Abstract
The latency-associated nuclear antigen (LANA) is constitutively expressed in cells infected with the Kaposi's sarcoma (KS) herpesvirus (KSHV), also referred to as human herpesvirus 8. KSHV is tightly associated with body cavity-based lymphomas (BCBLs) in immunocompromised patients infected with human immunodeficiency virus (HIV). LANA, encoded by open reading frame 73 of KSHV, is one of a small subset of proteins expressed during latent infection and was shown to be important in tethering the viral episome to host chromosomes. Additionally, it has been shown that LANA can function as a regulator of transcription. However, its role in the progression of disease is still being elucidated. Since KS is one of the most common AIDS-associated cancers in the United States and BCBLs appear predominantly in AIDS patients, we examined whether LANA is able to regulate the HIV type 1 (HIV-1) long terminal repeat (LTR). Using luciferase-based transient transfection assays, we found that LANA was able to transactivate the HIV-1 LTR in the human B-cell line BJAB, human monocytic cell line U937, and the human embryonic kidney fibroblast cell line 293T. Moreover, we observed that the virus-encoded HIV transactivator protein Tat cooperated with LANA in activation of the LTR in a dose-response fashion with increasing amounts of LANA. Surprisingly, LANA alone was sufficient to transactivate the HIV-1 LTR in BJAB cells. In similar assays using a HIV-1 LTR construct with the core enhancer elements deleted; the activity of LANA was diminished but not abolished, indicating a mechanism which involves the cooperation of the core enhancer elements and downstream elements which include Tat. Furthermore, transient transfection of an infectious clone of HIV with LANA demonstrated effects similar to those seen in the reporter assays based on Western blot analysis of HIV Gag polypeptide p24. Interestingly, we also demonstrated that the carboxy terminus of LANA associates with Tat in cells and in vitro. These experiments suggest a role for LANA in activating the HIV-1 LTR through association with cellular molecules targeting the core enhancer elements and Tat and may have important consequences in increasing the levels of HIV in infected individuals and, hence, the disease state.
Many individuals infected with human immunodeficiency virus type 1 (HIV-1) are also infected with Kaposi's sarcoma (KS) herpesvirus (KSHV) (4–6, 31). KSHV is thought to be the causative agent of all forms of KS: classical, endemic, transplantation associated, and HIV associated (37). HIV-associated KS is one of the clinically aggressive forms of KS which usually progress more rapidly than the classical types of KS (26–28). Furthermore, KS is the most common AIDS-associated cancer in the United States (9). While immunosuppression is an important contributor to the development of KS, the rapidly progressive KS seen in HIV-infected individuals is rare in HIV-negative patients, including those who are immunocompromised (5). Additionally, KSHV is associated with body cavity-based lymphoma (BCBL), a rare form of B-cell lymphoma appearing predominantly in AIDS patients (12, 14). The disease rapidly progresses in individuals coinfected with HIV-1 and KSHV, suggesting that KSHV is a critical cofactor in HIV infection and progression of the AIDS disease state. One possibility is that KSHV influences the activity of the HIV-1 LTR, contributing to a higher level of HIV-1 replication and a reduction in the clinical latency period.
Most cells harboring KSHV are latently infected, and several latency-associated viral gene products have been identified (52). One of these constitutively expressed KSHV-encoded viral antigens is the latency-associated nuclear antigen (LANA) encoded by open reading frame 73 of the KSHV genome (23, 30). LANA contains three major domains: an N-terminal proline-rich region, a central highly repetitive region of glutamine-rich acidic residues, and a basic leucine zipper motif (see Fig. 1). A number of putative nuclear localization signals have also been identified at the amino terminus (amino acids [aa] 8 to 24) and the carboxy terminus of the molecule (41, 47). These features function in the regulation of gene expression in other known regulators of transcription, indicating that LANA can function as a transcriptional regulator. Previous studies have determined that LANA localizes to the nucleus in infected cells and is important for KSHV episome maintenance (3, 15, 30). Moreover, recent evidence has shown that LANA can regulate the transcriptional activity of cellular and viral promoters (32, 35, 47). LANA has been shown to function as a transcriptional inhibitor of p53, protecting against cell death, and is able to repress Epstein-Barr virus (EBV) latent gene expression in primary effusion lymphoma cell lines by interacting with the mSin3 corepressor complex (21, 32). Additionally, LANA has been shown to interact with RING3, a member of the female sterile homeotic gene family, which has been implicated in the control of gene expression, and with Rb, which regulates E2F-responsive promoters (42, 43). More specifically, the carboxy-terminal leucine zipper domain of LANA has been shown to be involved in both dimerization and transcriptional repression when targeted to specific promoters (47).
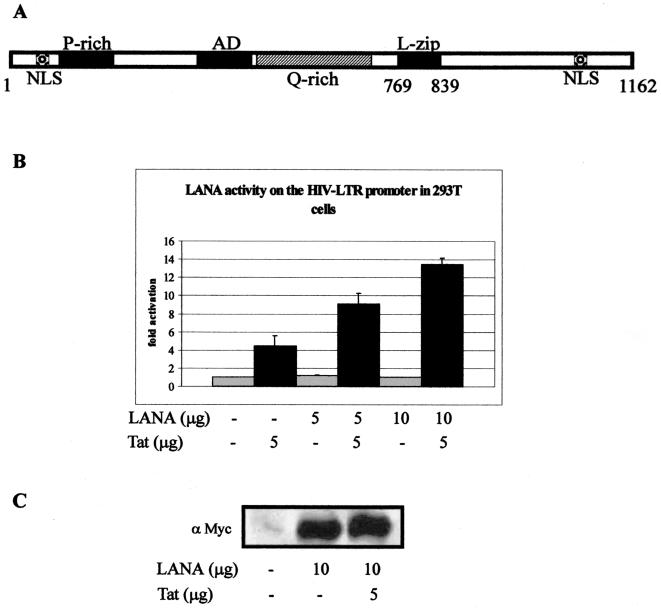
FIG. 1.
(A) Schematic representation of KSHV LANA. LANA contains three distinct regions: the proline-rich N-terminal region, a central region with glutamine-rich repeats, and the C-terminal region. A putative basic leucine zipper domain is located near the end of the central region. Two potential nuclear localization signals (NLS) have been identified, one near the amino terminus and the second near the carboxy terminus (41, 47). (B) LANA activates the HIV-1 LTR in HEK 293T cells. Results of transient-transfection assays using a subtype B HIV-1 LTR linked to the luciferase gene as the reporter in 293T cells are shown. Cells were transfected with 5 μg of reporter plasmid and LANA and/or Tat constructs. The total amount of DNA was normalized with empty vector DNA for each transfection and transfection efficiency. Cells were harvested 18 h after transfection. (C) Western blot analysis for detection of LANA in transiently transfected cells. One million cells were harvested, washed, and fractionated by SDS–6% PAGE gel and Western blotted for detection of the Myc epitope. The pA3M-LANA construct is fused at the carboxy terminus to the Myc epitope for detection of expression in these assays.
Based on this evidence, we sought to determine whether LANA's role as a transcriptional regulator may also extend to regulation of the long terminal repeat (LTR) of HIV-1. The LTR encodes the major transcriptional promoter elements of HIV-1, and activation of the LTR results in transcription of the HIV-1 genome (reviewed in reference 46). Moreover, transcription of the LTR depends upon the expression of host cell transcription factors, which bind to several cis-regulatory elements in the LTR promoter (29, 39, 40). The LTR also contains the transactivation response element (TAR), an RNA element located immediately downstream of the transcription initiation site (8). TAR encodes a stem-loop structure at the 5′ terminus of HIV-1 transcripts (7). The virus-encoded trans-activator protein Tat interacts with the TAR element, enhancing the processivity of full-length HIV-1 transcripts.
In this study, we tested the ability of LANA to modulate the activity of the HIV-1 LTR by using cotransfection experiments with reporter gene constructs, as well as in infectious clones in a number of human cell lines known to be infected with KSHV and HIV in vitro and in vivo (10, 22, 24, 45). Another recent study with COS-7 cells indicated a repression of the HIV-1 LTR in a chloramphenicol acetyltransferase reporter analysis (44). However, this cell line may not be the best representative of the effect of LANA on the HIV-1 LTR in the context of human cells known to be infected with HIV and KSHV. The HIV-1 LTR linked to a luciferase reporter construct was cotransfected with a plasmid containing full-length LANA into the B-cell line BJAB, human embryonic kidney (HEK) 293T cells, and the human monocytic cell line U937. Experiments were performed with and without the virus encoded transactivator protein Tat. We found that LANA was able to transactivate the HIV-1 LTR in BJAB cells at levels similar to or above those seen with equivalent amounts of Tat protein. Furthermore, LANA was able to synergize this increased transcriptional activity with Tat in both BJAB, U937, and 293T cells. These studies therefore suggest that LANA may have a role in enhancing transcriptional activities initiated from the HIV-1 LTR, which results in increased levels of HIV-1 transcripts in these human cell lines.
MATERIALS AND METHODS
Cell lines.
The EBV-negative human B-cell BJAB line isolated from a Burkitt's lymphoma patient was obtained from Elliott Kieff. The U937 cell line is a human monocytic cell line obtained from Robert Thomas. These cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin-streptomycin (5 U/ml and 5 μg/ml, respectively), and 20 μg of gentamicin per ml. HEK 293T cells were obtained from Jon Aster and cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin-streptomycin (5 U/ml and 5 μg/ml, respectively), and 20 μg of gentamicin per ml. All cell lines were maintained at 37°C in 5% CO2.
Plasmids and antibodies.
The LANA expression construct contains the entire open reading frame of LANA cloned into the pA3M vector under the control of the cytomegalovirus promoter (15). The pA3M vector contains a Myc tag (2). The pGL3-LAV and pGL3-SOE1 HIV-1 LTR luciferase and the pSV-Tat expression constructs were obtained from Robert Thomas (51). The pGL3-LAV plasmid contains the entire LTR from HIV-1 subtype B. The pGL3-SOE1 construct was derived from pGL3-LAV with a substitution of CCGCAGA for ATTTCAT at the NF-IL6 site and a 119-bp deletion just upstream of the TATAA box. Glutathione S-transferase (GST) fusions of Tat and pGEM-Tat were obtained from Fatah Kashanchi (George Washington University Medical School). LANA aa 1 to 756 and 1 to 950 were obtained from J. Choe (35). LANA aa 1 to 435 and 762 to 1162 were cloned into pA3M in frame with the Myc epitope tag (2).
Antibodies raised against LANA were obtained from Cocalico Inc. by inoculation of rabbits with a purified GST fusion of LANA (aa 762 to 1162). Ascites containing the Myc monoclonal antibody was produced by the University of Michigan hybridoma core facility using the 9E10 hybridoma. Anti-Myc ascites were used in Western blots at a 1:1,000 dilution in phosphate-buffered saline (PBS). Tat and p24 monoclonal and polyclonal antibodies, respectively, were obtained from the National Institutes of Health (NIH) AIDS Reagent Program (18).
Luciferase assays.
Ten million cells were used for each transient transfection. Transfections were performed by using 5 μg of an LTR-luciferase construct with or without a LANA expression construct (5 or 10 μg) with or without 5 μg of a Tat expression construct. Amounts of DNA for each transfection were normalized by using the empty pA3M vector, and transfection efficiencies were determined by using enhanced green fluorescent protein and counting the green fluorescence as a percentage of the transfected cells. Cells were electroporated at 210 V and 975 μF in 400 μl of complete medium, resuspended in 10 ml of medium, and incubated for 18 h. Cells were then washed with PBS and lysed by freeze-thawing after addition of 200 μl of reporter lysis buffer (Promega). Lysates from BJAB, U937, and 293T cells were diluted in reporter lysis buffer for measurement of luciferase activity within the linear range by using an Optocomp 1 luminometer (MGM Instruments). Twenty microliters of each sample was measured for 10 s. All experiments were done multiple times in triplicate, and the mean of each was plotted.
Western blotting.
Transient transfections and cell harvesting were performed as described above. One million cells were collected, washed once in PBS, lysed by mixing with an equal amount of sodium dodecyl sulfate (SDS) loading buffer, and heated at 95°C for 5 min. For detection of LANA, samples were subjected to SDS–6% polyacrylamide gel electrophoresis (PAGE) and transferred to a 0.45-μm nitrocellulose membrane. For fractionation of Tat and p24, samples were subjected to SDS–15% PAGE and transferred to nitrocellulose as described previously (11). Membranes were incubated overnight with a human polyclonal serum having reactivity to LANA, washed, and incubated with a 1:5,000 dilution of protein A-conjugated horseradish peroxidase (HRP) (11). Alternatively, LANA, which is tagged with a Myc epitope, was detected by using anti-Myc ascites at a 1:1,000 dilution. Tat and p24 were detected by using the monoclonal antiserum against Tat (18) at 1:1,000 and the polyclonal serum against p24 (Biomolecular Technologies Inc.) at 1:50,000 in PBS, respectively, and were obtained from the NIH AIDS Research and Reference Reagent Program. Anti-mouse antibody conjugated to HRP at 1:2,500 and anti-rabbit antibody conjugated to HRP at 1:10,000 were used as secondary antibodies. Signals were detected using the enhanced-chemiluminescence protocol supplied by the manufacturer (Amersham Inc.).
Immunofluorescence.
Transfections were performed as described above. Five hundred thousand cells were aliquoted and microcentrifuged at 5,000 rpm for 5 min at room temperature and further washed once with 1× PBS. Cells were resuspended in 20 μl of PBS, and approximately 1 μl of cells was spread on slides and allowed to air dry. Slides were fixed in 1:1 acetone-methanol at −20°C for 10 min and air dried. Cells were incubated with 20% goat serum at room temperature for 30 min, washed with PBS, and incubated with human KS serum reactive against LANA (1:500) or anti-Myc antibody for 2 h at room temperature. Slides were then washed four times for 5 min (each time) with PBS, incubated with anti-human immunoglobulin-fluorescein isothiocyanate (1:1,000), washed with PBS, and covered with Antifade for microscopic analysis. Photographs were taken with an Olympus BX60 fluorescence microscope and captured with the Espirit program v1.2.
Immunoprecipitation.
Ten million 293T cells were transfected with 30 μg of pSV-Tat and 30 μg of pA3M-LANA by electroporation at 210 V and 975 μF using a Bio-Rad electroporator. Transfected cells were incubated at 37°C with 5% CO2 for 24 hours, harvested, and then lysed in radioimmunoprecipitation assay buffer for 1 h on ice. Soluble protein was collected by clarification of cell debris and transfer of the soluble fraction to a fresh tube. Anti-Tat monoclonal antibody was added after preclearing, and the tubes were incubated at 4°C overnight with rotation. Twenty-five microliters of protein A Sepharose was added, and the reactions mixture were incubated further for 1 h. Complexes were collected by centrifugation and washed four times in radioimmunoprecipitation assay buffer. The isolated complexes were then solubilized by addition of SDS lysis buffer and heating at 95°C for 10 min. Proteins were fractionated by SDS–6% PAGE, transferred to a 0.45-μm nitrocellulose membrane, and blotted for detection of LANA by using a human polyclonal serum recognizing LANA.
GST binding assays.
Full-length LANA and truncated portions of LANA (aa 1 to 435, 1 to 756, 1 to 950, and 762 to 1162) were in vitro translated with [35S]methionine protein translabel (NEN-Dupont) using the Promega transcription and translation system (Promega, Inc.). GST-Tat was prepared by inducing cells in 500 ml of Luria-Bertani broth with 100 μg of ampicillin per ml and growing them to logarithmic phase with 1 M isopropyl-β-d-thiogalactopyranoside for 6 h. Cells were collected, washed, and sonicated in NETN buffer (16) with a cocktail of protease inhibitors and 1 mM phenylmethylsulfonyl fluoride. Debris was removed by centrifugation. GST-Tat was collected by incubating the soluble fraction with glutathione Sepharose beads for 12 h and collecting the beads complexed with GST-Tat by centrifugation. The bound beads were washed four times in NETN buffer with protease inhibitors as described above and stored at 4°C until use. In vitro binding assays were performed essentially as described previously (16, 49).
Transfection of 293T cells with infectious HIV clones and LANA.
To determine the levels of activation of the HIV-1 LTR in response to LANA in the context of the HIV-1 genome, we used infectious HIV-1 clone pYU2 obtained from the NIH AIDS Reagent Program (33, 34). Ten million 293T cells were grown to approximately 80% confluency, washed in PBS, and transfected as previously described for luciferase reporter assays. Cells were incubated at 37°C with 5% CO2, and aliquots were collected at 24 and 48 h posttransfection, respectively. One million cells were washed in PBS, resuspended in 25 μl of lysis buffer, and boiled for 10 min, and the soluble proteins were fractionated by SDS–15% PAGE. Proteins were transferred to 0.45-μm nitrocellulose membranes. Membranes were stained and photographed. Monoclonal antibody against the HIV-1 p24 antigen (NIH AIDS Reagent Program; supplied by Biomolecular Technologies Inc.) was used to detect changes in p24 levels in the cell lysates.
RESULTS
Previous studies indicated that LANA represses the HIV-1 LTR in an NF-κB-dependent fashion in COS-7 cells (44). Experiments in our laboratory indicate that these results differ in a number of human cells lines tested, including those known to be infected and capable of supporting replication of KSHV, as well as that of HIV-1 (10, 17, 20, 24, 45). A human B-cell line, a human monocytic cell line, and the HEK 293T cell line were used in our assays, and results from these experiments demonstrated that LANA could activate the HIV-1 LTR in these cells. Human monocytic and lymphocytic cells can be infected by HIV, as well as KSHV, and support the replication of these viruses (10, 22, 24). We used the human 293T cell line as another cell line that has been shown in at least two reports to support the replication of KSHV (17, 20). The fact that variations occur in the ability of LANA to activate or repress transcription in different cell lines suggests that LANA can associate with a number of transcription factors, altering their effects on transcription in the context of specific cell lines. As the cell lines used here are human cell lines, we believe that these results are important to document in terms of assigning a role for LANA in regulating the HIV-1 LTR in human cells.
LANA transactivates the HIV-1 LTR in HEK 293T cells.
To determine the effect of LANA on the regulation of the HIV-1 LTR, we performed transient-transfection experiments using a HIV-1 LTR luciferase reporter construct (pGL3-LAV) and a LANA expression vector (pA3M-LANA). HEK 293T cells were transiently transfected with a reporter plasmid and pA3M-LANA with or without pSV-Tat. The total amount of DNA was normalized with empty vector DNA for each transfection. Luciferase activity was measured 18 h after transfection. Under these conditions, Tat alone resulted in 4.4-fold activation of the HIV-1 promoter above basal levels in 293T cells. This transactivation was further enhanced at least twofold by the addition of LANA. Moreover, this activation was further increased by the addition of increasing amounts of LANA in the presence of Tat in a dose-dependent manner (Fig. 1B). When the levels of Tat remained constant, the luciferase activity increased to 9.0-fold and 13.4-fold above the basal activity when the amount of LANA was increased (Fig. 1B). Therefore, LANA is able to cooperate with Tat to increase transactivation of the HIV-1 LTR in 293T cells. LANA alone had little or no detectable effect on the transcription of the reporter construct in the absence of Tat in the 293T cell background (Fig. 1B). These results show that LANA can regulate the transcription of the HIV-1 LTR promoter in 293T cells and that the virus-encoded protein Tat is required for this activity.
To confirm the expression of LANA in our transfections, aliquots of 106 cells were removed and washed in PBS. The cell pellet was resuspended in SDS lysis buffer, heated to 95°C for 10 min, fractionated, and transferred to nitrocellulose membranes. LANA was clearly detected in the transfected cells by using anti-Myc monoclonal antibodies, which detect the carboxy-terminal Myc epitope tag of LANA, compared to the vector alone (Fig. 1C). LANA was not detected when 5 μg or less was transfected, as the signal was below the level of detection by Western blot assay. Similarly, little or no detectable signal for Tat was seen by Western blot assay and no change or an increase in the signal was seen in the presence of LANA (data not shown). However, Tat was clearly expressed, as indicated by the observed activation of the HIV-1 LTR (Fig. 1B).
LANA activates the HIV-1 LTR in BJAB cells.
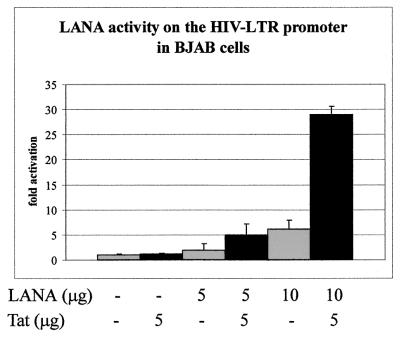
To determine whether the effect of LANA on the HIV-LTR is applicable to other human cell types, including B cells known to be associated with KSHV infection and disease, we performed similar transfection experiments with a human EBV-negative B cell line, BJAB. BJAB cells were transiently transfected with the reporter plasmid and pA3M-LANA with or without pSV-Tat. The total amount of DNA was normalized with empty vector DNA for each transfection, and luciferase activity was measured after 18 h of incubation. As in 293T cells, transcription was enhanced in BJAB cells in the presence of LANA and Tat, resulting in 5.0-fold and 29.0-fold increases with increasing amounts of LANA (Fig. 2). Interestingly, Tat alone did not activate the HIV-1 LTR as strongly in the BJAB cell line as in 293T cells. Furthermore, in contrast to our 293T cell experiments, LANA alone transactivated the HIV-1 LTR in the absence of Tat in the BJAB cell line. The luciferase activity was increased 1.9-fold and 6.9-fold when increasing amounts of LANA were transfected in the absence of Tat (Fig. 2). LANA alone was sufficient to transactivate the HIV-1 LTR at levels above those seen with the virus-encoded transactivator protein Tat under similar conditions. These results show that LANA functions to regulate transcription of the HIV-1 LTR promoter in BJAB cells both alone and in concert with the Tat protein and that this activity was synergized in the B-cell background. While LANA modulates the activity of the HIV-1 LTR promoter in both 293T and BJAB cells, there are distinct differences in the pattern of transactivation between the two cell lines. LANA can clearly transactivate the HIV-1 LTR in the absence of Tat in BJAB cells. Additionally, as seen quite strikingly in BJAB cells, the activity is more pronounced. Therefore, LANA is able to cooperate with Tat to synergize the transactivation activity of the HIV-1 LTR promoter, as well as activate the HIV-1 LTR in human B cells known to support the replication of KSHV and HIV (1, 22, 36, 45).
FIG. 2.
LANA activates the HIV-1 LTR in the Burkitt's lymphoma cell line BJAB. The subtype B HIV-1 LTR linked to a luciferase gene was used as the reporter in transient-transfection assays. Cells were transfected via electroporation with 5 μg of reporter plasmid and LANA and/or Tat constructs. Cells were normalized for the total amount of DNA with empty vector DNA for each experiment and also for transfection efficiency. Cells were harvested 18 h posttransfection. The results were plotted as the mean of three independent transfections.
Although HIV can be replicated in human B cells in vitro, the virus is not normally found in human B lymphocytes in infected individuals. Therefore, we wanted to determine if the effect we observed would be similar in U937 human monocytic cells known to be infected by HIV and able to support its replication (10, 22, 24). Our results demonstrate that LANA can activate the HIV-1 LTR in human monocytic cells in a manner similar to that seen in the other human cell lines described above (Fig. 3). As expected, Tat alone activated the HIV-1 LTR. However, in the presence of LANA, there was an approximately ninefold increase in activation. LANA alone had no observed effect on the promoter in these assays (Fig. 3). These results were similar to those obtained with 293T cells, suggesting that LANA may associate with similar cellular targets in these cell lines.
FIG. 3.
LANA activates the human monocytic cell line U937. The subtype B HIV-1 LTR linked to a luciferase gene was used as the reporter in transient-transfection assays. Cells were transfected via electroporation with 5 μg of reporter plasmid and LANA and/or Tat constructs. Cells were normalized for the total amount of DNA with empty vector DNA for each experiment and also for transfection efficiency. Cells were harvested 18 h posttransfection. The results were plotted as the mean of three independent transfections.
LANA is detected and localized to the nucleus in a manner similar to that seen in the KSHV-infected cell line BC-3.
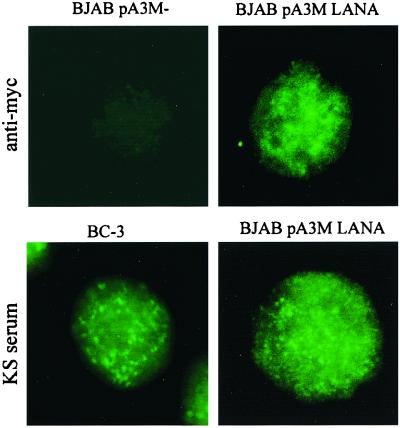
Immunofluorescence studies were carried out to determine if LANA is localized in the nucleus and expressed in a manner similar to that seen in KSHV-infected cells when it is transiently transfected in BJAB cells. The LANA expression construct used in our experiments is tagged at the carboxy terminus with a Myc epitope. LANA was detected in BJAB cells experimentally transfected with pA3M-LANA by using an anti-Myc antibody (Fig. 4, top right panel). BJAB cells transfected with the empty pA3M vector showed no staining with the anti-Myc antibody. BJAB cells transfected with pA3M-LANA also stained positive with human serum reactive against LANA (Fig. 4, bottom right panel). Immunofluorescence of a KSHV-infected cell line has previously established that LANA is distributed in a punctate pattern within the nucleus (23, 30, 48). Both anti-Myc and KS serum samples showed nuclear localization of LANA in the characteristic punctate pattern, indicating that in our transient reporter system, LANA is expressed in a manner similar to that observed in KSHV-infected cells. We also performed an immunofluorescence assay by using the same KS serum on the KSHV-positive, EBV-negative BCBL cell line BC-3 as a positive control (1). Immunofluorescence of BC-3 cells revealed a punctate staining pattern identical to that seen in the transiently transfected BJAB cells used in our experiments. These analyses indicate that LANA transiently transfected in this cell line was efficiently expressed and localized in the nucleus in a manner similar to that seen in KSHV-infected cells.
FIG. 4.
LANA is expressed and localizes to the nucleus in BJAB cells. BJAB cells transfected with pA3M-LANA were analyzed by immunofluorescence assay by using human KS serum reactive against LANA or an anti-Myc antibody. BJAB cells transfected with the empty pA3M vector served as a negative control. The BC-3 cell line (a KSHV-positive, EBV-negative BCBL line) was used as a positive control.
LANA transactivates the HIV-1 LTR with the core enhancer elements deleted.
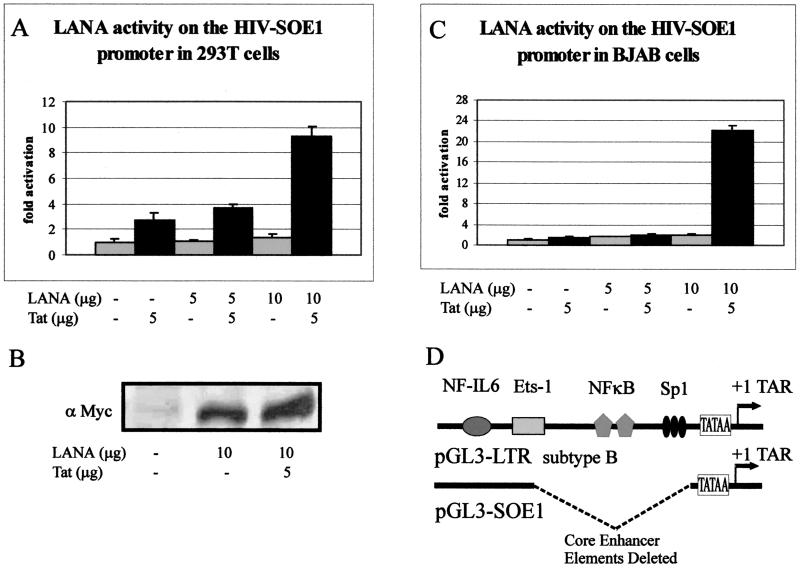
Transcriptional regulation of the HIV-1 LTR promoter is controlled by an enhancer region containing several transcription factor-binding sites (reviewed in references 38 and 46). The HIV-1 LTR used for our experiments is derived from the 5′ LTR of the subtype B HIV-1 genome (51). The major core transcription factor binding sites in the subtype B HIV-1 LTR include an NF-IL6, an Ets-1, two NFκB, and three Sp1 sites located upstream of the TATAA box and the TAR element (Fig. 5D). The LTR construct pGL3-SOE1 contains a 119-bp deletion in the enhancer region, which eliminates major Ets-1, NF-κB, and Sp1 sites (Fig. 5D). The nucleotide sequence in the NF-IL6 region is also altered to abolish the binding site (see Materials and Methods). This deletion construct was utilized to determine if LANA activity on the HIV-1 LTR promoter is dependent on the known transcription factors regulating the LTR and whether the activity would be abolished in the absence of these core enhancer elements.
FIG. 5.
LANA activates HIV-SOE1 with the core enhancer elements deleted in 293T cells (A) and is expressed when transiently transfected by Western blotting using the anti-Myc monoclonal antibody (B). This activity is also seen in BJAB cells (C). Transfections were carried out as described for the subtype B HIV-1 LTR construct. Basal reporter activity of the HIV-SOE1 promoter was reduced roughly 400-fold in 293T cells and about 23-fold in BJAB cells compared to the wild-type LTR construct. However, LANA activated the HIV-SOE1 reporter construct in both cell lines at fold activity similar to that seen with the parental HIV-1 LTR. Results were plotted as the mean of three independent experiments. (D) Schematic representation of the subtype B HIV-1 LTR constructs. The pGL3-SOE1 construct is derived from pGL3-LTR with a substitution of CCGCAGA for ATTTCAT at the NF-IL6 site and contains a 119-bp deletion just upstream of the TATAA box, which removes the entire core enhancer elements for Ets-1, NF-κB, and Sp1 (51). This removes the core enhancer elements but leaves the TAR element.
Transient transfections were performed by using this deletion reporter construct and pA3M-LANA, with or without pSV-Tat as described previously, for the full-length HIV-1 LTR construct. In 293T cells, Tat alone increased luciferase activity by 2.7-fold. In the presence of Tat, transcriptional activity was further increased 3.6-fold above the basal activity level and 9.3-fold with increased amounts of LANA (Fig. 5A). There was little or no detectable change in luciferase activity compared to basal levels when 293T cells were transfected with pA3M-LANA in the absence of Tat. Although LANA and Tat together showed a slightly lower increase in fold transactivation activity in the HIV-SOE1 construct compared to the wild-type HIV-1 LTR construct (compare Fig. 1 and 5A), the overall transcriptional activity was greatly decreased at basal levels for all combinations of LANA and Tat compared to the wild-type HIV-1 LTR construct. More specifically, the basal luciferase activity of the HIV-SOE1 construct was about 400-fold lower than that observed with the wild-type HIV-LTR construct. However, the fold activation over the promoter alone was surprisingly similar. Again, we analyzed the lysates as described above to detect the presence of LANA by Western blotting. LANA was detected, as expected (Fig. 5B); by an anti-Myc monoclonal antibody. Signals were clearly seen in the lanes where LANA was transfected (Fig. 5B). No signal was seen in the lane with the vector alone, where the reporter was transfected. Again, levels of Tat were undetectable due to the small amounts used in our assay and were unchanged in the presence of LANA (data not shown).
Based on the above-described results, we also wanted to determine if effects of the deletion were consistent in the B-cell background. In BJAB cells, the basal transcriptional activity of HIV-SOE1 was slightly reduced, to about 23-fold, compared to the basal level for the wild-type HIV-1 LTR (compare Fig. 2 and 5C). Activation by LANA alone in BJAB cells was significantly inhibited, with only a 1.7-fold and a 2.1-fold increase with increasing amounts of LANA in the absence of Tat (Fig. 5C) compared to the 1.9-fold and 6.9-fold activation seen in the wild-type HIV-1 LTR (compare Fig. 2 and 5C). In the presence of Tat, synergistic transactivation once again occurred with LANA, resulting in up to 22.1-fold greater activation than that achieved with the vector alone (Fig. 5C). These results suggest that although the activation seen in BJAB cells clearly involves the core enhancer elements, LANA is also capable of affecting regions or elements outside of the core enhancer elements. This region may include the TAR element, the target sequence for the HIV-encoded transactivator protein Tat, and other elements of the transcription activator complex involving the basal transcription factors.
LANA associates with Tat in transiently transfected 293T cells.
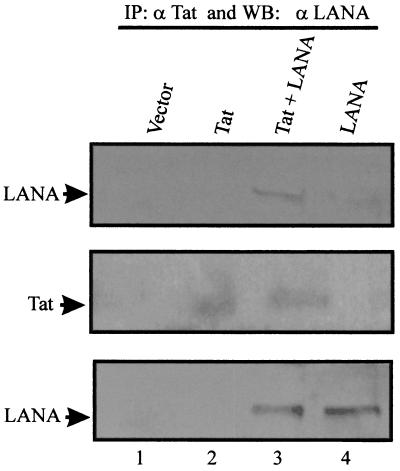
Since the transient-transfection assays indicated that LANA and Tat cooperate to activate the HIV-1 promoter, we further investigated this cooperation between LANA and Tat to determine if there is the potential for a direct association in complexes in cells. Tat and LANA expression vectors were transiently transfected into 293T cells. Cell lysates were immunoprecipitated with an anti-Tat antibody and Western blotted with human serum reactive to LANA to determine whether LANA and Tat can associate in human cells. Immunoprecipitation and Western blotting of complexes from cell lysates transfected with both LANA and Tat revealed that LANA is in a complex with Tat (Fig. 6, lane 3). Immunoprecipitation and Western blotting of cells transfected with the vector alone, Tat alone, or LANA alone showed no signals at the position where LANA migrates in the gel (Fig. 6, lanes 1, 2, and 4). Western blotting for Tat and LANA indicated that Tat was expressed in cells where the Tat expression construct was transfected and that LANA was expressed in a similar fashion in these transfections (Fig. 6, middle and lower panels). Therefore, LANA and Tat can associate in the same complex when transiently transfected and expressed in HEK 293T cells.
FIG. 6.
LANA associates with Tat transiently expressed in 293T cells. pA3M-LANA (25 μg) and pSVTat (25 μg) were transfected into 293T cells and incubated for 24 h. Cells were lysed, and the soluble fraction was incubated with anti-Tat monoclonal antibody. Bound complexes were collected and solubilized in SDS lysis buffer, fractionated by SDS–6% PAGE, and subjected to a Western blot (WB) assay for LANA using an adsorbed human polyclonal antiserum, which specifically recognizes LANA. Lane 3 of the top panel shows the specific LANA signal coimmunoprecipitated with Tat. The middle and lower panels show Western blot analyses of the lysate done to determine the expression of Tat and LANA, respectively. The arrows on the left indicate the positions of the specific signals for LANA and Tat in the Western blots. IP, immunoprecipitation.
Tat interacts with LANA at the carboxy terminus.
To determine if the association observed with LANA and Tat in cells was truly due to a direct interaction between the two proteins, we performed in vitro binding assays. Full-length LANA and truncated LANA (Fig. 7) were in vitro translated and labeled with [35S]methionine translabel. GST-Tat bound to glutathione Sepharose beads was incubated with the various polypeptides of LANA. The bound polypeptides were fractionated by SDS-PAGE, dried, and exposed to PhosphorImager screens (Molecular Dynamics). Full-length LANA bound to GST-Tat in this assay as well as did carboxy-terminal aa 762 to 1162 of LANA, which include the leucine zipper domain. Other polypeptides (aa 1 to 435, 1 to 756, and 1 to 950) did not bind in our assay. These results strongly demonstrate that LANA directly interacts with the HIV-1 Tat protein at its carboxy terminus (Fig. 7). Little or no detectable signal was seen when GST-Tat was incubated with the amino-terminal and central domains of LANA.
FIG. 7.
Tat interacts with the carboxy terminus of LANA. GST binding assays were performed by incubating 35S-labeled full-length (FL) LANA and truncated LANA with beads bound to GST-Tat. Complexes were analyzed by SDS-PAGE, dried, and exposed to PhosphorImager Screens (Molecular Dynamics). The specific amino acids for each truncation of LANA are indicated in the lower schematic showing the approximate region of binding for Tat. NLS, nuclear localization signal; AD, acidic domain.
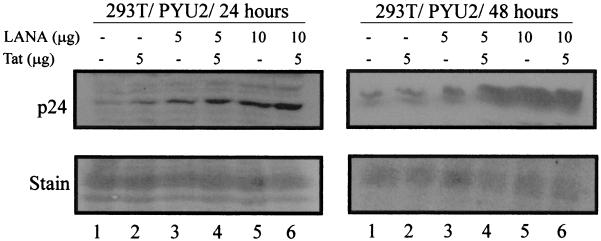
LANA induces expression of the HIV-1 p24 gag gene product when cotransfected with an infectious HIV-1 clone in 293T cells.
Based on the results of our reporter assays, we wanted to determine if the activation seen could also be translated to the level of the HIV-1 genome. Infectious HIV-1 clone pYU2 was transfected into 293T cells with LANA and Tat as described for the reporter assays. After 24 and 48 h, cells were harvested, lysed in SDS lysis buffer, boiled, and fractionated by SDS-PAGE. Fractionated proteins were transferred to nitrocellulose and Western blotted using a rabbit polyclonal antibody against Gag polypeptide p24. Our results convincingly show that after 24 h, the levels of p24 antigen increased in the presence of Tat (Fig. 8, lane 2). However, this increased levels of p24 was also seen in samples where LANA alone was transfected. Furthermore, the level was enhanced when LANA levels were increased and when LANA was cotransfected with Tat (Fig. 8). The levels of p24 antigen detected were further increased after 48 h (Fig. 8, right panels), consistent with increased production of p24, as well as an increased level of activation of the HIV-1 LTR. The membranes were stained with Ponceau to show that relatively equal amounts of protein had been loaded per lane in these experiments (Fig. 8, lower panels). These results were similar in multiple experiments and when using another infectious HIV-1 clone, p89.6 (data not shown). Therefore, we demonstrated that LANA can activate the HIV-1 LTR to drive the production of Gag polypeptides and that this effect is enhanced in cooperation with the HIV-1 Tat protein.
FIG. 8.
LANA induces the expression of Gag polypeptide p24 when transiently transfected in 293T cells. Ten million 293T cells were transfected with the Pyu2 infectious clone of HIV-1 with LANA and Tat. Five million cells were collected at 24 h posttransfection, and the remainder were collected after 48 h. Aliquots of 106 cells were washed and resuspended in SDS lysis buffer, boiled for 10 min, and subjected to SDS–15% PAGE. Proteins were transferred to nitrocellulose and Western blotted for p24 using the rabbit polyclonal antiserum at 1:50,000 in PBS. Signals were detected by using an HRP-protein A-conjugated secondary antibody at 1:5,000 in PBS and a chemiluminescence assay in accordance with the manufacturer's (Amersham) instructions. Activated membranes were then exposed to X-ray film. The top panels show the p24-specific signal, and the bottom panels show a section of the membrane Ponceau stained to demonstrate equivalent levels of protein loading in each assay.
DISCUSSION
By using transient-transfection assays, we have established that LANA is able to activate the HIV-1 LTR. This activation was documented in a number of human cell lines, the HEK 293T cell line, the B-cell line BJAB, and the human monocytic cell line U937. Our goal was to determine the effects of LANA on the HIV-1 LTR in the context of human cells known to be infected with KSHV and HIV. Our results indicate that in 293T and U937 cells, LANA cooperates with the HIV-encoded protein Tat to activate the HIV-1 promoter. Furthermore, in BJAB cells, we show that LANA is able to activate the HIV-1 LTR in the absence of Tat, as well as to synergistically activate this promoter in the presence of Tat. This suggests that LANA may act through at least two different mechanisms in BJAB cells. (i) LANA may be able to activate the promoter without the presence of Tat through molecules that target the core enhancer elements, and (ii) LANA is capable of enhancing the activity on the HIV-1 promoter through association with Tat, a known virus-encoded transactivator. KSHV has been shown to infect a variety of human cells, including endothelial cells and B lymphocytes (13). The differences seen between two different cell lines in our experiments suggest that the cellular environment, which contains cell type-specific proteins, may play an important contributing role in the activation of the HIV-1 LTR by LANA. LANA may interact with as yet unidentified cellular proteins which are expressed in B-lymphoid cell-derived cells such as BJAB cells and to a lesser extent in 293T cells or with cellular targets present in both 293T and BJAB cells to activate the HIV-1 LTR. These proteins may include, but are not limited to Sp1 and NF-κB, which target the core enhancer elements of the LTR. Moreover, LANA may interact with Tat in human monocytes to activate the HIV-1 LTR, as we have shown that in U937 cells, LANA activates the HIV-1 LTR. Moreover, HIV, as well as KSHV, infects human monocytes (10, 24). Therefore, this observation is physiologically relevant to the potential association of HIV and KSHV in HIV-positive patients with KS.
Recent studies with monkey COS-7 cells by Renne and colleagues found that transcription of the HIV-1 LTR was repressed in the presence of LANA (44). However, we have shown that LANA enhances the transcription of the HIV-1 LTR in the human U937, BJAB, and 293T cell lines. Differences in HIV-1 LTR activation by LANA clearly can be cell type specific, as indicated by the requirement of Tat for transactivation in U937 and 293T cells but not BJAB cells. Our experiments were performed with human cell lines with potentially greater cell specificity when comparing the cell types targeted by KSHV and HIV and relevant to understanding basic mechanisms of LANA–HIV-1 LTR interactions in vivo. It is clear from the previous studies that LANA represses the HIV-1 LTR in COS-7 cells (44). This result may reflect cell type specificity and the available cellular factors that are involved in the regulation of the HIV-1 LTR in these cells. Further experiments are needed to understand the extent of this variation in activities in these multiple cell lines. Our studies also demonstrate that LANA increases the levels of p24 when transiently transfected with an infectious HIV-1 clone. This strengthens the reporter assays and shows that the increase in activity results in an increase in HIV Gag transcription in the context of the HIV-1 genome.
Immunofluorescence assay of transfected BJAB cells for LANA showed the punctate nuclear localization pattern characteristic of LANA-specific staining, and this same pattern was observed in the KSHV-positive; EBV-negative cell line BC-3. This indicates that the transfected BJAB cells used in our studies express LANA and that it localizes to the nucleus in a manner similar to that seen in KSHV-infected cells. Furthermore, the results of the experiments presented here also address the role of Tat in the interaction between LANA and the HIV-1 LTR. Regardless of the cell type infected by KSHV, the role of Tat is a critical component, as it can be released from the cells where it is synthesized and taken up by other cells and is translocated to the nucleus, where it can affect the transcription of viral or cellular promoters (19, 50). Since Tat itself has been shown to transactivate cellular genes, including those for various cytokines, it may significantly influence the cellular environment including KSHV- and HIV-infected cells (reviewed in reference 50). Moreover, the fact that HIV and KSHV infect human monocytes provides strong evidence that LANA and Tat may cooperate to increase HIV replication in vivo, exacerbating the disease state (10, 24).
Notably, the synergistic activity with Tat is not abolished with the deletion construct. Therefore, we hypothesize that LANA at least partially transactivates the promoter by means other than signaling through the NF-IL6, Ets-1, NFκB, or Sp1 site. One possibility was that LANA affects the basal transcriptional machinery in cooperation with Tat to influence HIV gene expression. Transcriptional activation of the HIV-1 LTR by Tat involves interaction of Tat with the coactivator p300 or the related CREB binding protein CBP (25). Hence, LANA may work in concert with Tat to regulate the activity of the basal transcription complex through interaction with this complex. LANA has been shown to bind to ATF4, a member of the ATF-CREB family of transcription factors, in a yeast two-hybrid assay (35). It is possible that LANA interacts with other members of this family involved in activating the HIV-1 LTR. Recent studies with COS-7 cells found that LANA is able to transactivate a construct containing only the basic TATA box by using a chloramphenicol acetyltransferase assay, supporting the idea that LANA affects the basal transcriptional machinery (44). The experiments presented demonstrate that LANA is able to positively modulate the transcription of the HIV-1 LTR. Further studies intended to determine the specific cellular molecules involved in the mechanism of transactivation and how it is altered by the specific cellular milieu are under way.
Our in vitro studies further support the idea that LANA and Tat can functionally cooperate in cells, as observed by the luciferase studies. Immunoprecipitation using anti-Tat antibody and Western blotting with anti-LANA antibody produced a specific signal when both Tat and LANA expression vectors were transiently transfected into 293T cells. This also suggests that Tat and LANA can exist in a common complex in HIV- and KSHV-infected cells. Additionally, we demonstrated that Tat interacts with the carboxy terminus of LANA by in vitro binding assays. Formation of a complex with these two molecules is likely to be involved in the mechanism of synergistic HIV-1 promoter activation by LANA in cooperation with Tat. It is unknown what other proteins may contribute to the formation of this complex or the role they play in the activation of the HIV-1 LTR. Among the possibilities is the CBP/p300 coactivator protein, which has previously been shown to associate with Tat and modulate HIV-1 LTR activation (25). This study demonstrated through both luciferase reporter assays and immunoprecipitation that LANA associates and cooperates with Tat to influence the activity of the HIV-1 LTR. Furthermore, we found that LANA alone is able to induce activation of the HIV-1 promoter in the BJAB cell line through an unknown mechanism that does not involve Tat. Hence, further investigation to identify the specific cellular and viral proteins involved in this activation is critical. The details of such interactions will provide important clues as to how these two viruses may influence the disease state in dually infected immunocompromised individuals.
ACKNOWLEDGMENTS
We thank Elliott Kieff for the BJAB cell line and Kathy Collins for helphful suggestions. 293T cells were obtained from Jon Aster and Jeffrey Sklar. U937 cells were provided by Robert Thomas.
This work was supported by grants from the NIH (NCI CA072150-01 to E.S.R.) and from the Lymphoma and Leukemia Society of America. E.S.R. is a Scholar of the Lymphoma and Leukemia Society of America. M.A.C. is a fellow of the Lady Tata Memorial Trust. T.S.H. and M.A.C. are supported by the University of Michigan Medical Scientist Training Program (NIH T32 GM07863).
REFERENCES
- 1.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 2.Aster J C, Robertson E S, Hasserjian R P, Turner J R, Kieff E, Sklar J. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J Biol Chem. 1997;272:11336–11343. doi: 10.1074/jbc.272.17.11336. [DOI] [PubMed] [Google Scholar]
- 3.Ballestas M E, Kaye K M. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J Virol. 2001;75:3250–3258. doi: 10.1128/JVI.75.7.3250-3258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beral V. The epidemiology of cancer in AIDS patients. AIDS. 1991;5:S99–S103. doi: 10.1097/00002030-199101001-00014. [DOI] [PubMed] [Google Scholar]
- 5.Beral V. Epidemiology of Kaposi's sarcoma. Cancer Surv. 1991;10:5–22. [PubMed] [Google Scholar]
- 6.Beral V, Bull D, Jaffe H. Sexual spread of Kaposi's. Nurs Times. 1991;87:13. [PubMed] [Google Scholar]
- 7.Berkhout B, Jeang K-T. trans activation of human immunodeficiency virus type 1 is sequence specific for both the single-stranded bulge and loop of the trans-acting-responsive hairpin: a quantitative analysis. J Virol. 1989;63:5501–5504. doi: 10.1128/jvi.63.12.5501-5504.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkhout B, Silverman R H, Jeang K T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 9.Biggar R J, Rabkin C S. The epidemiology of AIDS—related neoplasms. Hematol Oncol Clin N Am. 1996;10:997–1010. doi: 10.1016/s0889-8588(05)70380-4. [DOI] [PubMed] [Google Scholar]
- 10.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Stürzl M. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan J, Pai S, Cotter M, Robertson E S. Distinct patterns of viral antigen expression in Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus coinfected body-cavity-based lymphoma cell lines: potential switches in latent gene expression due to coinfection. Virology. 1999;262:18–30. doi: 10.1006/viro.1999.9876. [DOI] [PubMed] [Google Scholar]
- 12.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman E, Knowles D M. Kaposi's sarcoma-associated herpesvirus: a lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Semin Diagn Pathol. 1997;14:54–66. . (Erratum, 14:161–162.) [PubMed] [Google Scholar]
- 14.Cesarman E, Nador R G, Aozasa K, Delsol G, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am J Pathol. 1996;149:53–57. [PMC free article] [PubMed] [Google Scholar]
- 15.Cotter M A, II, Robertson E S. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264:254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 16.Cotter M A, II, Robertson E S. Modulation of histone acetyltransferase activity through interaction of Epstein-Barr nuclear antigen 3C with prothymosin alpha. Mol Cell Biol. 2000;20:5722–5735. doi: 10.1128/mcb.20.15.5722-5735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delecluse H-J, Kost M, Feederle R, Wilson L, Hammerschmidt W. Spontaneous activation of the lytic cycle in cells infected with a recombinant Kaposi's sarcoma-associated virus. J Virol. 2001;75:2921–2928. doi: 10.1128/JVI.75.6.2921-2928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingwall C, Ernberg I, Gait M J, Green S M, Heaphy S, Karn J, Lowe A D, Singh M, Skinner M A, Valerio R. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci USA. 1989;86:6925–6929. doi: 10.1073/pnas.86.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R A, Wingfield P, Gallo R C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman K E, Friborg J, Jr, Kong W P, Woffendin C, Polverini P J, Nickoloff B J, Nabel G J. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N Engl J Med. 1997;336:163–171. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- 21.Friborg J, Jr, Kong W, Hottiger M O, Nabel G J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 22.Fritsch L, Marechal V, Schneider V, Barthet C, Rozenbaum W, Moisan-Coppey M, Coppey J, Nicolas J C. Production of HIV-1 by human B cells infected in vitro: characterization of an EBV genome-negative B cell line chronically synthetizing a low level of HIV-1 after infection. Virology. 1998;244:542–551. doi: 10.1006/viro.1998.9120. [DOI] [PubMed] [Google Scholar]
- 23.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, Detels R, Chang Y, Moore P S. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 24.Heinzinger N, Baca-Regen L, Stevenson M, Gendelman H E. Efficient synthesis of viral nucleic acids following monocyte infection by HIV-1. Virology. 1995;206:731–735. doi: 10.1016/s0042-6822(95)80097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hottiger M O, Nabel G J. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500–517. [PubMed] [Google Scholar]
- 27.Iscovich J, Boffetta P, Winkelmann R, Brennan P. Classic Kaposi's sarcoma as a second primary neoplasm. Int J Cancer. 1999;80:178–182. doi: 10.1002/(sici)1097-0215(19990118)80:2<178::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Iscovich J, Fischbein A, Fisher-Fischbein J, Freedman L S, Eng S M, Boffetta P, Vudovich A, Glasman C, Goldschmidt R, Livingston M, Heger-Maslansky B, Brennan P, Moore P S. Seroprevalence of Kaposi's sarcoma-associated herpesvirus in healthy adults in Israel. Anticancer Res. 2000;20:2119–2122. [PubMed] [Google Scholar]
- 29.Jones K A, Kadonaga J T, Luciw P A, Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986;232:755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- 30.Kedes D H, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J Clin Investig. 1997;100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. . (Erratum, 2:1041.) [DOI] [PubMed] [Google Scholar]
- 32.Krithivas A, Young D B, Liao G, Greene D, Hayward S D. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J Virol. 2000;74:9637–9645. doi: 10.1128/jvi.74.20.9637-9645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Hui H, Burgess C J, Price R W, Sharp P M, Hahn B H, Shaw G M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hahn B H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim C, Sohn H, Gwack Y, Choe J. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J Gen Virol. 2000;81:2645–2652. doi: 10.1099/0022-1317-81-11-2645. [DOI] [PubMed] [Google Scholar]
- 36.Monini P, Colombini S, Sturzl M, Goletti D, Cafaro A, Sgadari C, Butto S, Franco M, Leone P, Fais S, Melucci-Vigo G, Chiozzini C, Carlini F, Ascheri G, Cornali E, Zietz C, Ramazzotti E, Ensoli F, Andreoni M, Pezzotti P, Rezza G, Yarchoan R, Gallo R C, Ensoli B. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood. 1999;93:4044–4058. [PubMed] [Google Scholar]
- 37.Moore P A, Chang Y. Kaposi's sarcoma-associated herpesvirus. I. New York, N.Y: Churchhill Livingston; 1997. [Google Scholar]
- 38.Pereira L A, Bentley K, Peeters A, Churchill M J, Deacon N J. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res. 2000;28:663–668. doi: 10.1093/nar/28.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins N D, Agranoff A B, Pascal E, Nabel G J. An interaction between the DNA-binding domains of RelA(p65) and Sp1 mediates human immunodeficiency virus gene activation. Mol Cell Biol. 1994;14:6570–6583. doi: 10.1128/mcb.14.10.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins N D, Edwards N L, Duckett C S, Agranoff A B, Schmid R M, Nabel G J. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piolot T, Tramier M, Coppey M, Nicolas J-C, Marechal V. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J Virol. 2001;75:3948–3959. doi: 10.1128/JVI.75.8.3948-3959.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt G M, Simpson G R, Mittnacht S, Schulz T F. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J Virol. 1999;73:9789–9795. doi: 10.1128/jvi.73.12.9789-9795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radkov S A, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene hras transforms primary rat cells. Nat Med. 2000;6:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 44.Renne R, Barry C, Dittmer D, Compitello N, Brown P O, Ganem D. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J Virol. 2001;75:458–468. doi: 10.1128/JVI.75.1.458-468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roebuck K A, Saifuddin M. Regulation of HIV-1 transcription. Gene Expr. 1999;8:67–84. [PMC free article] [PubMed] [Google Scholar]
- 47.Schwam D R, Luciano R L, Mahajan S S, Wong L, Wilson A C. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J Virol. 2000;74:8532–8540. doi: 10.1128/jvi.74.18.8532-8540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson G R, Schulz T F, Whitby D, Cook P M, Boshoff C, Rainbow L, Howard M R, Gao S J, Bohenzky R A, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder R S, Weller I V, Weiss R A, Moore P S. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 49.Subramanian C, Cotter M A, 2nd, Robertson E S. Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23–H1: a molecular link to cancer metastasis. Nat Med. 2001;7:350–355. doi: 10.1038/85499. [DOI] [PubMed] [Google Scholar]
- 50.Watson K, Edwards R J. HIV-1-trans-activating (Tat) protein: both a target and a tool in therapeutic approaches. Biochem Pharmacol. 1999;58:1521–1528. doi: 10.1016/s0006-2952(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 51.Zachar V, Ebbesen P, Thomas R A, Zacharova V, Goustin A S. Basal and Tat-transactivated expression from the human immunodeficiency virus type 1 long terminal repeat in human placental trophoblast rules out promoter-enhancer activation as the partial block to viral replication. J Gen Virol. 1994;75:1461–1468. doi: 10.1099/0022-1317-75-6-1461. [DOI] [PubMed] [Google Scholar]
- 52.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]