Abstract
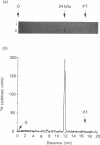
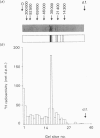
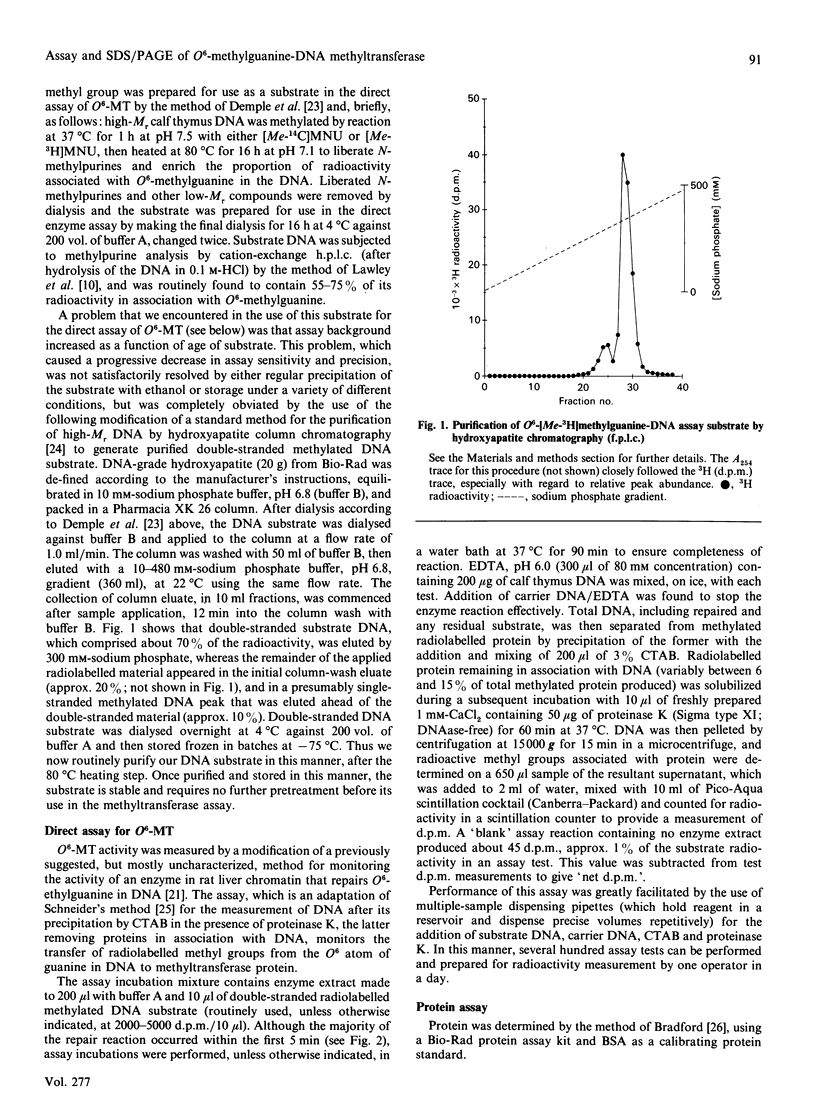
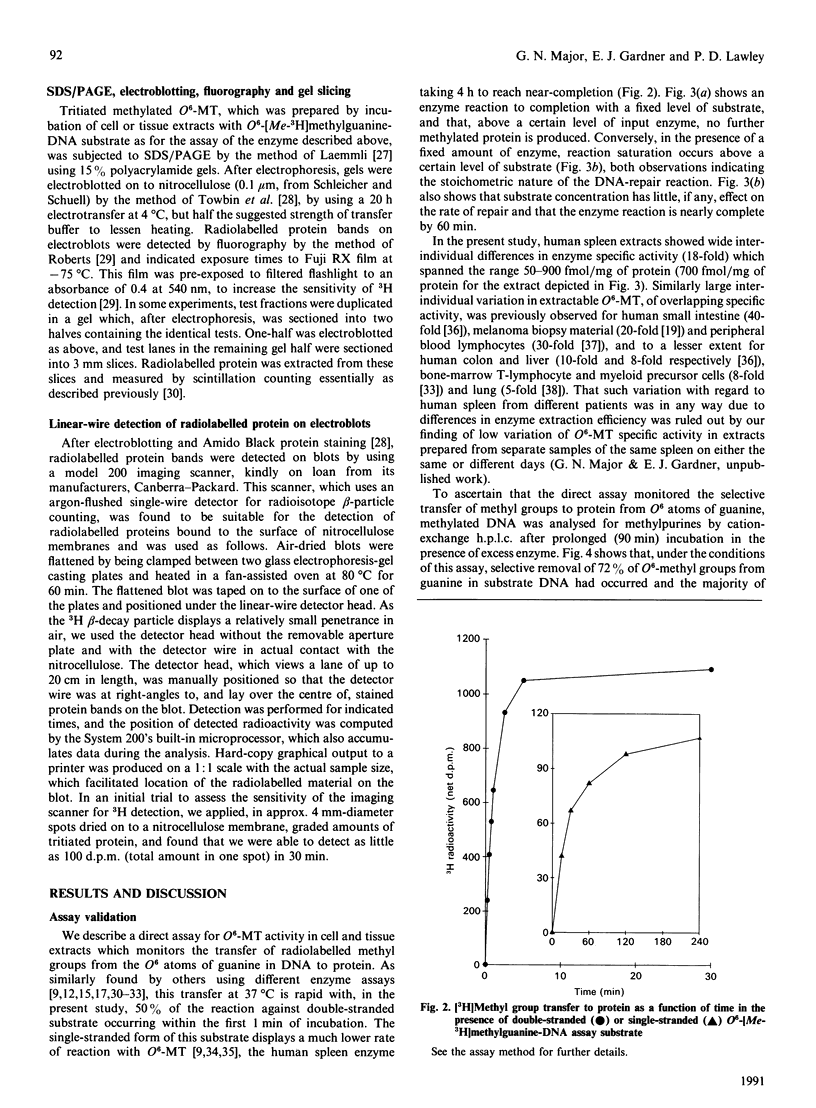
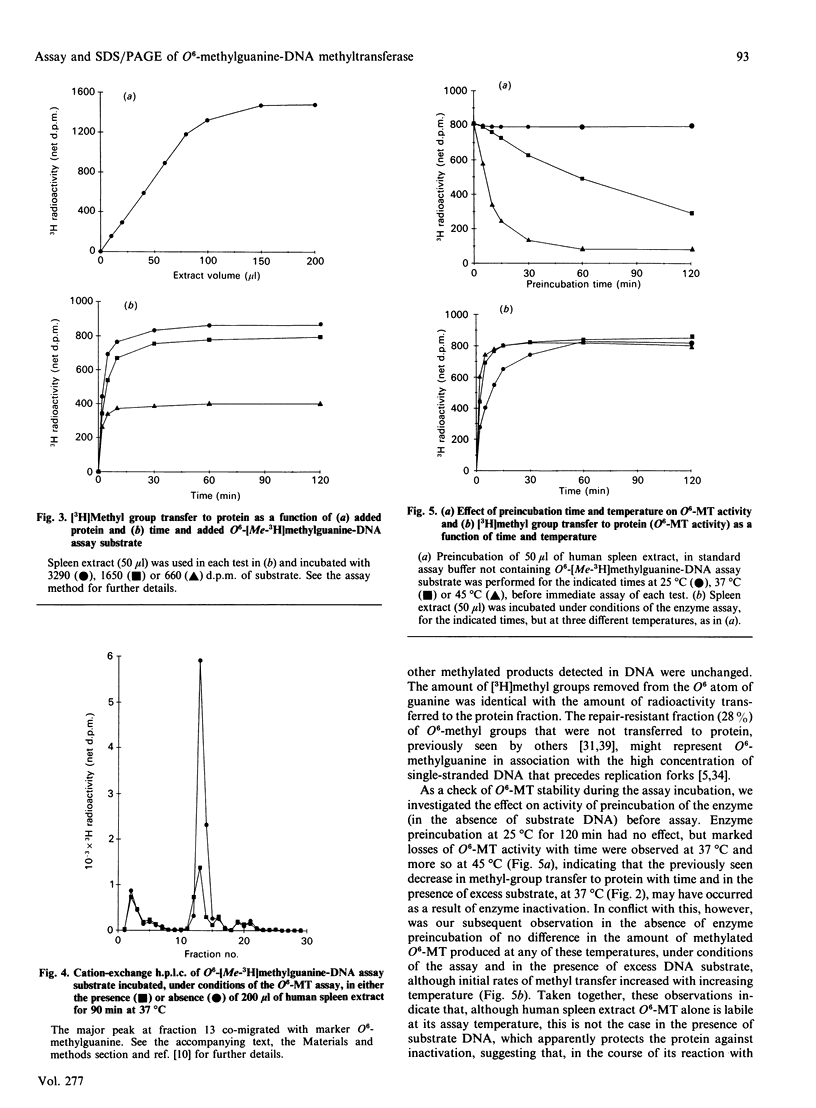
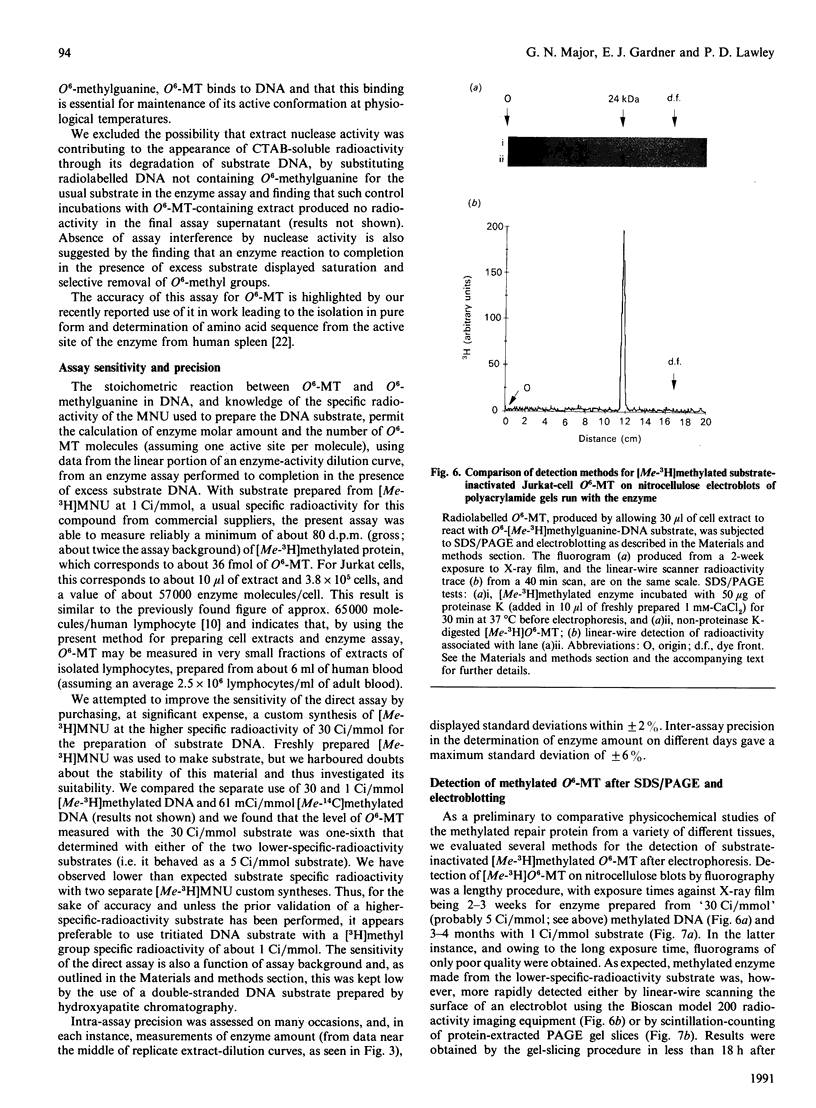
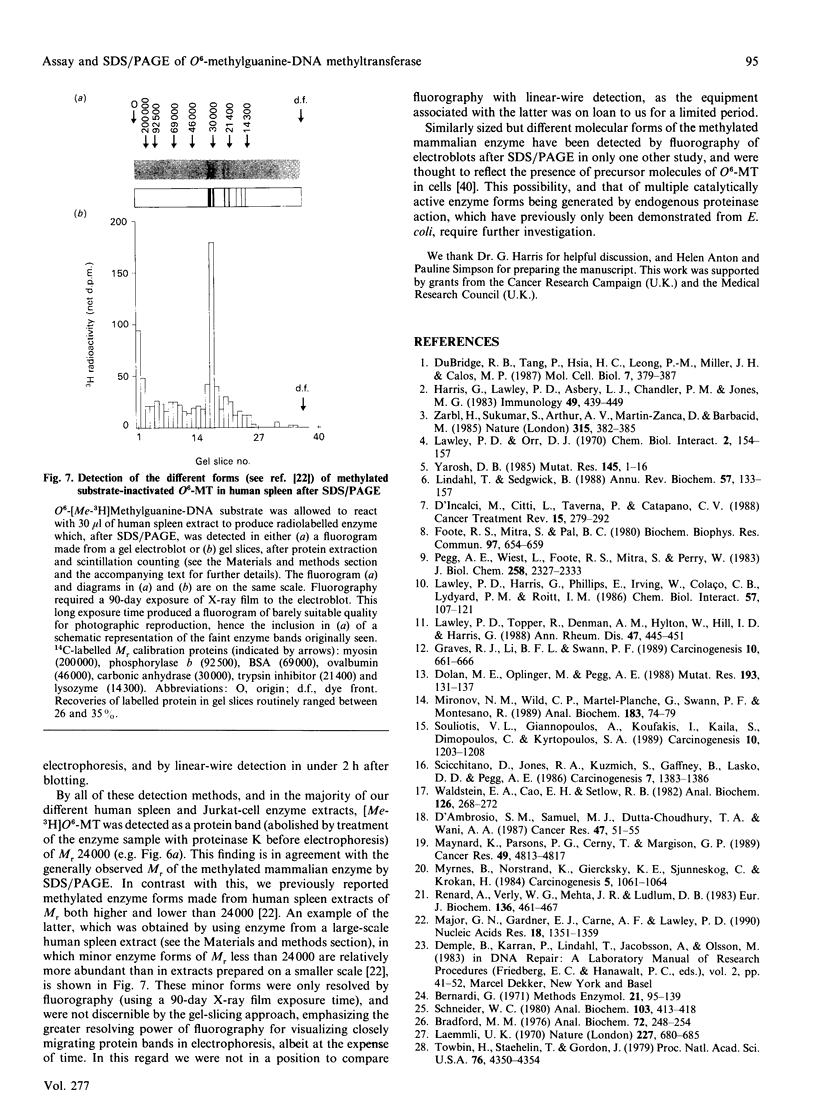
We describe in detail a direct assay for the substrate-inactivated DNA-repair enzyme, O6-methylguanine-DNA methyltransferase (O6-MT), which measures the transfer of radiolabelled methyl groups from a prepared O6-methylguanine-DNA substrate to the protein fraction of an enzyme-containing cell/tissue extract. This assay, a modification of a previously suggested method for monitoring O6-ethylguanine-DNA repair [Renard, Verly, Mehta & Ludlum (1983) Eur. J. Biochem. 136, 461-467], is sensitive, highly reproducible, accurate and is, as described here and relative to previously published methods, well suited for use with a large number of test samples. We identified two problems with the O6-[Me-3H]methylguanine-DNA substrate used in the present work and in other reported assay: firstly, that of progressively higher assay backgrounds with increasing age of substrate, which was nullified by once-only purification of the double-stranded substrate by hydroxyapatite chromatography; secondly, a substrate of high specific radioactivity (30 Ci/mmol), made with freshly prepared tritiated methylnitrosourea, behaved as a substrate of 5 Ci/mmol when referenced against radiolabelled O6-methylguanine-DNA made with either [3H]- or [14C]-methylnitrosourea at the lower specific radioactivities of 1 Ci/mmol and 61 mCi/mmol respectively. This apparently stemmed from the known instability of high-specific-radioactivity [3H]methylnitrosourea and indicated that an expected increase in sensitivity of the assay does not necessarily result from increasing the specific radioactivity of substrates above approx. 1 Ci/mmol. Although O6-MT was stable to preincubation at 25 degrees C, marked losses of activity were observed at 37 degrees C, and more so at 45 degrees C. Enzyme lability at the higher temperatures was not, however, seen during preincubation in the presence of its substrate. O6-[Me-3H]methylguanine-DNA, which apparently protected O6-MT against thermal inactivation. As previously seen with other human cells and tissues, extracts of human spleen in the present study showed wide interindividual differences in O6-MT specific activity (18-fold), which spanned the range 50-900 fmol/mg of protein. Cultured human lymphoblastoid Jurkat cells contained approx. 57,000 enzyme molecules/cell. Substrate-inactivated [Me-3H]methylated O6-MT was analysed by SDS/PAGE and electroblotting. The different but similarly sized forms of this enzyme that we previously detected in human spleen [Major, Gardner, Carne & Lawley (1990) Nucleic Acids Res. 18, 1351-1359] were clearly resolved by fluorography of electroblots, but only at considerable expense of time. As expected, scintillation counting of the protein extracted from gel slices and linear-wire scanning of enzyme-associated radioactivity on electroblots were quicker methods for detecting the [Me-3H]methylated inactivated O6-MT.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boiteux S., Laval F. Repair of O6-methylguanine, by mammalian cell extracts, in alkylated DNA and poly(dG-m5dC).(poly dG-m5dC) in B and Z forms. Carcinogenesis. 1985 May;6(5):805–807. doi: 10.1093/carcin/6.5.805. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Samuel M. J., Dutta-Choudhury T. A., Wani A. A. O6-methylguanine-DNA methyltransferase in human fetal tissues: fetal and maternal factors. Cancer Res. 1987 Jan 1;47(1):51–55. [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Oplinger M., Pegg A. E. Use of a dodecadeoxynucleotide to study repair of the O4-methylthymine lesion. Mutat Res. 1988 Mar;193(2):131–137. doi: 10.1016/0167-8817(88)90043-0. [DOI] [PubMed] [Google Scholar]
- DuBridge R. B., Tang P., Hsia H. C., Leong P. M., Miller J. H., Calos M. P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987 Jan;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote R. S., Mitra S., Pal B. C. Demethylation of O6-methylguanine in a synthetic DNA polymer by an inducible activity in Escherichia coli. Biochem Biophys Res Commun. 1980 Nov 28;97(2):654–659. doi: 10.1016/0006-291x(80)90314-9. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Miller K., Berger N. A. O6 alkylguanine-DNA alkyltransferase activity in human myeloid cells. J Clin Invest. 1985 Dec;76(6):2106–2114. doi: 10.1172/JCI112215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstrom R. C., Pegg A. E., Trump B. F., Harris C. C. O6-alkylguanine-DNA alkyltransferase activity in normal human tissues and cells. Cancer Res. 1984 Jul;44(7):2855–2857. [PubMed] [Google Scholar]
- Graves R. J., Li B. F., Swann P. F. Repair of O6-methylguanine, O6-ethylguanine, O6-isopropylguanine and O4-methylthymine in synthetic oligodeoxynucleotides by Escherichia coli ada gene O6-alkylguanine-DNA-alkyltransferase. Carcinogenesis. 1989 Apr;10(4):661–666. doi: 10.1093/carcin/10.4.661. [DOI] [PubMed] [Google Scholar]
- Hall J., Brésil H., Montesano R. O6-Alkylguanine DNA alkyltransferase activity in monkey, human and rat liver. Carcinogenesis. 1985 Feb;6(2):209–211. doi: 10.1093/carcin/6.2.209. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Harris G., Lawley P. D., Asbery L. J., Chandler P. M., Jones M. G. Autoimmune haemolytic disease in mice after exposure to a methylating carcinogen. Immunology. 1983 Jul;49(3):439–449. [PMC free article] [PubMed] [Google Scholar]
- Hora J. F., Eastman A., Bresnick E. O6-methylguanine methyltransferase in rat liver. Biochemistry. 1983 Aug 2;22(16):3759–3763. doi: 10.1021/bi00285a007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Harris G., Phillips E., Irving W., Colaço C. B., Lydyard P. M., Roitt I. M. Repair of chemical carcinogen-induced damage in DNA of human lymphocytes and lymphoid cell lines--studies of the kinetics of removal of O6-methylguanine and 3-methyladenine. Chem Biol Interact. 1986 Jan;57(1):107–121. doi: 10.1016/0009-2797(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Topper R., Denman A. M., Hylton W., Hill I. D., Harris G. Increased sensitivity of lymphocytes from patients with systemic autoimmune diseases to DNA alkylation by the methylating carcinogen N-methyl-N-nitrosourea. Ann Rheum Dis. 1988 Jun;47(6):445–451. doi: 10.1136/ard.47.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Major G. N., Gardner E. J., Carne A. F., Lawley P. D. Purification to homogeneity and partial amino acid sequence of a fragment which includes the methyl acceptor site of the human DNA repair protein for O6-methylguanine. Nucleic Acids Res. 1990 Mar 25;18(6):1351–1359. doi: 10.1093/nar/18.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard K., Parsons P. G., Cerny T., Margison G. P. Relationships among cell survival, O6-alkylguanine-DNA alkyltransferase activity, and reactivation of methylated adenovirus 5 and herpes simplex virus type 1 in human melanoma cell lines. Cancer Res. 1989 Sep 1;49(17):4813–4817. [PubMed] [Google Scholar]
- Mironov N. M., Wild C. P., Martel-Planche G., Swann P. F., Montesano R. Measurement of the removal of O6-methylguanine and O4-methylthymine from oligodeoxynucleotides using an immunoprecipitation technique. Anal Biochem. 1989 Nov 15;183(1):74–79. doi: 10.1016/0003-2697(89)90173-5. [DOI] [PubMed] [Google Scholar]
- Myers K. A., Saffhill R., O'Connor P. J. Repair of alkylated purines in the hepatic DNA of mitochondria and nuclei in the rat. Carcinogenesis. 1988 Feb;9(2):285–292. doi: 10.1093/carcin/9.2.285. [DOI] [PubMed] [Google Scholar]
- Myrnes B., Giercksky K. E., Krokan H. Interindividual variation in the activity of O6-methyl guanine-DNA methyltransferase and uracil-DNA glycosylase in human organs. Carcinogenesis. 1983 Dec;4(12):1565–1568. doi: 10.1093/carcin/4.12.1565. [DOI] [PubMed] [Google Scholar]
- Myrnes B., Norstrand K., Giercksky K. E., Sjunneskog C., Krokan H. A simplified assay for O6-methylguanine-DNA methyltransferase activity and its application to human neoplastic and non-neoplastic tissues. Carcinogenesis. 1984 Aug;5(8):1061–1064. doi: 10.1093/carcin/5.8.1061. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Renard A., Verly W. G., Mehta J. R., Ludlum D. B. Properties of the chromatin repair activity against O6-ethylguanine lesions in DNA. Mechanism of the reaction. Eur J Biochem. 1983 Nov 15;136(3):461–467. doi: 10.1111/j.1432-1033.1983.tb07764.x. [DOI] [PubMed] [Google Scholar]
- Roberts P. L. Comparison of fluorographic methods for detecting radioactivity in polyacrylamide gels or on nitrocellulose filters. Anal Biochem. 1985 Jun;147(2):521–524. doi: 10.1016/0003-2697(85)90308-2. [DOI] [PubMed] [Google Scholar]
- Sagher D., Karrison T., Schwartz J. L., Larson R. A., Strauss B. Heterogeneity of O6-alkylguanine-DNA alkyltransferase activity in peripheral blood lymphocytes: relationship between this activity in lymphocytes and in lymphoblastoid lines from normal controls and from patients with Hodgkin's disease or non-Hodgkin's lymphoma. Cancer Res. 1989 Oct 1;49(19):5339–5344. [PubMed] [Google Scholar]
- Schneider W. C. Simplified isolation and quantitation of cytoplasmic DNA from rat liver. Anal Biochem. 1980 Apr;103(2):413–418. doi: 10.1016/0003-2697(80)90632-6. [DOI] [PubMed] [Google Scholar]
- Scicchitano D., Jones R. A., Kuzmich S., Gaffney B., Lasko D. D., Essigmann J. M., Pegg A. E. Repair of oligodeoxynucleotides containing O6-methylguanine by O6-alkylguanine-DNA-alkyltransferase. Carcinogenesis. 1986 Aug;7(8):1383–1386. doi: 10.1093/carcin/7.8.1383. [DOI] [PubMed] [Google Scholar]
- Souliotis V. L., Giannopoulos A., Koufakis I., Kaila S., Dimopoulos C., Kyrtopoulos S. A. Development and validation of a new assay for O6-alkylguanine-DNA-alkyltransferase based on the use of an oligonucleotide substrate, and its application to the measurement of DNA repair activity in extracts of biopsy samples of human urinary bladder mucosa. Carcinogenesis. 1989 Jul;10(7):1203–1208. doi: 10.1093/carcin/10.7.1203. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein E. A., Cao E. H., Setlow R. B. Direct assay for O6-methylguanine-acceptor protein in cell extracts. Anal Biochem. 1982 Nov 1;126(2):268–272. doi: 10.1016/0003-2697(82)90514-0. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B., Fornace A. J., Day R. S., 3rd Human O6-alkylguanine-DNA alkyltransferase fails to repair O4-methylthymine and methyl phosphotriesters in DNA as efficiently as does the alkyltransferase from Escherichia coli. Carcinogenesis. 1985 Jul;6(7):949–953. doi: 10.1093/carcin/6.7.949. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B., Rice M., Day R. S., 3rd, Foote R. S., Mitra S. O6-Methylguanine-DNA methyltransferase in human cells. Mutat Res. 1984 Jan;131(1):27–36. doi: 10.1016/0167-8817(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B. The role of O6-methylguanine-DNA methyltransferase in cell survival, mutagenesis and carcinogenesis. Mutat Res. 1985 Jan-Mar;145(1-2):1–16. doi: 10.1016/0167-8817(85)90034-3. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]