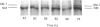Abstract
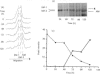
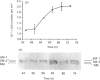
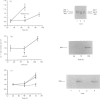
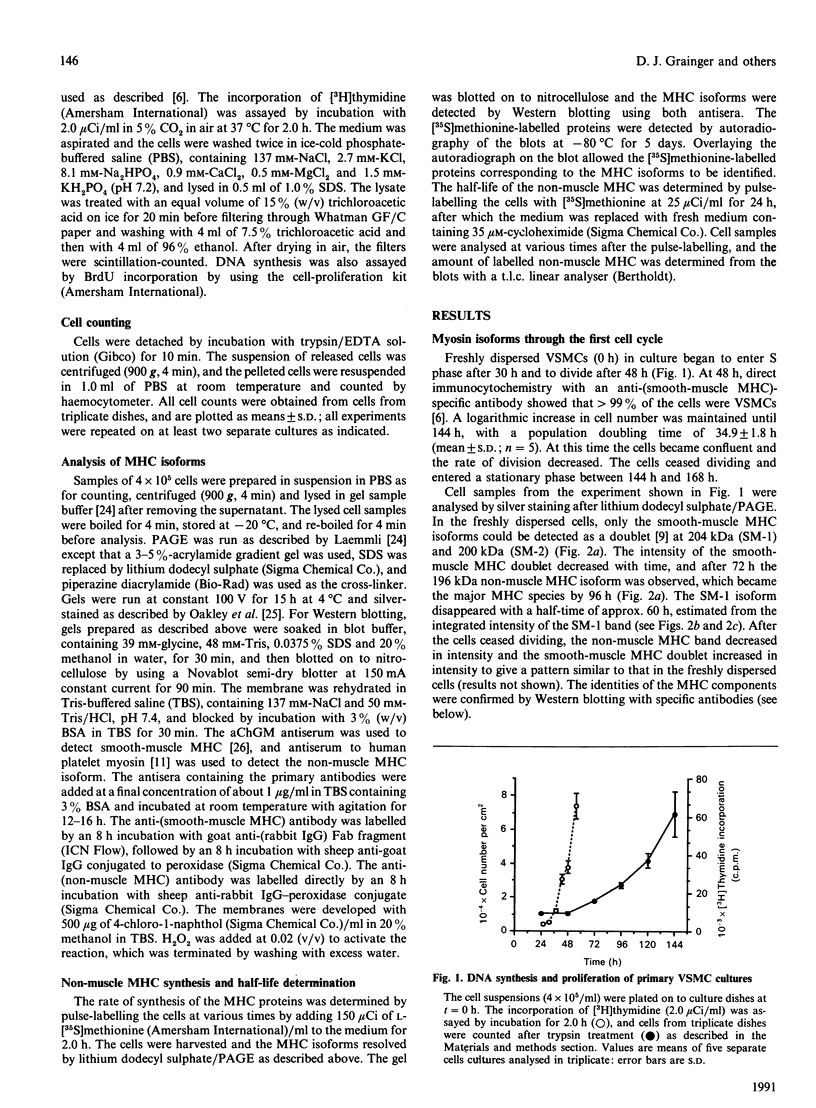
Vascular smooth-muscle cells (VSMCs) from rat aortae contained very little non-muscle myosin heavy chain (MHC) immediately after dispersal, and the protein did not accumulate if the cells were held in G0/G1 phase by withholding serum or were held in first S phase by the addition of bromodeoxyuridine (BrdU). However, non-muscle MHC accumulated by greater than 20-fold per cell during first M phase, when over 80% of the cells divided between 48 h and 72 h after addition of serum. Delaying the addition of serum caused a delay in the accumulation of the non-muscle MHC until the cells subsequently entered M phase. If the cells were held in M phase at the metaphase/anaphase boundary by nocadazole, the accumulation of non-muscle myosin still occurred, although division was blocked. When the cells were pulse-labelled with [35S]methionine, it was found that non-muscle MHC was one of the major proteins being made and that its synthesis occurred at similar rates throughout the cell cycle. This implied that the rate of degradation of the protein before first M phase was much faster than in M phase, when the protein accumulated rapidly. This was confirmed by direct measurements of the rate at which [35S]methionine-labelled non-muscle MHC disappeared from the cells, which gave a half-life for the protein of about 8 h before M phase but about 5 days in post-mitotic cells, i.e. an increase of approx. 15-fold. These data are consistent with the hypothesis that there is a mechanism in VSMCs which shortens the half-life of the protein before first M phase and that the accumulation of non-muscle MHC which results from the increase in half-life at first M phase may be necessary for division of these cells.
Full text
PDF
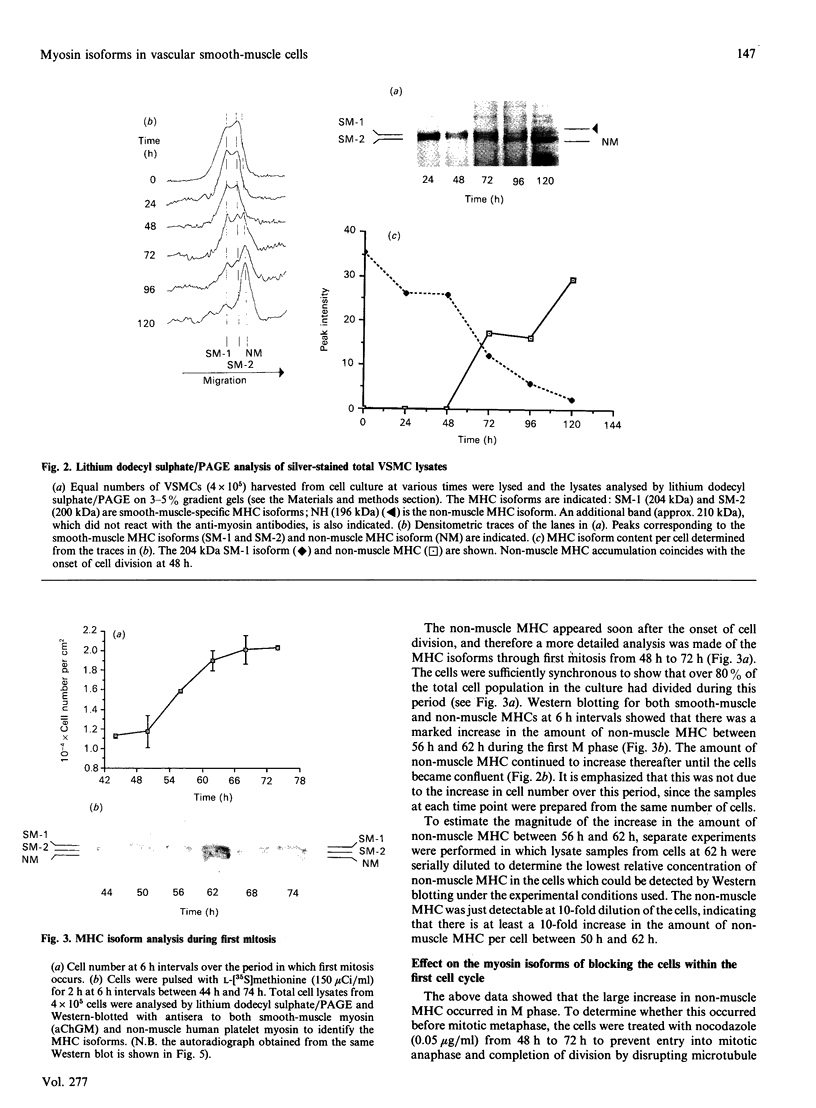


Images in this article
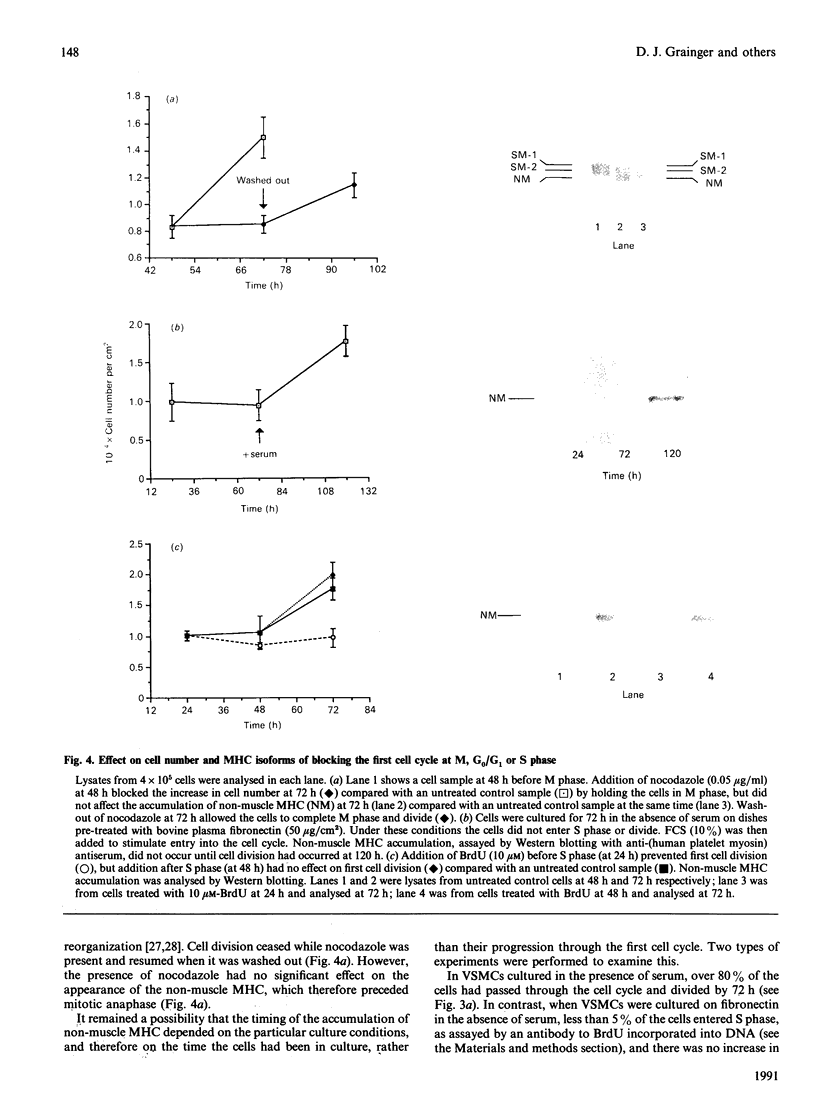
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang A. H., Tachas G., Campbell J. H., Bateman J. F., Campbell G. R. Collagen synthesis by cultured rabbit aortic smooth-muscle cells. Alteration with phenotype. Biochem J. 1990 Jan 15;265(2):461–469. doi: 10.1042/bj2650461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Kocher O., Skalli O., Gabbiani G., Campbell G. R. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells. Correlation with cell density and proliferative state. Arteriosclerosis. 1989 Sep-Oct;9(5):633–643. doi: 10.1161/01.atv.9.5.633. [DOI] [PubMed] [Google Scholar]
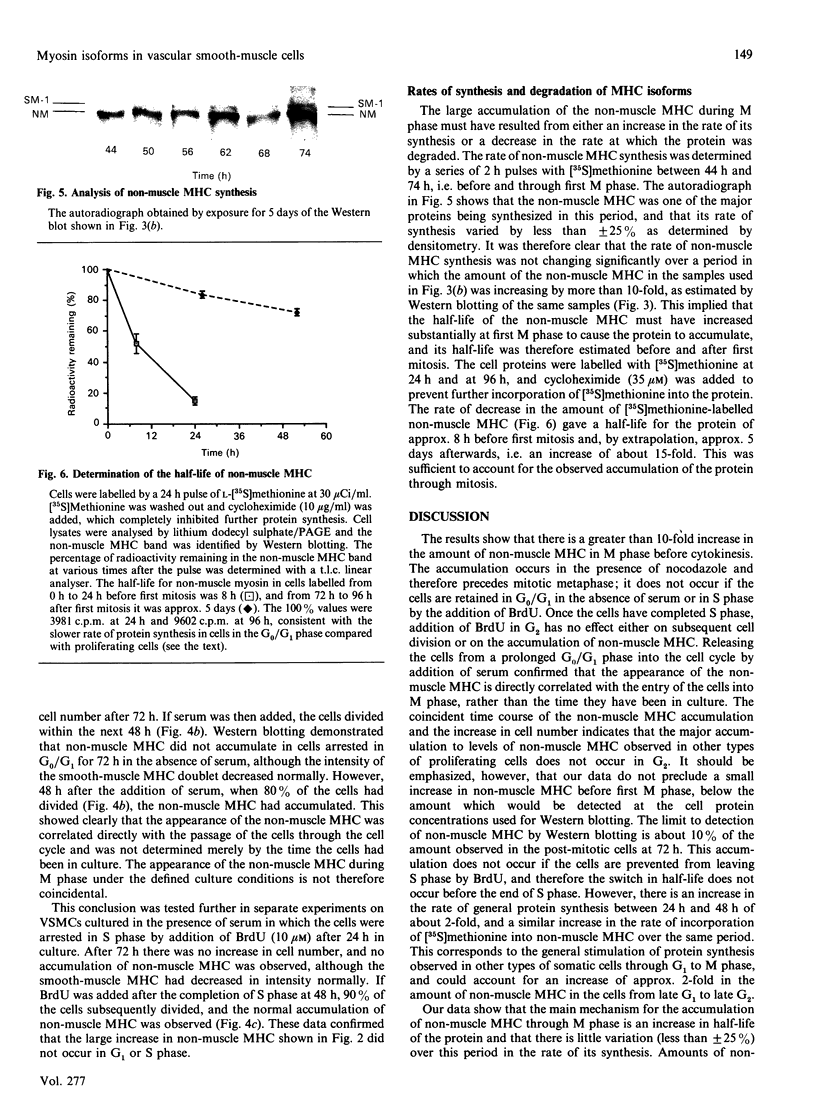
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., Burnstock G. An analysis of the interactions between sympathetic nerve fibers and smooth muscle cells in tissue culture. Dev Biol. 1973 Aug;33(2):344–361. doi: 10.1016/0012-1606(73)90142-5. [DOI] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., McConnell J. D., Gröschel-Stewart U. Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tissue Res. 1977 Feb 14;177(4):503–522. doi: 10.1007/BF00220611. [DOI] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R. Mitosis of contractile smooth muscle cells in tissue culture. Exp Cell Res. 1974 Mar 15;84(1):105–110. doi: 10.1016/0014-4827(74)90385-1. [DOI] [PubMed] [Google Scholar]
- Corjay M. H., Thompson M. M., Lynch K. R., Owens G. K. Differential effect of platelet-derived growth factor- versus serum-induced growth on smooth muscle alpha-actin and nonmuscle beta-actin mRNA expression in cultured rat aortic smooth muscle cells. J Biol Chem. 1989 Jun 25;264(18):10501–10506. [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylinn B. D., Eddinger T. J., Martino P. A., Monical P. L., Hunt D. F., Murphy R. A. Expression of nonmuscle myosin heavy and light chains in smooth muscle. Am J Physiol. 1989 Nov;257(5 Pt 1):C997–1004. doi: 10.1152/ajpcell.1989.257.5.C997. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Chamley J. H., Campbell G. R., Burnstock G. Changes in myosin distribution in dedifferentiating and redifferentiating smooth muscle cells in tissue culture. Cell Tissue Res. 1975 Dec 29;165(1):13–22. doi: 10.1007/BF00222796. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Magel E., Paul E., Neidlinger A. C. Pig brain homogenates contain smooth muscle myosin and cytoplasmic myosin isoforms. Cell Tissue Res. 1989 Jul;257(1):137–139. doi: 10.1007/BF00221643. [DOI] [PubMed] [Google Scholar]
- Hedin U., Bottger B. A., Forsberg E., Johansson S., Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988 Jul;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsmeyer K., de Cino P., White R. Spontaneous contractions of dispersed vascular muscle in cell culture. In Vitro. 1976 Sep;12(9):628–634. doi: 10.1007/BF02797461. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Beaven M. A., Rogers J., Burke B., Warren G. B. Stimulated release of histamine by a rat mast cell line is inhibited during mitosis. J Cell Biol. 1984 Jun;98(6):2250–2254. doi: 10.1083/jcb.98.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Adelstein R. S. Characterization of myosin heavy chains in cultured aorta smooth muscle cells. A comparative study. J Biol Chem. 1987 May 25;262(15):7282–7288. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray T. R., Marshall B. E., Macarak E. J. Contraction of vascular smooth muscle in cell culture. J Cell Physiol. 1990 Apr;143(1):26–38. doi: 10.1002/jcp.1041430105. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Rovner A. S., Murphy R. A., Owens G. K. Expression of smooth muscle and nonmuscle myosin heavy chains in cultured vascular smooth muscle cells. J Biol Chem. 1986 Nov 5;261(31):14740–14745. [PubMed] [Google Scholar]
- Sartore S., De Marzo N., Borrione A. C., Zanellato A. M., Saggin L., Fabbri L., Schiaffino S. Myosin heavy-chain isoforms in human smooth muscle. Eur J Biochem. 1989 Jan 15;179(1):79–85. doi: 10.1111/j.1432-1033.1989.tb14523.x. [DOI] [PubMed] [Google Scholar]
- Seidel C. L., Wallace C. L., Dennison D. K., Allen J. C. Vascular myosin expression during cytokinesis, attachment, and hypertrophy. Am J Physiol. 1989 Apr;256(4 Pt 1):C793–C798. doi: 10.1152/ajpcell.1989.256.4.C793. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Nilsson J., Palmberg L., Sjölund M. Adult human arterial smooth muscle cells in primary culture. Modulation from contractile to synthetic phenotype. Cell Tissue Res. 1985;239(1):69–74. doi: 10.1007/BF00214904. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Turnbull D., Mullins J. M., McIntosh J. R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980 Apr;126(2):397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]