expressed in Normalized Protein eXpression (NPX) units, which are expressed on a log2 scale, with a 1 NPX difference translating into a doubling of protein concentration. Change from baseline was calculated by subtracting the baseline NPX from the NPX at each time point, resulting in the log2 fold change from baseline (log2FC). Non-normal measures were transformed to log2 scale when appropriate. The log2FC from baseline for each post-baseline time point was derived and used as the dependent variable in the model fitting. For each outcome, linear mixed-effects models (LMMs) were fit separately to each protein using the lmerTest70 v3.1-3 package in R. Log2FC was used as the dependent variable. The main fixed factor in the LMMs was REZPEG treatment cohort arm (i.e. REZPEG dose level or placebo, defined as ‘arm’ in the model). Age, gender, ethnicity, and race were the covariates empirically included in the model. Visit was incorporated as a random factor to account for the variability in the averages of log2FC at different visits for days 15, 22, 29, and 85. Significance of the model terms was determined with an F-test using Satterthwaite degrees of freedom and type III sum of squares implemented with the lmerTest package in R. P-values for the model terms of interest were adjusted across the 1461 proteins within each model term using the Benjamini-Hochberg (BH) method. 347 proteins had the model term ‘arm’ showing statistical significance with the BH adjusted p values < 0.05. All pairs of comparisons under the model term ‘arm’ were performed separately for each of the 347 proteins and the adjusted p values based on the Tukey method were calculated within each protein by the emmeans71 v1.8.7 package in R. After adjustment, 329 proteins were significant between either treatment group and placebo. These Tukey-adjusted p values were presented for the Y-axis in the volcano plots. The ggplot2 v3.4.2 package in R was used to generate the two types of graphs. Pathway enrichment analysis was performed using the Reactome knowledgebase30.
Statistical analysis
The sample size for this trial was empirically selected based on the typical numbers for Phase 1 studies to evaluate safety, pharmacokinetics, and PD, and was not powered on the basis of statistical hypothesis testing. No adjustment for Type I error over the multiple efficacy outcomes was performed. Data from placebo groups were pooled across cohorts. All randomized patients were included in the intention-to-treat (ITT) population and those who received at least one dose of REZPEG or placebo were included in the safety analysis adjusted to exclude 1 patient for the PsO study and 4 patients for the AD study due to GCP noncompliance at one study site. Those who also had adequate data were also included in PD analyses. Quantitative displays were summarized using descriptive statistics. Continuous clinical endpoints were additionally analyzed using mixed effect models for repeated measures with baseline score as the covariate, treatment arm and protocol-defined visit time and their interaction as the fixed factors. Visit time was used as the repeated measure to account for within-patient variability. Differences of treatment vs placebo and corresponding confidence intervals were derived based on least-squares means. P value for testing the null hypothesis of no difference between treatment and placebo group was based on the t-test using SAS version 9.4 PROC MIXED procedure. For binary/categorical clinical data, frequency counts and percentages were provided. Missing outcomes due to reasons including early treatment discontinuation were imputed with non-responder imputation (NRI) for categorical endpoints. The statistical analysis plans for both studies are included as Supplementary Data 3 and Supplementary Data 4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This research was sponsored by Eli Lilly and Company and Nektar Therapeutics. ClinicalTrials.gov identifiers NCT04119557 and NCT04081350. The authors thank the patients for their participation in these studies. Medical writing/editorial assistance was provided by Jaime S. Horton of CD Chunn & Associates and was funded by Nektar Therapeutics according to the Good Publication Practice guideline. PsO List of Investigators Seth Forman, Lawrence Parish, Stacy Smith, Cindy Owen, Stephen Schleicher, Scott Fretzin, Jarad Levin, George Murakawa, Jamie Weisman, Timothy Rodgers, Neal Bhatia, and Michelle Chambers. AD List of Investigators Stephen Schleicher, Timothy Rodgers, Leon Kircik, Lawrence Parish, Mark Lee, Lauren Campbell, Seth Forman, Scott Fretzin, Jill Waibel, Stacy Smith, Jarad Levin George Murakawa, Neal Bhatia, Michelle Chambers, Bartley Gill, and Paul Yamauchi.
Author contributions
C.F., C.S., A.N., B.K., and J.Z. contributed to the study concept and design. S.S. and C.S. acquired data. C.F., J.L., D.Y., and J.Z. contributed to the biomarker analysis. Y.L. and D.Y. conducted the statistical analyses on the data. J.S., D.R., R.C., T.B., L.B., M.G., S.C., T.M., and M.T. provided additional interpretation of the clinical data and critical feedback on the manuscript. All authors approved the final manuscript for submission and are accountable for the accuracy and integrity of the manuscript.
Peer review
Peer review information
Nature Communications thanks Brian Kim and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data are provided in the Source Data file. Source data are provided with this paper.
Competing interests
JS has received honoraria as a consultant and/or advisory board member for Abbvie, Aldena, Amgen, AObiome, Apollo, Arcutis, Arena, Asana, Aslan, Attovia, BioMX, Biosion, Bodewell, Boehringer-Ingelheim, Bristol Meyers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, Corevitas, Dermavant, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Invea, Kiniksa, Leo Pharma, My-Or Diagnostics, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi-Genzyme, Shaperon, TARGET-RWE, Union, UpToDate; speaker for Abbvie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, Sanofi-Genzyme; stock/stock options for Connect, Verdant; institution received grants from Galderma, Incyte, Pfizer DR has consulted, spoken for, or conducted trials for the following companies: AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer-Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Nektar, Novartis, Pfizer, RAPT, Regeneron, Recludix, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, VielaBio, Zura Bio. RC has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Amgen, AnaptysBio, Apogee Therapeutics, Arcutis, Argenx, ASLAN Pharmaceuticals, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, Eli Lilly and Company, FIDE, Formation Bio, Galderma, Genentech, GSK, Incyte, LEO Pharma, L’Oréal, Nektar Therapeutics, Novartis, Opsidio, Pfizer Inc., RAPT, Regeneron, Sanofi, Sitryx, Takeda, TRexBio, and UCB. TB has been a speaker and/or consultant and/or Investigator for AbbVie, Affibody, Almirall, Amagma, AnaptysBio, AOBiom, Apogee, Arena, Aristea, Artax, Asana Biosciences, ASLAN pharma, Astria, Attovia, Bayer Health, Biofilm control, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Daichi-Sanyko, Dermavant, DICE Therapeutics, Domain Therapeutics, DS Pharma, EQRx, Galderma, Galapagos, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, L´Oréal, MSD, Medac, Micreos, Nektar, Novartis, Numab, OM-Pharma, Overtone, Pfizer, Pierre Fabre, Q32bio, RAPT, Samsung Bioepis, Sanofi/Regeneron, TIRmed, UCB, Union Therapeutics, UPStream Bio, YUHAN. He is founder and chairman of the board of the non-profit biotech Davos Biosciences within the international Kühne-Foundation. SS has received research funding from Eli Lilly and Company, Amgen, Galderma, HighLight Therapeutics, Encube, Cara Therapeutics, Vyne, Novan, AstraZeneca. Honoria for Nektar. Consulting/Advisory Role for Nektar. LB has been a consultant and/or investigator for Abbvie, Allakos, Amgen, Arcutis, Arena Pharmaceuticals, AstraZeneca, Astria Therapeutics, Evelo Biosciences, Escient Pharma, Galderma, Incyte, Invea Therapeutics, Janssen, LEO Pharma, Merck, Nektar Therapeutics, Novartis, Numab Therapeutics, Pfizer, Rapt Therapeutics, Regeneron Pharmaceuticals Inc., Ribon Therapeutics, Sanofi-Aventis/Genzyme, Sitryx Therapeutics, Stealth BioTherapeutics, Trevi Therapeutics, UCB Pharma, Union therapeutics, and Xencor. MG has been an investigator, speaker and/or advisor for: AbbVie, Acelyrin, Amgen, Akros, Arcutis, Aristea, AnaptysBio, Apogee, Bausch Health, BMS, Boehringer Ingelheim, Cara, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, InMagene, JAMP Pharma, Janssen, Kyowa Kirin, LEO Pharma, L’Oreal, MedImmune, Meiji, Moonlake, Nektar, Nimbus, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Tarsus, Takeda, UCB, Union, Ventyx and Vyne. SC, CF, DY, YL, BK, TM, MT, and JZ are employees and stockholders at Nektar Therapeutics. MT also has a leadership role and is a shareholder in Enzo Biochem and RayzeBio. CS and AN are shareholders at Eli Lilly and Company. AN is a shareholder at Recludix Pharma. JL is a stockholder in Eli Lilly and Company.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53384-1.
References
- 1.Liu, Y. et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling. Sci. Immunol.7, eabl9165 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalgard, F. J. et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Invest. Dermatol.135, 984–991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langenbruch, A. et al. Quality of health care of atopic eczema in Germany: results of the national health care study AtopicHealth. J. Eur. Acad. Dermatol. Venereol.28, 719–726 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Adamson, A. S. The Economics Burden of Atopic Dermatitis. Adv. Exp. Med. Biol.1027, 79–92 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Finlay, A. Y. The burden of skin disease: quality of life, economic aspects and social issues. Clin. Med. (Lond.)9, 592–594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W. Y. et al. Annoying Psoriasis and Atopic Dermatitis: A Narrative Review. Int. J. Mol. Sci.23, 4898 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roediger, B. & Schlapbach, C. T cells in the skin: Lymphoma and inflammatory skin disease. J. Allergy Clin. Immunol.149, 1172–1184 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Carretero, M. et al. Differential Features between Chronic Skin Inflammatory Diseases Revealed in Skin-Humanized Psoriasis and Atopic Dermatitis Mouse Models. J. Inv. Dermatol.136, 136–145 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Paller, A. S. et al. Efficacy and Safety of Tralokinumab in Adolescents With Moderate to Severe Atopic Dermatitis: The Phase 3 ECZTRA 6 Randomized Clinical Trial. JAMA Dermatol159, 596–605 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson, E. L. et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med.375, 2335–2348 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero, A., Picone, V., Martora, F., Fabbrocini, G. & Megna, M. Guselkumab, Risankizumab, and Tildrakizumab in the Management of Psoriasis: A Review of the Real-World Evidence. Clin. Cosmet. Investig. Dermatol.15, 1649–1658 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simopoulou, T., Tsiogkas, S. G., Zafiriou, E. & Bogdanos, D. P. Secukinumab, ixekizumab, bimekizumab and brodalumab for psoriasis and psoriatic arthritis. Drugs Today (Barc.)59, 135–167 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Chima, M. & Lebwohl, M. TNF inhibitors for psoriasis. Semin. Cutan. Med. Surg.37, 134–142 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Ferreira, S., Guttman-Yassky, E. & Torres, T. Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: Focus on Upadacitinib and Abrocitinib. Am. J. Clin. Dermatol.21, 783–798 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Megna, M. et al. JAK Inhibitors in Psoriatic Disease. Clin. Cosmet. Investig. Dermatol.16, 3129–3145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ujiie, H. et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med.9, 875492 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevyrev, D. & Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol.10, 3100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell133, 775–787 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum, L., Chen, Y. L. & Ogg, G. S. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br. J. Dermatol.184, 14–24 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Sharabi, A. et al. Regulatory T cells in the treatment of disease. Nat. Rev. Drug Discov.17, 823–844 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Nedoszytko, B. et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part II: The Treg role in skin diseases pathogenesis. Postepy. Dermatol. Alergol.34, 405–417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas, A. K., Trotta, E., D, R. S., Marson, A. & Bluestone, J. A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol.3, eaat1482 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Rosenzwajg, M. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis.78, 209–217 (2019). [DOI] [PubMed] [Google Scholar]
- 24.He, J. et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis.79, 141–149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuoka, K. et al. Low-Dose Interleukin-2 Therapy Restores Regulatory T Cell Homeostasis in Patients with Chronic Graft-Versus-Host Disease. Sci. Transl. Med.5, 179ra143–179ra143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konrad, M. W. et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 50, 2009–2017 (1990). [PubMed] [Google Scholar]
- 27.Dixit, N. et al. NKTR-358: A novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J. Transl. Autoimmun.4, 100103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanton, C. et al. Selective expansion of regulatory T cells by NKTR-358 in healthy volunteers and patients with systemic lupus erythematosus. J. Transl. Autoimmun.5, 100152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverberg, J. I. et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br. J. Dermatol.184, 888–895 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 50, D687–d692 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raeber, M. E., Sahin, D., Karakus, U. & Boyman, O. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. EBioMedicine90, 104539 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayati, F. et al. The Therapeutic Potential of Regulatory T Cells: Challenges and Opportunities. Front. Immunol.11, 585819 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutcher, J. P. et al. High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014. J. Immunother. Cancer2, 26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blauvelt, A. et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet389, 2287–2303 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Stein Gold, L. et al. Safety of Lebrikizumab in Adults and Adolescents with Moderate-to-Severe Atopic Dermatitis: An Integrated Analysis of Eight Clinical Trials. Am. J. Clin. Dermatol.24, 595–607 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wollenberg, A. et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br. J. Dermatol.186, 453–465 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Qiao, Z. et al. Low-dose Interleukin-2 For Psoriasis Therapy Based on the Regulation of Th17/Treg Cell Balance in Peripheral Blood. Inflammation46, 2359–2373 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Z., Li, D., Tsun, A. & Li, B. FOXP3+ regulatory T cells and their functional regulation. Cell. Mol. Immunol.12, 558–565 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luci, C. et al. Peripheral natural killer cells exhibit qualitative and quantitative changes in patients with psoriasis and atopic dermatitis. Br. J. Dermatol.166, 789–796 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Kabashima, K. & Weidinger, S. NK cells as a possible new player in atopic dermatitis. J. Allergy Clin. Immunol.146, 276–277 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Mack, M. R. et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci. Transl. Med.12, eaay1005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Möbus, L. et al. Blood transcriptome profiling identifies 2 candidate endotypes of atopic dermatitis. J. Allergy Clin. Immunol.150, 385–395 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Lee, M. et al. CD56bright natural killer cells preferentially kill proliferating CD4+ T cells. Discov. Immunol.2, kyad012 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel, T. et al. Human CD56bright NK Cells: An Update. J. Immunol.196, 2923–2931 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Guttman-Yassky, E. et al. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet401, 204–214 (2023). [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto, S. et al. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J. Dermatol. Sci.44, 93–99 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Bissonnette, R. et al. Clinical and molecular effects of oral CCR4 antagonist RPT193 in atopic dermatitis: A Phase 1 study. Allergy79, 924–936 (2024). [DOI] [PubMed] [Google Scholar]
- 48.Karlen, H., Yousefi, S., Simon, H.-U. & Simon, D. IL-15 Expression Pattern in Atopic Dermatitis. Int. Arch. Allergy Immunol.181, 417–421 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Chong, S., Lan, H., Zeng, K. & Zhao, X. Serum Fractalkine (CX3CL1) Concentration Correlates with Clinical Severity in Pediatric Atopic Dermatitis Patients. Ann. Clin. Lab. Sci.46, 168–173 (2016). [PubMed] [Google Scholar]
- 50.Renert-Yuval, Y. et al. Biomarkers in atopic dermatitis—a review on behalf of the International Eczema Council. J. Allergy Clin. Immunol.147, 1174–1190.e1171 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Günther, C., Carballido-Perrig, N., Kaesler, S., Carballido, J. M. & Biedermann, T. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J. Invest. Dermatol.132, 626–634 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Chen, L. & Shen, Z. Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell. Mol. Immunol.17, 64–75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokura, Y., Phadungsaksawasdi, P., Kurihara, K., Fujiyama, T. & Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol.11, 618897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fanton, C. & Zalevsky, J. Recent patents in allergy and immunology: The interleukin-2 receptor pathway agonist rezpegaldesleukin (REZPEG) for the rescue of regulatory T cells in chronic inflammatory and autoimmune disease. Allergy79, 2565–2566 (2024). [DOI] [PubMed]
- 55.Silverberg, J. I. et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann. Allergy Asthma Immunol.126, 40–45 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Worm, M. et al. Efficacy and Safety of Multiple Dupilumab Dose Regimens After Initial Successful Treatment in Patients With Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol156, 131–143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gooderham, M. J. et al. Durability of Response to Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis After Treatment Discontinuation in a Phase 2b Trial. Dermatol. Ther. (Heidelb.)12, 2077–2085 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blauvelt, A. et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J. Am. Acad. Dermatol.86, 104–112 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Engstrom, L. et al. Characterization of a murine keyhole limpet hemocyanin (KLH)-delayed-type hypersensitivity (DTH) model: role for p38 kinase. Int. Immunopharmacol.9, 1218–1227 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Eichenfield, L. F. et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol.70, 338–351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowler, J. F. Jr., Hebert, A. A. & Sugarman, J. DFD-01, a Novel Medium Potency Betamethasone Dipropionate 0.05% Emollient Spray, Demonstrates Similar Efficacy to Augmented Betamethasone Dipropionate 0.05% Lotion for the Treatment of Moderate Plaque Psoriasis. J. Drugs Dermatol.15, 154–162 (2016). [PubMed] [Google Scholar]
- 62.Kimball, A. B. et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol.175, 157–162 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Naegeli, A. N., Flood, E., Tucker, J., Devlen, J. & Edson-Heredia, E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int. J. Dermatol.54, 715–722 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Hanifin, J. M. et al. The Eczema Area and Severity Index-A Practical Guide. Dermatitis33, 187–192 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scarisbrick, J. J. & Morris, S. How big is your hand and should you use it to score skin in cutaneous T-cell lymphoma? Br. J. Dermatol.169, 260–265 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Bieber, T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov.21, 21–40 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basra, M. K., Salek, M. S., Camilleri, L., Sturkey, R. & Finlay, A. Y. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology230, 27–33 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Khilji, F. A., Gonzalez, M. & Finlay, A. Clinical meaning of change in Dermatology Life Quality Index scores. Br. J. Dermatol.147, 50 (2002). [Google Scholar]
- 69.Charman, C. R., Venn, A. J., Ravenscroft, J. C. & Williams, H. C. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br. J. Dermatol.169, 1326–1332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft.82, 1–26 (2017). [Google Scholar]
- 71.Lenth, R. V. emmeans: Estimated marginal means, aka least-squares means. https://rvlenth.github.io/emmeans/ (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data are provided in the Source Data file. Source data are provided with this paper.