Abstract
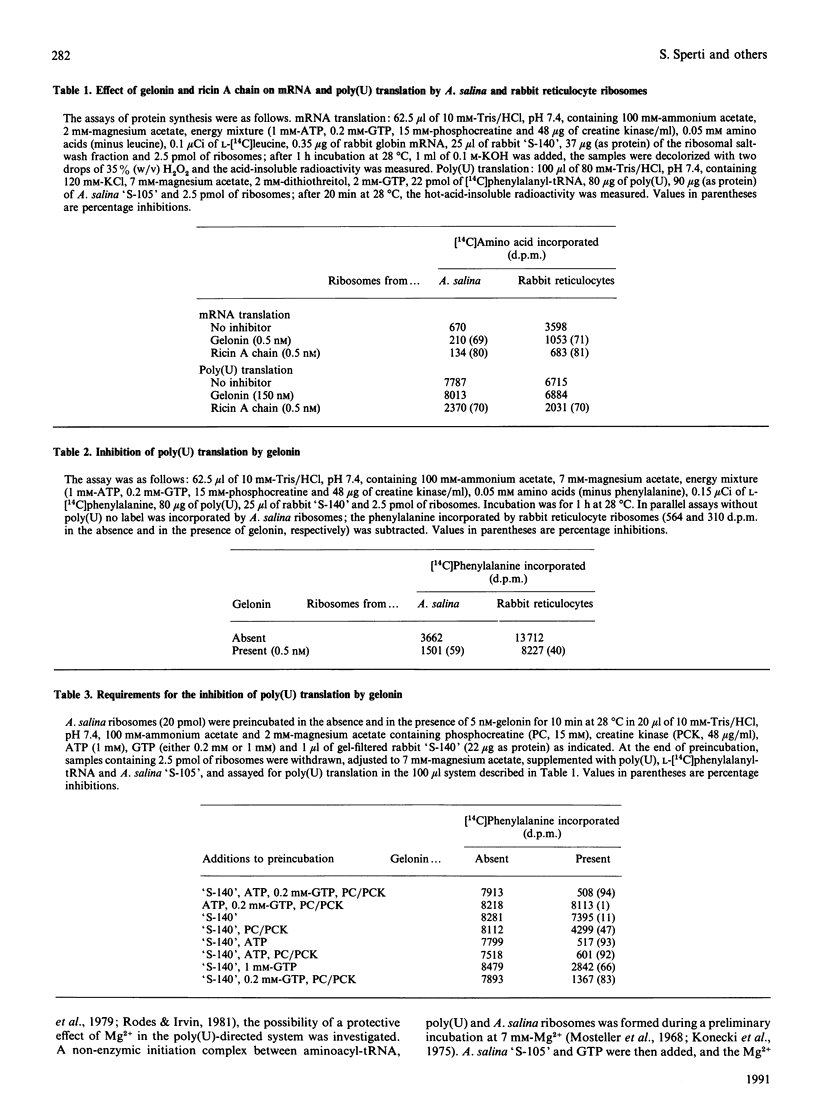
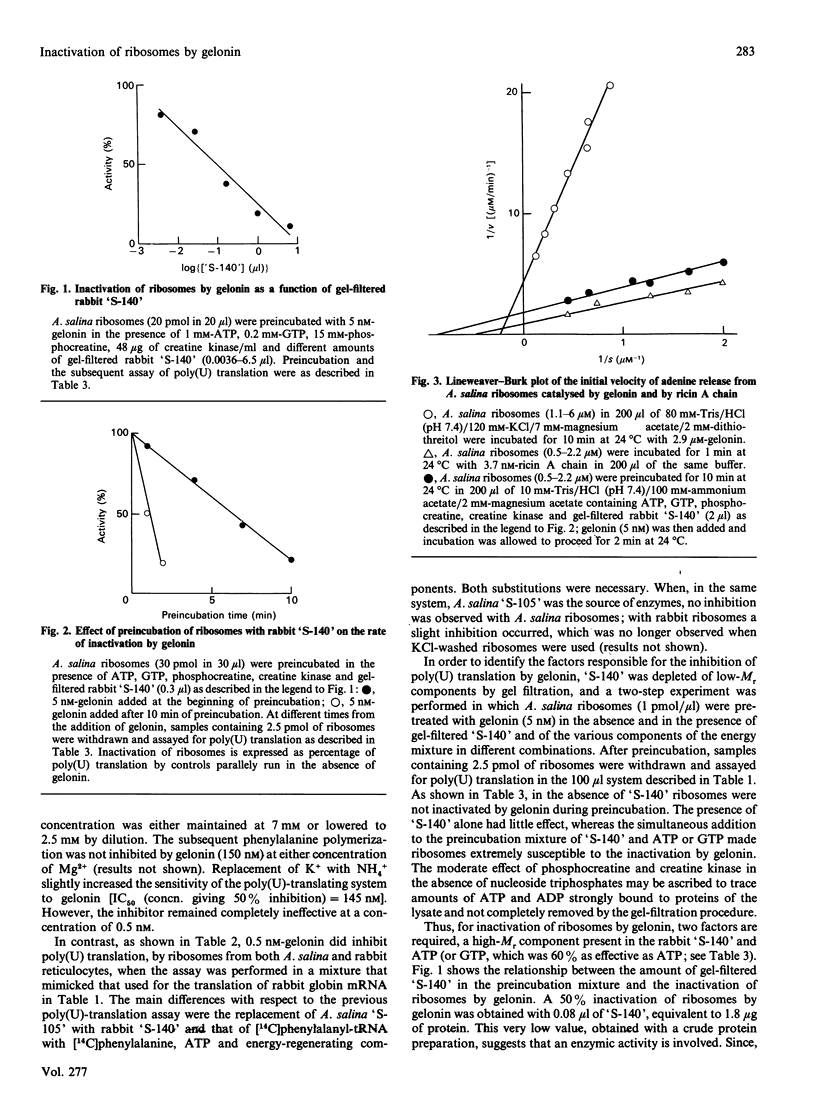
Inactivation of Artemia salina and rabbit ribosomes by gelonin requires ATP and a high-Mr factor present in the rabbit reticulocyte-lysate post-ribosomal supernatant. The kinetic constants of the gelonin-catalysed release of adenine from A. salina ribosomes are Km = 4.35 microM and Kcat. = 0.1 min-1 in the absence of cofactors, and Km = 1.15 microM and Kcat. = 108 min-1 in their presence. The last two values are similar to those measured for ricin A chain in the absence of cofactors (Km = 2.02 microM and Kcat. = 317 min-1).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. H., SCHWEET R. S. Synthesis of hemoglobin in a cell-free system. I. Properties of the complete system. J Biol Chem. 1962 Mar;237:760–767. [PubMed] [Google Scholar]
- Brigotti M., Rambelli F., Zamboni M., Montanaro L., Sperti S. Effect of alpha-sarcin and ribosome-inactivating proteins on the interaction of elongation factors with ribosomes. Biochem J. 1989 Feb 1;257(3):723–727. doi: 10.1042/bj2570723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley D. B., Hedblom M. L., Houston L. L. Protection and rescue of ribosomes from the action of ricin A chain. Biochemistry. 1979 Jun 12;18(12):2648–2654. doi: 10.1021/bi00579a034. [DOI] [PubMed] [Google Scholar]
- Coleman W. H., Roberts W. K. Factor requirements for the tritin inactivation of animal cell ribosomes. Biochim Biophys Acta. 1981 Jun 26;654(1):57–66. doi: 10.1016/0005-2787(81)90136-2. [DOI] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Lambert J. M. The site of action of six different ribosome-inactivating proteins from plants on eukaryotic ribosomes: the RNA N-glycosidase activity of the proteins. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1032–1036. doi: 10.1016/0006-291x(88)90733-4. [DOI] [PubMed] [Google Scholar]
- Hedblom M. L., Cawley D. B., Boguslawski S., Houston L. L. Binding of ricin A chain to rat liver ribosomes: relationship to ribosome inactivation. J Supramol Struct. 1978;9(2):253–268. doi: 10.1002/jss.400090210. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Konecki D., Kramer G., Pinphanichakarn P., Hardesty B. Polyamines are necessary for maximum in vitro synthesis of globin peptides and play a role in chain initiation. Arch Biochem Biophys. 1975 Jul;169(1):192–198. doi: 10.1016/0003-9861(75)90332-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCann W. P., Hall L. M., Siler W., Barton N., Whitley R. J. High-pressure liquid chromatographic methods for determining arabinosyladenine-5'-monophosphate, arabinosyladenine, and arabinosylhypoxanthine in plasma and urine. Antimicrob Agents Chemother. 1985 Aug;28(2):265–273. doi: 10.1128/aac.28.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Morrisey J., Hardesty B. Met-tRNA hydrolase from reticulocytes specific for Met-tRNA f Met on 40S ribosomal subunits. Arch Biochem Biophys. 1972 Sep;152(1):385–397. doi: 10.1016/0003-9861(72)90228-7. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Culp W. J., Hardesty B. Deacylated transfer ribonucleic acid as a factor for peptide chain initiation in rabbit reticulocyte systems. J Biol Chem. 1968 Dec 25;243(24):6343–6352. [PubMed] [Google Scholar]
- Ready M., Bird S., Rothe G., Robertus J. D. Requirements for antiribosomal activity of pokeweed antiviral protein. Biochim Biophys Acta. 1983 May 20;740(1):19–28. doi: 10.1016/0167-4781(83)90116-1. [DOI] [PubMed] [Google Scholar]
- Rodes T. L., 3rd, Irvin J. D. Reversal of the inhibitory effects of the pokeweed antiviral protein upon protein synthesis. Biochim Biophys Acta. 1981 Jan 29;652(1):160–167. doi: 10.1016/0005-2787(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Sierra J. M., Meier D., Ochoa S. Effect of development on the translation of messenger RNA in Artemia salina embryos. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2693–2697. doi: 10.1073/pnas.71.7.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperti S., Montanaro L., Rambelli F. Dye affinity chromatography of ricin subunits. Biosci Rep. 1986 Dec;6(12):1035–1040. doi: 10.1007/BF01141024. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Bailey S., Miller S. P., Bodley J. W. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988 Feb 25;16(4):1349–1357. doi: 10.1093/nar/16.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Hughes R. C. Specificity of ribosome-inactivating proteins with RNA N-glycosidase activity. Biochem J. 1989 Sep 15;262(3):1001–1002. doi: 10.1042/bj2621001b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Olsnes S., Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980 Jul 25;255(14):6947–6953. [PubMed] [Google Scholar]
- Zamboni M., Brigotti M., Rambelli F., Montanaro L., Sperti S. High-pressure-liquid-chromatographic and fluorimetric methods for the determination of adenine released from ribosomes by ricin and gelonin. Biochem J. 1989 May 1;259(3):639–643. doi: 10.1042/bj2590639. [DOI] [PMC free article] [PubMed] [Google Scholar]


