Abstract
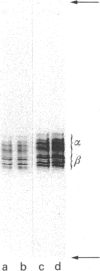
The principle proteins present in twice-cycled chick brain microtubule protein were characterized. The protein consists of a stoichiometric mixture of MAP2 and tubulin, together with a number of minor components. Its composition remains unaltered after a third cycle of assembly in a buffer supplemented with 67 mM-NaCl, with the exception of the phosphorylation of MAP2 to a low level (congruent to 1 mol.mol-1). The inclusion of 67 mM-NaCl dissociates the MAP2-tubulin oligomers, and restricts the assembly to the MAP2-dependent addition and loss of tubulin dimers, such that the assembly kinetics approximate to a simple pseudo-first-order reaction. The assembled microtubules exhibit dynamic instability, with no evidence for end-to-end annealing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce C. A., Barra H. S. Association of tubulinyl-tyrosine carboxypeptidase with microtubules. FEBS Lett. 1983 Jun 27;157(1):75–78. doi: 10.1016/0014-5793(83)81119-3. [DOI] [PubMed] [Google Scholar]
- Barton J. S., Vandivort D. L., Heacock D. H., Coffman J. A., Trygg K. A. Microtubule assembly kinetics. Changes with solution conditions. Biochem J. 1987 Nov 1;247(3):505–511. doi: 10.1042/bj2470505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley P. M., Butler F. M., Clark D. C., Manser E. J., Martin S. R. The assembly of microtubule protein in vitro. The kinetic role in microtubule elongation of oligomeric fragments containing microtubule-associated proteins. Biochem J. 1985 Apr 15;227(2):439–455. doi: 10.1042/bj2270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Kim H., Caceres A., Payne M. R., Rebhun L. I. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5613–5617. doi: 10.1073/pnas.81.17.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett C. R., Foster K. E., Johnson L., Gull K. Use of monoclonal antibodies to analyse the expression of a multi-tubulin family. FEBS Lett. 1985 Aug 5;187(2):211–218. doi: 10.1016/0014-5793(85)81244-8. [DOI] [PubMed] [Google Scholar]
- Bordas J., Mandelkow E. M., Mandelkow E. Stages of tubulin assembly and disassembly studied by time-resolved synchrotron X-ray scattering. J Mol Biol. 1983 Feb 15;164(1):89–135. doi: 10.1016/0022-2836(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Bré M. H., Karsenti E. Effects of brain microtubule-associated proteins on microtubule dynamics and the nucleating activity of centrosomes. Cell Motil Cytoskeleton. 1990;15(2):88–98. doi: 10.1002/cm.970150205. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K., Chapman R. The multiple phosphorylation of the microtubule-associated protein MAP2 controls the MAP2:tubulin interaction. Eur J Biochem. 1984 Jun 15;141(3):609–615. doi: 10.1111/j.1432-1033.1984.tb08236.x. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Modulation of the kinetic parameters of microtubule assembly by MAP-2 phosphorylation, the GTP/GDP occupancy of oligomers, and the tubulin tyrosylation status. Ann N Y Acad Sci. 1986;466:340–356. doi: 10.1111/j.1749-6632.1986.tb38405.x. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Nucleosidediphosphate kinase associates with rings but not with assembled microtubules. Eur J Biochem. 1981 Jul;117(3):515–519. doi: 10.1111/j.1432-1033.1981.tb06367.x. [DOI] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Phosphorylation of the microtubule-associated protein MAP2 by GTP. Biochem J. 1984 Dec 1;224(2):623–627. doi: 10.1042/bj2240623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. G. Kinetics of GTP hydrolysis during the assembly of chick brain MAP2-tubulin microtubule protein. Biochem J. 1991 Jul 1;277(Pt 1):239–243. doi: 10.1042/bj2770239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. G. Stoichiometry of estramustine phosphate binding to MAP2 measured by the disassembly of chick brain MAP2:tubulin microtubules. Cell Motil Cytoskeleton. 1990;17(3):167–173. doi: 10.1002/cm.970170304. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Hill T. L., Chen Y. Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proc Natl Acad Sci U S A. 1984 Feb;81(3):771–775. doi: 10.1073/pnas.81.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Cote R. H., Anderson C. F., Borisy G. G. Formulation of the general rate equation for subunit flux at steady-state. J Mol Biol. 1981 Aug 25;150(4):599–602. doi: 10.1016/0022-2836(81)90383-1. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Desbruyères E., Gros F., Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990 Jan 5;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Assembly of microtubules from preformed, ring-shaped protofilaments and 6-S tubulin. J Supramol Struct. 1974;2(2-4):393–411. doi: 10.1002/jss.400020228. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farrell K. W., Jordan M. A., Miller H. P., Wilson L. Phase dynamics at microtubule ends: the coexistence of microtubule length changes and treadmilling. J Cell Biol. 1987 Apr;104(4):1035–1046. doi: 10.1083/jcb.104.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Vallano M. L., DeLorenzo R. J. Phosphorylation of microtubule-associated protein 2 at distinct sites by calmodulin-dependent and cyclic-AMP-dependent kinases. J Neurochem. 1985 Sep;45(3):900–905. doi: 10.1111/j.1471-4159.1985.tb04078.x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Islam K., Burns R. G. Microtubules and nucleoside diphosphate kinase. Nucleoside diphosphate kinase binds to co-purifying contaminants rather than to microtubule proteins. Biochem J. 1985 Dec 15;232(3):651–656. doi: 10.1042/bj2320651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam K., Burns R. G. The GTP concentration modulates the association rate constant for microtubule assembly. Ann N Y Acad Sci. 1986;466:639–641. doi: 10.1111/j.1749-6632.1986.tb38441.x. [DOI] [PubMed] [Google Scholar]
- Islam K., Burns R. Multiple phosphorylation sites of microtubule-associated protein (MAP2) observed at high ATP concentrations. FEBS Lett. 1981 Jan 26;123(2):181–185. doi: 10.1016/0014-5793(81)80282-7. [DOI] [PubMed] [Google Scholar]
- Jameson L., Caplow M. Modification of microtubule steady-state dynamics by phosphorylation of the microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3413–3417. doi: 10.1073/pnas.78.6.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977 Nov 25;117(1):1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- Karr T. L., Purich D. L. A microtubule assembly/disassembly model based on drug effects and depolymerization kinetics after rapid dilution. J Biol Chem. 1979 Nov 10;254(21):10885–10888. [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C., Weingarten M., Gerhart J. C. Microtubules from mammalian brain: some properties of their depolymerization products and a proposed mechanism of assembly and disassembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1159–1163. doi: 10.1073/pnas.71.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristofferson D., Mitchison T., Kirschner M. Direct observation of steady-state microtubule dynamics. J Cell Biol. 1986 Mar;102(3):1007–1019. doi: 10.1083/jcb.102.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Johnson K. A., Borisy G. G. Role of tubulin-associated proteins in microtubule nucleation and elongation. J Mol Biol. 1977 Nov 25;117(1):33–52. doi: 10.1016/0022-2836(77)90021-3. [DOI] [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F., Simon C., Batelier G. Mechanism of tubulin assembly: role of rings in the nucleation process and of associated proteins in the stabilization of microtubules. Biochemistry. 1981 Aug 4;20(16):4709–4716. doi: 10.1021/bi00519a029. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell S. W., Grasser W. A., Baker H. N., Murphy D. B. The relative contributions of polymer annealing and subunit exchange to microtubule dynamics in vitro. J Cell Biol. 1987 Aug;105(2):863–874. doi: 10.1083/jcb.105.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell S. W., Grasser W. A., Murphy D. B. End-to-end annealing of microtubules in vitro. J Cell Biol. 1986 Feb;102(2):619–627. doi: 10.1083/jcb.102.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele R. B., Borisy G. G. Electron microscopy of metal-shadowed and negatively stained microtubule protein. Structure of the 30 S oligomer. J Biol Chem. 1978 Apr 25;253(8):2846–2851. [PubMed] [Google Scholar]
- Sullivan K. F., Wilson L. Developmental and biochemical analysis of chick brain tubulin heterogeneity. J Neurochem. 1984 May;42(5):1363–1371. doi: 10.1111/j.1471-4159.1984.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Symmons M. F., Burns R. G. Assembly of chick brain MAP2-tubulin microtubule protein. Analysis of tubulin subunit flux rates by immunofluorescence microscopy. Biochem J. 1991 Jul 1;277(Pt 1):245–253. doi: 10.1042/bj2770245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyama S., Bramblett G. T., Huang K. P., Flavin M. Calcium/phospholipid-dependent kinase recognizes sites in microtubule-associated protein 2 which are phosphorylated in living brain and are not accessible to other kinases. J Biol Chem. 1986 Mar 25;261(9):4110–4116. [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. Electron microscopy of MAP 2 (microtubule-associated protein 2). J Ultrastruct Res. 1982 Sep;80(3):374–382. doi: 10.1016/s0022-5320(82)80051-8. [DOI] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandosell F., Serrano L., Hernández M. A., Avila J. Phosphorylation of tubulin by a calmodulin-dependent protein kinase. J Biol Chem. 1986 Aug 5;261(22):10332–10339. [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Sandoval I. V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo. J Cell Biol. 1983 Nov;97(5 Pt 1):1467–1475. doi: 10.1083/jcb.97.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]