Abstract
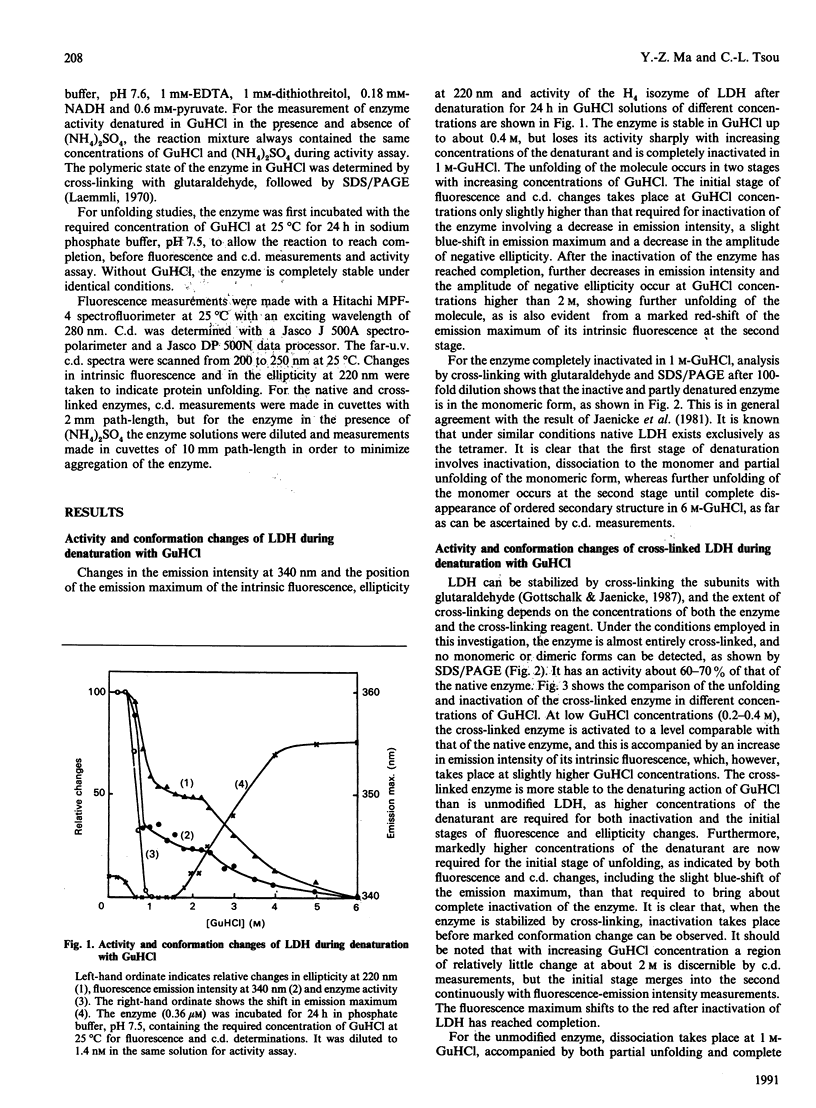
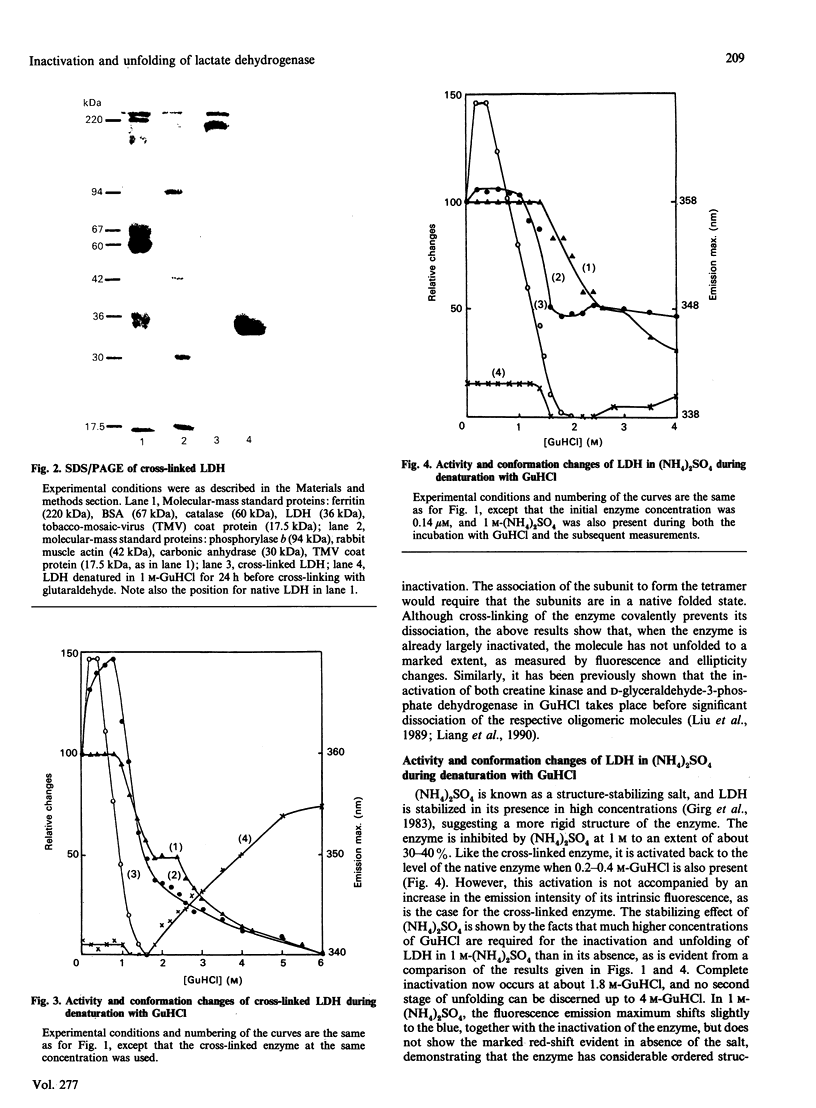
The inactivation and unfolding of lactate dehydrogenase (LDH) during denaturation by guanidinium chloride (GuHCl) under diverse conditions have been compared. Unfolding of the native conformation, as monitored by fluorescence and c.d. measurements, occurs in two stages with increasing GuHCl concentrations, and the inactivation approximately coincides with, but slightly precedes, the first stage of unfolding. The enzyme is inhibited to about 60-70% of its original activity by cross-linking with glutaraldehyde or in the presence of 1 M-(NH4)2SO4, with its conformation stabilized as shown by the requirement for higher GuHCl concentrations to bring about both inactivation and unfolding. Low concentrations of GuHCl (0.2-0.4 M) activate the cross-linked and the (NH4)2SO4-inhibited enzyme back to the level of the native enzyme. For the enzyme stabilized by (NH4)2SO4 or by cross-linking with glutaraldehyde, inactivation occurs at a markedly lower GuHCl concentration than that required to bring about its first stage of unfolding. It is concluded that the active site of LDH is situated in a limited region relatively fragile in conformation as compared with the molecule as a whole. The GuHCl activation of LDH stabilized in (NH4)2SO4 or by cross-linking with glutaraldehyde suggests that this fragility and consequently flexibility of the active site is required for its catalytic activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELLA E., MARKERT C. L. Dissociation of lactate dehydrogenase into subunits with guanidine hydrochloride. Biochem Biophys Res Commun. 1961 Nov 20;6:171–176. doi: 10.1016/0006-291x(61)90123-1. [DOI] [PubMed] [Google Scholar]
- BRAND L., EVERSE J., KAPLAN N. O. Structural characteristics of dehydrogenases. Biochemistry. 1962 May 25;1:423–434. doi: 10.1021/bi00909a009. [DOI] [PubMed] [Google Scholar]
- Biringer R. G., Fink A. L. Intermediates in the refolding of ribonuclease at subzero temperatures. 1. Monitoring by nitrotyrosine absorbance. Biochemistry. 1988 Jan 12;27(1):301–311. doi: 10.1021/bi00401a046. [DOI] [PubMed] [Google Scholar]
- Busby T. F., Atha D. H., Ingham K. C. Thermal denaturation of antithrombin III. Stabilization by heparin and lyotropic anions. J Biol Chem. 1981 Dec 10;256(23):12140–12147. [PubMed] [Google Scholar]
- Creighton T. E. Pathways and mechanisms of protein folding. Adv Biophys. 1984;18:1–20. doi: 10.1016/0065-227x(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Doster W., Hess B. Reversible solvent denaturation of rabbit muscle pyruvate kinase. Biochemistry. 1981 Feb 17;20(4):772–780. doi: 10.1021/bi00507a019. [DOI] [PubMed] [Google Scholar]
- Girg R., Rudolph R., Jaenicke R. The dimeric intermediate on the pathway of reconstitution of lactate dehydrogenase is enzymatically active. FEBS Lett. 1983 Oct 31;163(1):132–135. doi: 10.1016/0014-5793(83)81179-x. [DOI] [PubMed] [Google Scholar]
- Gottschalk N., Jaenicke R. Chemically crosslinked lactate dehydrogenase: stability and reconstitution after glutaraldehyde fixation. Biotechnol Appl Biochem. 1987 Oct;9(5):389–400. [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Koberstein R., Teuscher B. The enzymically active unit of lactic dehydrogenase. Molecular properties of lactic dehydrogenase at low-protein and high salt concentrations. Eur J Biochem. 1971 Nov 11;23(1):150–159. doi: 10.1111/j.1432-1033.1971.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Vogel W., Rudolph R. Dimeric intermediates in the dissociation of lactic dehydrogenase. Eur J Biochem. 1981 Mar;114(3):525–531. doi: 10.1111/j.1432-1033.1981.tb05176.x. [DOI] [PubMed] [Google Scholar]
- Johnson C. M., Price N. C. Denaturation and renaturation of the monomeric phosphoglycerate mutase from Schizosaccharomyces pombe. Biochem J. 1987 Jul 15;245(2):525–530. doi: 10.1042/bj2450525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang S. J., Lin Y. Z., Zhou J. M., Tsou C. L., Wu P. Q., Zhou Z. K. Dissociation and aggregation of D-glyceraldehyde-3-phosphate dehydrogenase during denaturation by guanidine hydrochloride. Biochim Biophys Acta. 1990 Apr 19;1038(2):240–246. doi: 10.1016/0167-4838(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Lin Y. Z., Liang S. J., Zhou J. M., Tsou C. L., Wu P. Q., Zhou Z. K. Comparison of inactivation and conformational changes of D-glyceraldehyde-3-phosphate dehydrogenase during thermal denaturation. Biochim Biophys Acta. 1990 Apr 19;1038(2):247–252. doi: 10.1016/0167-4838(90)90212-x. [DOI] [PubMed] [Google Scholar]
- Liu W., Tsou C. L. Activity change during unfolding of bovine pancreatic ribonuclease A in guanidine. Biochim Biophys Acta. 1987 Dec 18;916(3):455–464. doi: 10.1016/0167-4838(87)90192-0. [DOI] [PubMed] [Google Scholar]
- Mori E., Mikami B., Morita Y., Jirgensons B. Circular dichroism and the conformational properties of soybean beta-amylase. Arch Biochem Biophys. 1981 Oct 1;211(1):382–389. doi: 10.1016/0003-9861(81)90468-9. [DOI] [PubMed] [Google Scholar]
- Nieto M., Ayala J. A. Optical properties and denaturation by guanidinium chloride and urea of the adenosine triphosphatase of Micrococcus lysodeikticus. A comparison of four molecular forms of the enzyme. Biochem J. 1977 Feb 1;161(2):321–331. doi: 10.1042/bj1610321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H., Ikai A., Oshima T., Noda H. Reversible thermal unfolding of thermostable phosphoglycerate kinase. Thermostability associated with mean zero enthalpy change. J Mol Biol. 1977 Nov 5;116(3):429–442. doi: 10.1016/0022-2836(77)90078-x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Jaenicke R. Kinetics of reassociation and reactivation of pig-muscle lactic dehydrogenase after acid dissociation. Eur J Biochem. 1976 Apr 1;63(2):409–417. doi: 10.1111/j.1432-1033.1976.tb10242.x. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Vecchio G., Pasta P., Mazzola G., Carrea G. Multiparameter study of denaturation of 20 beta-hydroxysteroid dehydrogenase by urea in the presence of stabilizing agents. Biochim Biophys Acta. 1987 Aug 5;914(2):122–126. doi: 10.1016/0167-4838(87)90054-9. [DOI] [PubMed] [Google Scholar]
- Withka J., Moncuse P., Baziotis A., Maskiewicz R. Use of high-performance size-exclusion, ion-exchange, and hydrophobic interaction chromatography for the measurement of protein conformational change and stability. J Chromatogr. 1987 Jul 10;398:175–202. doi: 10.1016/s0021-9673(01)96504-5. [DOI] [PubMed] [Google Scholar]
- Xie G. F., Tsou C. L. Conformational and activity changes during guanidine denaturation of D-glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1987 Jan 5;911(1):19–24. doi: 10.1016/0167-4838(87)90265-2. [DOI] [PubMed] [Google Scholar]
- Yao Q. Z., Tian M., Tsou C. L. Comparison of the rates of inactivation and conformational changes of creatine kinase during urea denaturation. Biochemistry. 1984 Jun 5;23(12):2740–2744. doi: 10.1021/bi00307a032. [DOI] [PubMed] [Google Scholar]



