Abstract
Cytotoxic T lymphocytes (CTL) play a major role in the recovery from primary viral infections and the accompanying tissue injuries. However, it is unclear to what extent the two main cytolytic pathways, perforin-granzyme A and B exocytosis and Fas ligand (FasL)-Fas interaction, contribute to these processes. Here we have employed mouse strains with either spontaneous mutations or targeted gene defects in one or more components of either of the two cytolytic pathways to analyze the molecular basis of viral clearance and induction of hepatitis during lymphocytic choriomeningitis virus infection. Our results reveal that viral clearance is solely dependent on perforin but that virus-induced liver damage only occurs when both the FasL/Fas and the perforin pathways, including granzymes A and B, are simultaneously activated. The finding that development of hepatitis but not viral clearance is dependent on the concomitant activation of FasL-Fas and perforin-granzymes may be helpful in designing novel strategies to prevent hepatic failures during viral infections.
Viral clearance and tissue injury are thought to be two interrelated consequences of the cellular immune response, in particular, of cytolytic leukocytes, induced during viral infection (19, 27). However, despite detailed knowledge on the various cytolytic and noncytolytic functions of CD8+ cytotoxic T lymphocytes (CTL) and natural killer (NK) cells as observed in vitro, it is still unclear whether these cytolytic lymphocytes use the same or distinct effector pathways for virus elimination and disease pathogenesis. Mouse studies with natural pathogens, such as the cytopathic orthopoxvirus ectromelia (Ect) (5) and the noncytopathic viruses lymphocytic choriomeningitis virus (LCMV) (37, 74), mouse cytomegalovirus (34), and Sendai virus (11), as well as the non-mouse pathogens influenza virus (43, 68), herpes simplex virus (62), and hepatitis B virus (HBV) (19), have not revealed any common principle governing CTL and/or NK-mediated virus control and organ destruction (6, 26, 33, 46).
CTL can control virus infections and induce disease pathogenesis in two possible ways: by secreting cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) and by using either or both of two cytolytic pathways (29, 39). One, granule exocytosis, is mediated by perforin (perf [23, 56]) and serine proteases, i.e., granzymes (gzm's) A and B (64, 69) and appears to be the dominant mechanism in recovery from virus infections (26, 46). The second one, designated the Fas-mediated pathway, functions by binding of the Fas ligand (FasL) on CTL or NK cells to the Fas-receptor on target cells. Although this pathway was suggested to be involved in processes of homeostasis and tolerance (35, 50, 59), its definite biological role has yet to be uncovered (73).
Studies with perf−/− mice demonstrated the critical role of perf in recovery from LCMV (25, 71) and Ect (46, 57). However, the potential contribution of other CTL-associated effector functions could not be evaluated. Subsequent studies with mice lacking either gzmA, gzmB, or both showed that the two gzms are indispensable effector molecules acting in concert with perf in recovery from Ect infection (46). In the mouse cytomegalovirus system (58), both perforin and gzm's participate in the control of viral replication during acute infection. On the other hand, mice with mutations in either Fas or FasL, lpr and gld (36, 51), controlled LCMV like normal B6 mice, indicating that the Fas pathway is not essential for clearance of this virus (28).
In addition to the cytolytic pathways, a role for cytokines has been shown in the CTL-mediated control of certain virus infections (30, 44, 48). For LCMV, IFN-γ is essential in recovery from infection (53). It was shown to clear, together with TNF-α, virus from hepatocytes but not from nonparenchymal cells or splenocytes in the absence of cell lysis (18). Using a transgenic mouse model of HBV infection, it was demonstrated that the antiviral activity of CTL in the liver is mainly mediated by IFN-γ and TNF-α (20), most probably via induction of nitric oxide (21). Thus, different effector mechanisms of CTL and/or NK cells function in the control of different virus infections, and they may also be differentially active toward one and the same virus in distinct tissues during infection and at different stages of the pathologic process.
Besides recovery from viral infection, CTL are involved in virus-induced immunopathology (12). For hepatotropic LCMV and for HBV-induced hepatitis in mice, an association of liver cell damage with CTL activity in the respective organ has been documented (2, 16, 75). However, the precise contribution of individual CTL-derived effector molecules in this process is unclear. Although both IFN-γ and TNF-α have been implicated in virus- or drug-induced liver injury (2, 15, 67), causality for apoptotic cell death in vivo is absent. Evidence for virus-induced immunopathology as a result of cytolytic function has been obtained in a number of mouse models. perf−/− mice are resistant to LCMV-induced hepatitis (25) and CTL from perf−/− mice do not cause hepatitis in a mouse model of HBV-mediated hepatitis (20, 52). This contrasts with the observations that CTL-induced liver disease in HBV transgenic mice can be blocked by a soluble form of Fas (33) and that no hepatolysis occurred in the same mouse strain upon transfer of virus-specific CTL from FasL-mutant mice (52). These results indicate that Fas-dependent and perf–gzmA and -B-dependent mechanisms are critical for the induction of liver disease in viral infections and suggested that both cytolytic pathways may be required simultaneously for liver damage in vivo (33, 52).
In this report we identify the requirements for simultaneous activation of the two key pathways of cytotoxicity and the concomitant involvement of gzm's in CTL-mediated liver damage and virus clearance during LCMV infection.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6 (B6), gld and lpr (B6) mice, and mouse strains deficient for perf (perf−/−) (25), gzmA (gzmA−/−) (14), gzmB (gzmB−/−) (24), gzmA and -B (gzmA×B) (gzmA×B−/−) (63), and perf×gzmA×B (perf×A×B−/− [65]) were maintained at the Max-Planck-Institut für Immunbiologie, Freiburg, Germany, and the John Curtin School of Medical Research under pathogen-free conditions. All single knockout mice, except gzmB−/− mice (C57BL/6×129) (24), originated from B6 embryonic stem cells; mice with the deficient gzmB gene were bred onto the B6 background (eight backcrosses). Fas-deficient mice were generated by S. Nagata's lab (1) and were kindly provided by M. Klein (Zurich, Switzerland). Only mice of the same sex (male) were used in the experiments at 8 to 12 weeks of age. For detection of the respective mutations, DNA was analyzed by PCR, as described earlier (63). All mutant and normal B6 mice were analyzed for their gzmA, gzmB, perf, and Fas genotypes prior to experimentation, as described previously (63). Animal studies were conducted in accordance with the guidelines of Federation of European Laboratory Animal Science Associations.
Cells and cell cultures.
Cells were maintained in minimal essential medium medium (10% heat-inactivated fetal calf serum) as described earlier (63). The following cell lines were used as target cells: EL4.F15 (EL4; thymoma, H-2b) and B6:SV40 (transformed fibroblast, H-2b) (13).
Primary, LCMV-immune cytolytic effector cells were generated by infecting mice intraperitoneally (i.p.) with 105 PFU of LCMV strain WE as described earlier (17, 38). The cytolytic potential of splenocytes and liver-derived mononulear cells (MNC) was tested at day 8 postinfection (p.i.).
Liver-derived MNC were isolated as described previously (72). Accordingly, mice previously infected with LCMV for 8 to 15 days were anesthetized, and the liver was removed after total bleeding. The organ was cut into small pieces and pressed through a metal grid. MNC were enriched by Ficoll density (1.090 × g) separation.
Primary mouse hepatocytes were isolated by a two-step procedure with collagenase digestion as described earlier (60). Parenchymal hepatocytes were separated from nonparenchymal cells by centrifugation (300 rpm/3 min) and resuspended in William's medium. Cell viability was always 80% or higher, as assessed by trypan blue exclusion.
Disease model.
Mice were infected i.p. with 105 PFU of LCMV strain WE according to established protocols (17, 38) and analyzed for (i) virus titers in tissue, (ii) liver damage (transaminase activity in serum), (iii) histopathological alterations, (iv) phenotypic analysis of liver-associated leukocytes, (v) expression of transcripts specific for effector molecules in affected tissue by reverse transcription-PCR [RT-PCR]), and (vi) cytolytic potential of ex vivo-derived LCMV-specific CTL.
(i) Virus titers.
Aliquots of spleen and liver tissues were used for the determination of virus titers on MC57G fibroblasts as described elsewhere (3) (with the exception of that for staining, goat anti-rat immunoglobulin-alkaline phosphatase (Biozol, Eching, Germany) as secondary antibody and a specific phosphatase substrate (KPL) were used.
(ii) Determination of transaminase activity in serum.
For the detection of hepatocellular injury, the levels of liver enzymes, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in serum were monitored at various time points p.i., as described elsewhere (38, 75). Accordingly, mice were bled from the tail vein, and the activities of ALT and AST in serum were measured by an optimized method as recommended by the German Society for Clinical Chemistry with a Hitachi Modular Analyser (Roche Diagnostics).
(iii) Histopathological analysis.
Mice were infected i.p. with 105 PFU LCMV strain WE. At the indicated days p.i., the livers were removed and fixed in phosphate-buffered saline (PBS)-buffered formaldehyde (4%) before being embedded in paraffin. Sections were stained with hematoxylin-eosin and embedded in Entellan (Merck, Darmstadt, Germany). Samples were coded and examined under double-blind conditions.
(iv) Phenotypic analysis of liver-associated leukocytes.
Liver-derived MNC were isolated as described above. For phenotypic analysis, MNC were stained for the expression of CD3, CD4, CD8, B220, NK1.1, and Mac1 using the following monoclonal antibodies (MAbs; PharMingen, Hamburg, Germany): CD3-Bio (clone 500A2); CD4-FITC (clone H129.19), Mac1-FITC (clone M1/70), B220-PE (clone RA3-6B2), NK1.1-PE (clone PK-136), and CD8-APC (clone 53-6.7). The cell-bound MAb CD3-Bio was stained with SA-PerCP (Becton Dickinson). All fluorescence-conjugated MAbs were diluted to a concentration of 2 to 5 μg/ml in anti-FcR (clone 2.4G2) supernatant. Stained cells were fixed in PBS with 1% paraformaldehyde and examined with the FACSCalibur (Becton Dickinson). Data were analyzed by using CellQuest software.
(v) RT-PCR analysis.
Tissue samples were homogenized with the RiboLyser Cell disruptor (Hybaid, Heidelberg, Germany) for 15 s, in the presence of peqGOLDTriFast (PeqLab; Biotechnologie GmbH, Erlangen, Germany). RNA was isolated according to the instructions of the manufacturer. After treatment with DNase (Roche Molecular Biochemicals, Mannheim, Germany), 2 μl (100 ng) of RNA were incubated with oligo(dT)12–18 primer (500 ng; Pharmacia, Freiburg, Germany) and Omniscript RT (4 U; Qiagen, Hilden, Germany). RT-PCR was done as described by Qiagen. The cDNA was used as a template for PCR amplification of the following gene products: gzmA (13), gzmB (5′-ATG AAG ATC CTC CTG CTA CTG C-3′ and 5′-AGT CCG ACG ACT AGG AAC TAG C-3′; 135 bp), perf (5′-GAG CCC CTG CAC ACA TTA CTG GAA -3′ and 5′-ACA TTC TCA AAG TCC ATC T -3′; 380 bp), FasL (66), Fas (8), and IFN-γ and TNF-α (49). PCR products were amplified with 35 cycles (55°C), separated by 1.5% agarose gel electrophoresis, and visualized by ethidium bromide staining. Semiquantitative analysis of specific mRNA expression was done using the Light Cycler system. Accordingly, anti-Taq antibody was mixed with the DNA Master SYBR Green I, incubated in the dark for 5 min, and subsequently mixed with oligonucleotide primers according to the instructions of the manufacturer (Light Cycler-DNA Master SYBR Green I; Roche Molecular Biochemicals). These master mixes were pipetted into capillaries (Roche Molecular Biochemicals), and finally 2 μl (20 ng) of the above cDNA preparations (20 μl, final) was added, centrifuged, and analyzed according to manufacturer's instructions (Roche Molecular Biochemicals). The primer pairs used were as mentioned above exception for FasL (5′-TAG ACA GCA GTG CCA CTT CAT-3′ and 5′-AAC TCA CGG AGT TCT GCC AGT T-3′).
(vi) Cytotoxicity assay.
All cytotoxicity assays were performed in cell culture medium, in which fetal calf serum was replaced by bovine serum albumin (2 mg/ml). The 51Cr release assay was performed for 4 to 8 h, essentially as described previously (13). For detection of LCMV-specific CTL, target cells were either infected with LCMV or pretreated with 10−6 M synthetic peptide (p33) derived from the glycoprotein of LCMV for 1 h at 37°C, as described previously (54). In some experiments, effector cells (2 × 107 cells/ml) were pretreated with 200 nM concanamycin A (CMA; Sigma, Deisenhofen, Germany) for 2 h prior to the cytolytic assay. In other experiments, anti-FasL MAb (MFL-3; 10 μg/ml, final; PharMingen) or control antibody (hamster immunoglobulin G; Dianova, Hamburg, Germany) was added for 30 min to cell cultures prior to incubation. After the indicated time periods, 25 μl of supernatant was harvested onto a solid scintillator plate (LumaPlate; Packard, Dreieich, Germany), dried, and counted with TopCount (Packard). The percent specific lysis was calculated by use of the following formula: percent specific lysis = {(experimental release − medium release)/(maximum release [2% Triton ×−100] − medium release)} × 100. The data are the means of triplicate determinations. Standard errors of the mean were always <5%.
Statistical analysis.
Statistical significance was calculated by the two-tailed Student's t test for comparison of means with unequal variances. P values < 0.05 were considered statistically significant.
RESULTS
Involvement of perf and gzmA and -B and FasL/Fas in the clearance of LCMV from affected tissues.
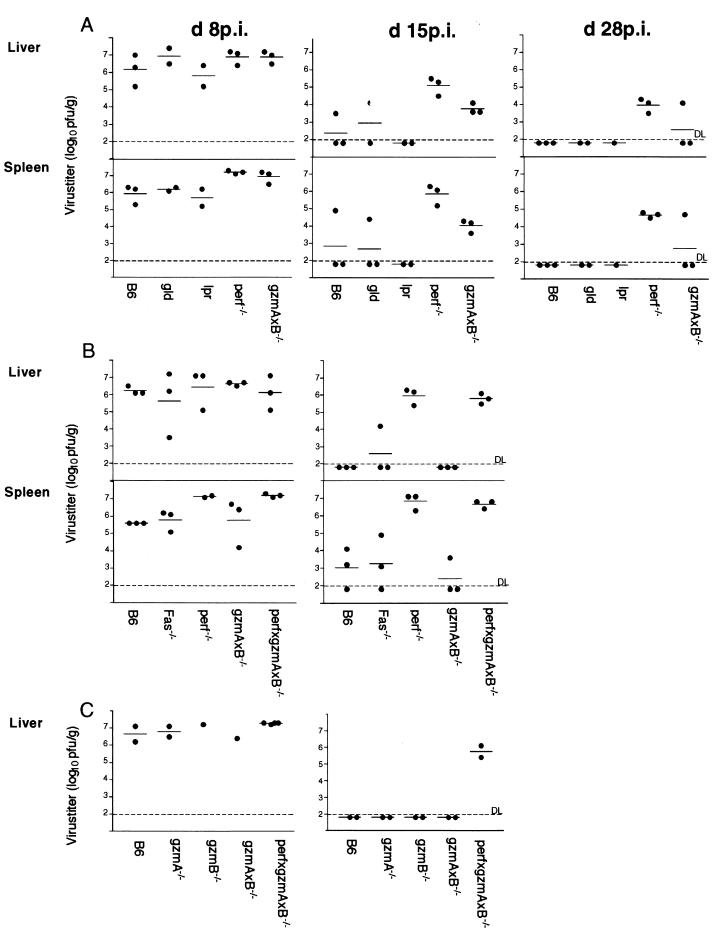
The importance of perf in recovery from LCMV infection has been documented (25, 71) and is corroborated and extended here. LCMV titers were monitored in liver and spleen of mouse strains deficient in one or more components of either the FasL/Fas or the exocytosis pathway at 8, 15, and /or 28 days p.i., (dpi) Figure 1 presents the combined results of three independent experiments (A to C). At 8 dpi (105 PFU of LCMV, i.p.), virus titers of 106 to 107 PFU/g of tissue were detected in the livers and/or spleens of normal B6 and all mutant mice independent of their defects in either FasL/Fas, perf, gzmA, and/or gzmB. At 15 and/or 28 dpi, virus titers were reduced by ca. 4 logs in both organs of B6 mice and mice defective in the Fas pathway (gld, lpr, and Fas−/−; Fig. 1A and B) or in either of the two gzm's (Fig. 1C; see also reference 13), with few exceptions. In gzmA×B−/− mice, virus titers were either reduced by 3 to 4 logs at 15 and 28 dpi (Fig. 1A) or undetectable in the liver and/or spleen at 15 dpi (Fig. 1B and C). This contrasts with perf-deficient mice, which had still high virus titers at 15 and 28 dpi, independent of gzmA and -B expression (perf−/− versus perf×gzmA×B−/−). Thus, early recovery from LCMV infection is mainly controlled by perf and, only marginally (if at all), by gzmA (13) and gzmB. The dispensability of the FasL/Fas pathway for LCMV clearance (28) is further supported by the fact that the kinetics of virus elimination in B6 mice was not altered upon treatment with anti-FasL MAbs, which were shown before to inhibit FasL/Fas-mediated cytolysis in vitro (data not shown). The finding that LCMV titers also decreased to some extent in the liver and spleens of perf−/− mice at 28 dpi suggests, however, that additional molecules, such as cytokines and/or neutralizing antibodies, contribute to the clearance of virus (18, 53, 55).
FIG. 1.
LCMV titers in the livers and spleens of infected B6 and mutant mice. Mice were infected i.p. with 105 PFU of LCMV and killed on the indicated days (A to C, independent experiments). Virus titers in the livers and spleens of individual mice were determined as described in Materials and Methods and are given as the log10 PFU/gram of tissue.
Involvement of perf and gzmA and -B and FasL/Fas in LCMV-induced hepatitis.
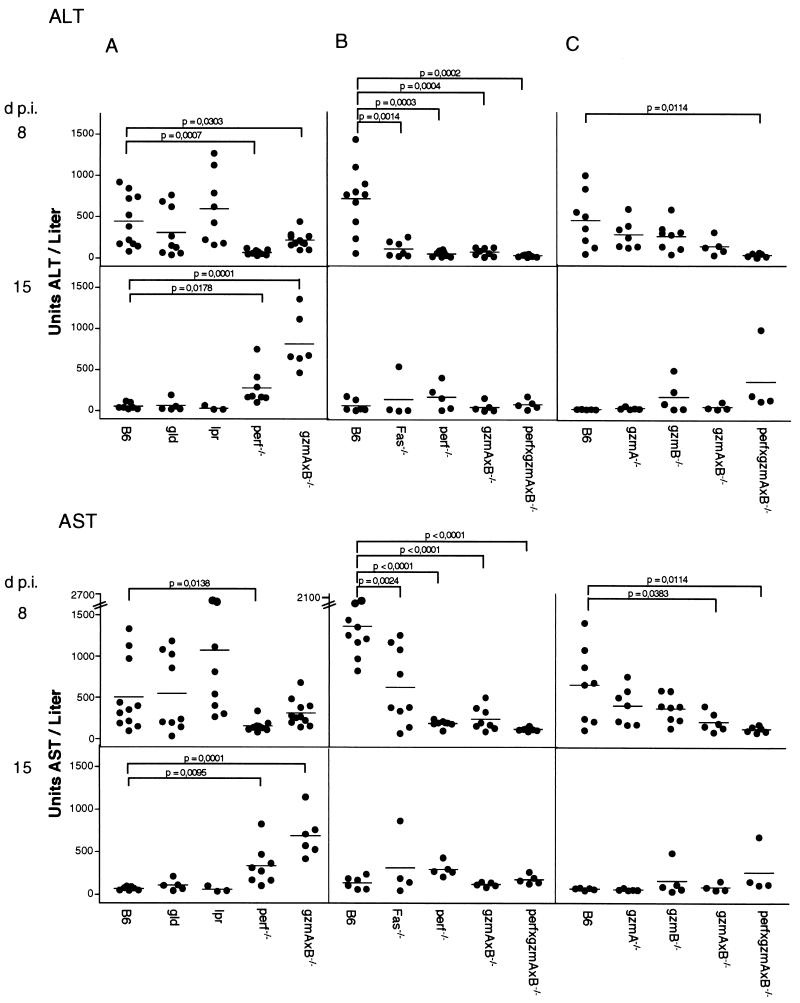
To examine the individual contribution(s) of FasL/Fas, perf, and the two gzm's in LCMV-induced hepatitis, we assayed the levels of liver enzymes, ALT and AST, which are indicators of hepatocyte damage early in the infection process (38, 75), in the sera of B6 and mutant mice (Fig. 2). As in earlier reports (38), B6 mice, after infection with 105 PFU of LCMV i.p., have detectable ALT and AST levels by as early as 3 dpi (data not shown), reaching peak values at 8 to 9 dpi, and declining to background levels by 15 dpi (Fig. 2). At 8 dpi, ALT and AST concentrations in the sera of lpr mice were a little higher and in in the sera of gld mice were a little lower, but the levels were not significantly different from those of B6 mice (Fig. 2A). In contrast, no or only marginal amounts of ALT and AST were detected at this time point in infected perf−/−, perf×gzmA×B−/−, and gzmA×B−/− mice in three independent experiments (Fig. 2). At 15 dpi, the levels of ALT and AST in serum were significantly higher in perf−/− and in gzmA×B−/− mice compared to B6 mice in one experiment (Fig. 2A) but not in two subsequent experiments (Fig. 2B and C). At 28 dpi ALT and AST activities in serum were back to the levels in uninfected mice for all five mouse strains tested (data not shown). The great variations in ALT and AST activities obtained between individual infected animals within a group is in line with previous observations and is probably influenced by the weight of the recipients (references 45 and 75; and our own observations).
FIG. 2.
Course of LCMV-induced hepatitis in B6 and mutant mice. Mice were infected i.p. with 105 PFU of LCMV (A to C, independent experiments). On the days indicated, mice were bled and the levels of ALT and AST in sera were determined as described in Materials and Methods. Dots indicate the individual mice. The mean ALT and AST values of uninfected B6 mice (n = 10) and mutant mice (at least five determinations for each strain) were for 13.6 ± 5.8 and 28.3 ± 1.3 U/liter, respectively.
The data obtained with lpr and gld mice suggested that early during LCMV infection perf is the principal cause of liver damage. However, the finding that in infected gzmA×B−/− mice, which express perf and FasL/Fas (63), only low levels of ALT/AST were observed in serum (Fig. 2) indicated the contribution of additional factors, including gzmA and/or gzmB, in the lysis of hepatocytes. Further evidence for the involvement of both gzm's in hepatolysis is provided in Fig. 2C. Compared to B6 mice, ALT and AST levels in serum are reduced in both infected gzmA−/− or gzmB−/− mice and are hardly detectable in gzmA×B−/− mice at 8 dpi.
To further explore the putative contribution of FasL/Fas-mediated lysis in LCMV-induced hepatitis, we examined mice with a deletion (Fas−/− [1]) rather than a mutation in Fas. In contrast to lpr and gld mice (Fig. 2A), at 8 dpi the ALT and AST levels in sera from infected Fas−/− mice were significantly lower than in those from infected B6 mice (Fig. 2B). These results were confirmed in two additional experiments (data not shown). Treatment of previously infected B6 mice with anti-FasL MAbs, which were shown to be effective in vitro (data not shown) but not with control antibody, led to highly variable reduction of ALT and AST activities in individual mice in two independent experiments (data not shown). Whether this was due to an insufficient amount of antibodies administered or to their altered potential in vivo is not known at present. These results indicate that in LCMV-induced hepatitis, both cytolytic pathways, including FasL/Fas, perf, gzmA, and gzmB, have to be functional in order to induce hepatolysis in the early stage of LCMV infection.
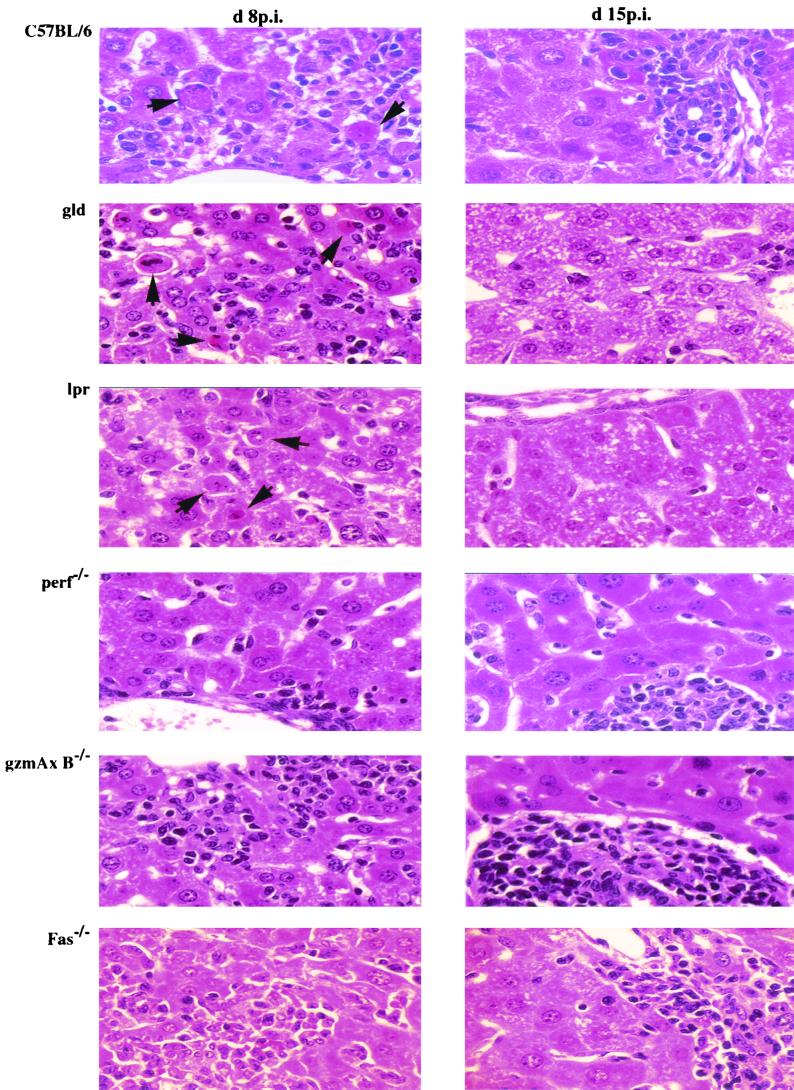
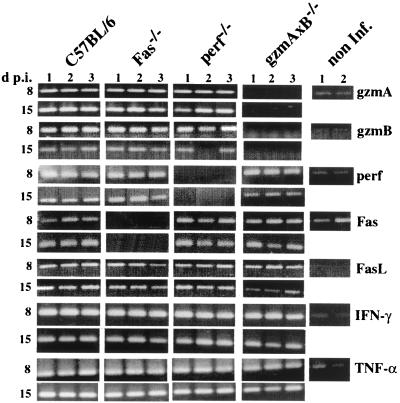
Histological examination of liver tissue from LCMV-infected B6 and mutant mice (lpr, gld, perf−/−, gzmA−/−, gzmB−/−, gzmA×B−/−, and Fas−/−) at 8 dpi revealed marked accumulations of MNC, which were mainly scattered in the liver lobules but were also seen in and around the portal tracts, comparable to the levels seen in control B6 mice (Fig. 3, Table 1). The amount of MNC as well as the ratios of T cells, B cells, NK cells, and Mac1+ cells recovered from infected liver tissues at day 8 p.i. were comparable in all mouse strains tested and were not affected by their genetic defects (Table 2). At this time point, many apoptotic hepatocytes were observed scattered in the liver lobules and the periportal tracts of B6 mice (as described before [2, 18, 38, 75]) and also in those of gld and lpr mice. In contrast, only few (if any) and significantly lower numbers of apoptotic cells were seen in livers from infected Fas−/−, perf−/−, gzmA−/−, gzmB−/−, or gzmA×B−/− mice (Fig. 3, Table 1). At 15 dpi, inflammatory infiltrations were greatly reduced in liver from infected B6, lpr, gld, Fas−/−, gzmA−/−, and gzmB−/− mice but were even more pronounced in those from perf−/− or gzmA×B−/− mice compared to that seen at 8 dpi (Fig. 3, Table 1). At this stage of disease, the uniform acinar distribution changed to a predominant portal pattern in all investigated mouse strains, as indicated by enlarged portal tracts densely filled with MNC (data not shown), as described before for B6 mice (38). Again, no major differences were found in the numbers of T cells, B cells, NK cells, and Mac1+ cells recovered from infected liver tissues of perf−/−, gzmA−/−, gzmB−/−, gzmA×B−/−, and Fas−/− mice, with a preponderance for CD8+ cells (>73%; data not shown). At this time point, no or only a few apoptotic hepatocytes were seen in the liver parenchyme of either B6 or any of the mutant mice, independent on the severity of the cellular infiltrate (Table 1). Finally, a comparison of the expression pattern of mRNAs specific for perf, gzm's, Fas, FasL, TNF-α, and IFN-γ in liver tissues at days 8 and 15 p.i., as determined by RT-PCR analysis, revealed the presence of all transcripts, except the specific deletions, in all mouse strains (Fig. 4). Quantitative analysis of the respective RT-PCR products using the Light Cycler system showed that all mRNAs in liver tissue, with the exception of Fas-specific transcripts, were strongly upregulated (>100-fold compared to the uninfected control) in the course of LCMV infection (at days 8 and 15 p.i., data not shown).
FIG. 3.
Histopathological analysis of the liver. B6, gld, lpr, perf−/−, gzmA×B−/−, and Fas−/− mice were infected i.p. with 105 PFU of LCMV per mouse. Liver sections stained with hematoxylin-eosin at 8 and 15 dpi. Arrowheads indicate the apoptotic cells. Magnification, ×400.
TABLE 1.
Histopathological findings in the liver tissues of LCMV-infected mice
| Mouse strain | Liver infiltrationa at:
|
Mean no. of apoptotic cells/10 HPFb ± SD (n) at:
|
||
|---|---|---|---|---|
| 8 dpi | 15 dpi | 8 dpi | 15 dpi | |
| B6 | ++ | + | 21,0 ± 9,1 (6) | 0,5 ± 0,8 (6) |
| gld | ++ | + | 58,0 ± 3,5 (2)∗ | 1,0 ± 1,4 (2) |
| lpr | + | +/− | 35,0 ± 28,3 (2) | 1,0 ± 1,4 (2) |
| Fas−/− | ++ | + | 0,7 ± 0,8 (3)∗ | 0,0 ± 0,0 (3) |
| gzmA−/− | ++ | + | 4,3 ± 1,7 (3)∗ | 0,5 ± 0,5 (2) |
| gzmB−/− | ++ | + | 1,0 ± 1,0 (2)∗ | 0,5 ± 0,5 (2) |
| gzmA×B−/− | ++ | ++ | 3,3 ± 3,4 (6)∗ | 0,0 ± 0,0 (6) |
| perf−/− | + | ++ | 1,0 ± 1,2 (5)∗ | 0,2 ± 0,4 (6) |
Inflammatory infiltrates were assessed on an arbitrary scale ranging from slight (+/−) to moderate (+) to marked (++).
HPF, high-power field (×400 enlargement). The mean number of apoptotic cells/10 different HPF/mouse (n = 2 to 6) is shown. ∗, the numbers of apoptotic cells counted were significantly lower or higher than in B6 mice.
TABLE 2.
Cell numbers and phenotypic characterization of liver-derived leukocytes from LCMV-infected B6 and knockout mice isolated at day 8 p.i.
| Mouse strain | Total cell no. (105) | % Cells staining positive for:
|
||||
|---|---|---|---|---|---|---|
| CD4a | CD8a | NK1.1b | B220 | Mac1 | ||
| B6 | 80 | 7 | 86 | 11 | 15 | 37 |
| 90 | 6 | 89 | 7 | 17 | 38 | |
| 90 | 8 | 90 | 5 | 17 | 38 | |
| Fas−/− | 70 | 5 | 93 | 6 | 11 | 42 |
| 70 | 4 | 94 | 7 | 11 | 35 | |
| 20 | 9 | 89 | 5 | 14 | 36 | |
| perf−/− | 10 | 12 | 86 | 12 | 17 | 40 |
| 100 | 8 | 89 | 7 | 13 | 44 | |
| 60 | 10 | 87 | 12 | 13 | 52 | |
| gzmA×B−/− | 20 | 10 | 87 | 5 | 16 | 43 |
| 100 | 8 | 89 | 4 | 11 | 50 | |
| 60 | 8 | 89 | 3 | 9 | 51 | |
| perf×gzmA×B−/− | 50 | 5 | 91 | 10 | 19 | 42 |
| 200 | 3 | 95 | 4 | 9 | 51 | |
| 50 | 8 | 89 | 6 | 17 | 42 | |
Double staining with anti-CD3.
A total of 20 to 40% of the NK cells are NKT cells (i.e., NK1.1+ plus CD3+).
FIG. 4.
Detection of gene expression in liver tissue from LCMV-infected mice. Total RNA was isolated from liver tissues of LCMV-infected B6, Fas−/−, perf−/−, and gzmA×B−/− mice at 8 and 15 dpi, as well as from uninfected B6 mice. Specific mRNA for the indicated parameters was analyzed by RT-PCR as described in Materials and Methods.
Cytotoxicity of ex vivo-derived LCMV-specific CTL against hepatocytes and nonhepatocytes.
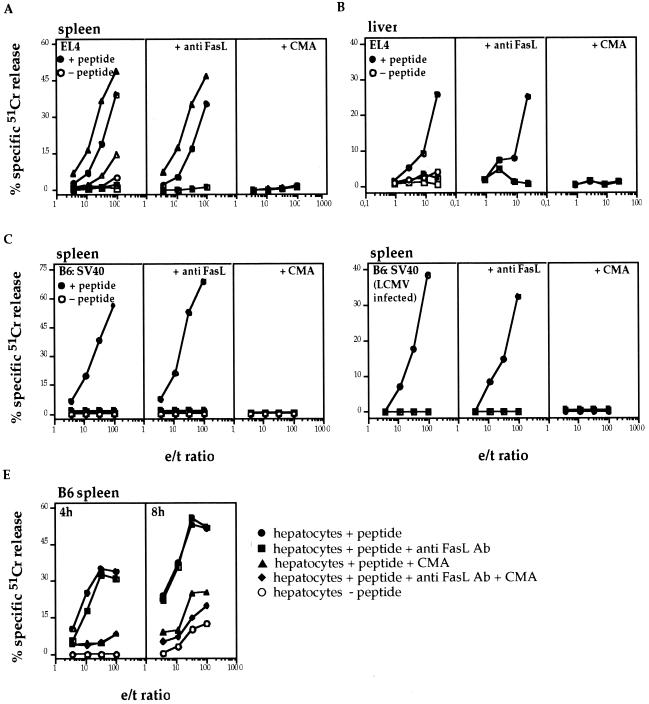
Finally, the contribution of the two main cytolytic pathways in the lysis of target cells by LCMV-immune spleen cells and/or liver-derived leukocytes was analyzed in vitro. As seen in Fig. 5A, immune spleen cells (8 dpi) from B6 and gld mice efficiently lysed LCMV peptide-pulsed EL4 target cells. The cytotoxic activities of the two effector populations were totally inhibited upon pretreatment of effector cells with CMA, which has been shown to selectively inhibit exocytosis (31), or with CMA + anti-FasL MAb, but not when effector and target cells were incubated in the presence of anti-FasL MAb alone. The fact that the same anti-FasL MAb was able to inhibit FasL/Fas-mediated cytolysis of perf×gzmA×B−/− CTL on Fas-transfected L1210 and L929 cells and that the EL4 target cells used express high amounts of functionally active Fas and are readily killed by the FasL+ hybridoma d11 (70; also data not shown) suggests a dominant role of perf in the lysis of target cells by ex vivo-derived LCMV-specific CTL. Similar results were obtained with immune B6 or perf×gzmA×B−/− splenocytes on peptide-loaded or LCMV-infected and Fas-expressing (data not shown) B6:SV40 target cells (Fig. 5C and D), as well as with liver-derived MNC from B6 mice, when tested on peptide-loaded EL4 target cells (Fig. 5B). In contrast, immune splenocytes (Fig. 5A and C) and liver-derived MNC (Fig. 5B) from perf×gzmA×B−/− mice were not or only marginally cytolytic for any of the peptide-pulsed or infected target cells, even after an extended incubation time (8 h; data not shown). Immune B6 splenocytes also showed a high cytolytic activity on peptide-pulsed ex vivo-derived Fas+ hepatocytes (Fig. 5E; 4 h), which further increased with time (8 h; Fig. 5E). Again, cytotoxicity on the latter target cells was not affected significantly in the presence of anti-FasL MAb at both time points (4 and 8 h). Pretreatment of B6 effector cells with CMA totally abrogated hepatolysis after 4 h but not after 8 h of incubation. The fact that application of both anti-FasL MAb and CMA led to a further reduction of cytolysis at 8 h indicates that at the later stages of cytolysis the FasL/Fas pathway contributes to hepatolysis in vitro.
FIG. 5.
In vitro cytolytic activity of LCMV-immune cells. B6 (circles; A to E), gld (triangles; A), and perf×gzmA×B−/− (squares; A to D) were infected i.p. with 105 PFU of LCMV. Primary LCMV-immune cells (B6, gld, and perf×gzmA×B−/−) were collected from the spleen and/or the liver on d 8 p.i. and then tested for their cytolytic activity. (A) Primary LCMV-immune cells from the spleen of B6, gld, and perf×gzmA×B−/− mice were tested on EL4 target cells with (solid symbols) or without (open symbols) previous incubation of the EL4 target cell with LCMV-specific peptide. Aliquots of effector cells were exposed to anti-FasL antibody (30 min) or, alternatively, CMA (2 h) to test which cytotoxic pathways are involved in the killing process. (B) Same as panel A, except that primary LCMV-immune cells from the liver of B6 and perf×gzmA×B−/− mice were used. (C) Same as panel A for B6 and perf×gzmA×B−/−, except that the target cells were B6:SV40. (D) Primary LCMV-immune cells from the spleen of B6 and perf×gzmA×B−/− mice were tested on LCMV-infected B6:SV40 target cells (24 h) alone or in the presence of anti-FasL antibody or CMA. (E) Primary LCMV-immune cells from the spleen of B6 mice were tested on ex vivo hepatocytes in the presence (●) or absence (○) of LCMV-specific peptide. The cytolytic activities were tested after 4 and 8 h. Aliquots of effector cells were exposed to anti-FasL antibody (■) or, alternatively, CMA (▴). Finally, effector cells were incubated with anti-FasL antibody and CMA together (⧫).
DISCUSSION
In this study we show that LCMV-induced hepatitis and recovery from infection are controlled by different cytolytic mechanisms of the same effector cell population. Whereas CTL-mediated lysis of hepatocytes is critically dependent on the simultaneous activation of the FasL/Fas and the perf-mediated cytolytic pathways of CTL, including functionally active gzmA and gzmB, virus elimination is mainly controlled by perf alone and is independent of FasL/Fas and of both gzm's.
Recent reports that perf−/− mice are unable to clear LCMV (25, 71) and Ect poxvirus (46) already emphasized the critical role of perf as an effector molecule in antiviral immune defense. The present findings that mice deficient for either Fas (lpr, Fas−/−) or FasL (gld) or those deleted of both gzmA and gzmB are able to recover from LCMV infection with kinetics similar to those for normal B6 mice demonstrate in addition that the two gzm's, as well as FasL/Fas-mediated cytotoxicity, are not essential for the eradication of LCMV. This contrasts with the results obtained with cytopathic Ect mousepox virus, which documented the absolute requirement for both gzm's, in addition to perf, in the recovery from infection (47). The differential involvement of gzm's in viral elimination may be due to the distinctly different replication strategies of the two viruses. Infection of target cells by LCMV and Ect and the release of progeny virus from infected cells, i.e., budding (LCMV) versus cytopathic release (Ect) are governed by distinct membrane-associated processes (9, 14, 40) which may be differentially affected by perf. In the absence of gzm's, integration of limiting amounts of perf into the cell membrane may lead to its mere perturbation rather than disintegration of target cells in vivo; such a process may interfere with the budding process of noncytopathic LCMV (9, 40) but not with the release of mature cytopathic Ect virions (14). In the latter case, virus replication and/or transmission may be blocked by gzm-induced and perf-facilitated fragmentation of DNA (4, 24, 47, 61, 63).
The decrease of LCMV titers observed between days 8 and 28 even in the absence of perf suggests that perf-independent mechanisms contribute to viral clearance at later time points of infection. In fact, there is ample evidence that recovery from LCMV infection is also controlled by neutralizing antibodies (55) and cytokines (18, 53). Thus, with respect to CTL- and NK-dependent mechanisms, LCMV replication seems to be curtailed by two independent mechanisms: a perf-mediated cytolytic process acting on infected nonparenchymal target cells and a noncytolytic process, elicited by IFN-γ and/or TNF-α, operating on infected hepatocytes (18).
The assumption that both the FasL/Fas and the perforin–gzmA and -B systems must be activated in CTL to kill hepatocytes during virus infection was suggested before (33, 52). This was inferred from the findings that the administration of soluble Fas (Fas-Fc) prevents T-cell-induced liver disease (33), that perf−/− mice are resistant to LCMV-induced hepatitis (25), and that CTL from perf−/− mice do not cause hepatitis in a mouse model of HBV-mediated hepatitis (20). Moreover, it was found that neither HBV-specific CTL from gld nor perf−/− mice were able to cause liver disease in Fas-competent HBV-transgenic animals (52). Furthermore, the fact that cotransfer of gld and perf−/− CTL also did not induce hepatolysis indicated that both death pathways—FasL/Fas and perf—must be executed by the same CTL to destroy liver cells in vivo (52). Our present findings that Fas−/− and gzmA×B−/− mice are as resistant as perf−/− mice to LCMV-induced hepatitis demonstrate that, during LCMV infections, CTL not only must use FasL and perf but also gzmA and gzmB in order to cause early hepatolysis in vivo.
The data on differential liver injury in normal versus mutant mice is corroborated by histopathological analysis. Apoptotic cells were only observed in the livers of LCMV-infected mice which also had increased levels of ALT and/or AST in serum, i.e. B6 mice, but not in the sera hepatitis-free perf−/−, Fas−/−, gzmA−/−, gzmB−/−, and gzmA×B−/− mice. This finding supports the concept that CTL-induced hepatitis is initiated by the induction of apoptosis in hepatocytes, followed by necrosis (7, 32) and, as outlined above, requires the concert action of FasL/Fas, perf, and the two gzm's. Further evidence for a critical role of these five components in LCMV-induced hepatitis is derived from the fact that neither apoptotic cell death nor ALT and AST release were observed in any of the infected knockout mice, even in cases in which liver lobules were characterized by massive infiltrations of inflammatory cells, including CD8+ effector cells (Fig. 3, 15 dpi; see also reference 42).
The finding that apoptotic cells were also observed in the liver lobules and periportal tracts of lpr and gld mice in numbers comparable to those in B6 mice, but not those in Fas−/− mice, requires some discussion. In lpr mice, the expression of Fas is greatly reduced but is not abolished (41). Thus, the limiting amount of functional active Fas expressed on the cell surface of hepatocytes seems to allow sufficient signaling via the two cytolytic pathways for cytolysis to occur. The significance of findings with gld mice, which carry a mutation in FasL resulting in an impaired ability to interact successfully with Fas to cause apoptosis, is less clear (36, 51). The possibility that a still-undiscovered ligand may substitute for FasL and trigger effector function via Fas cannot be discounted. Such apparent redundancy has recently been documented in the TNF-TNF receptor system (22).
The lack of liver disease observed in LCMV-infected Fas−/− mice or in LCMV-infected gzmA−/− and/or gzmB−/− mice is astounding, especially since their ex vivo-derived splenocytes and liver-derived leukocytes express high cytolytic activity on various target cells, including hepatocytes, even in the presence of blocking anti-FasL MAb (Fig. 5 and data not shown; see also reference 63). On the other hand, the absence of hepatitis in LCMV-infected perf−/− (Fig. 2 [25]) or perf×gzmA×B−/− mice is in line with the inability of their CTL to lyse Fas+ target cells, including hepatocytes, in vitro. These findings indicate that the in vitro cytotoxic assays do not necessarily reflect the complex cytolytic processes by which CTL elicit their function in vivo.
Why are both cytolytic pathways needed for the apoptosis of hepatocytes? The liver regulates vital processes such as the storage and release of energy; the neutralization of exogenous materials, including toxins and pathogens; and the clearance of effector lymphocytes (10). It is therefore possible that liver cells developed strategies to safeguard against cytolytic injury by CTL. The fact that hepatocytes are rapidly cleared from LCMV by a cytokine-induced and noncytopathic mechanism (18) supports this assumption.
The present finding that disease pathogenesis during LCMV infection, but not viral clearance, is dependent on simultaneous activation of the two main cytolytic pathways of CTL and/or NK cells, including FasL/Fas, perf, and both gzm's, may be of clinical value. It implies that virus-induced hepatitis can be efficiently treated or prevented by using appropriate drugs without affecting virus elimination.
ACKNOWLEDGMENTS
The generous help of Sabine MacNelly in preparing primary hepatocytes and of S. Merz in the analysis of liver enzymes is gratefully acknowledged. We also thank Ann Prins for excellent histological work and Jürgen Löhler and Hans-Eckart Schaefer for helpful discussions.
This study was in part supported by the Deutsche Forschungsgesellschaft (Si 214/7-1).
REFERENCES
- 1.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 2.Ando K, Moriyama T, Guidotti L G, Wirth S, Schreiber R D, Schlicht H J, Huang S N, Chisari F V. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel R M. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. . (Errata, 35:115, 1991, and 38:263, 1992.) [DOI] [PubMed] [Google Scholar]
- 4.Beresford P J, Xia Z, Greenberg A H, Lieberman J. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity. 1999;10:585–594. doi: 10.1016/s1074-7613(00)80058-8. [DOI] [PubMed] [Google Scholar]
- 5.Blanden R V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971;133:1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerny A, Chisari F V. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 7.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 8.Chu J L, Drappa J, Parnassa A, Elkon K B. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med. 1993;178:723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compans R W. Arenavirus ultrastructure and morphogenesis. New York, N.Y: Plenum Press, Inc.; 1993. [Google Scholar]
- 10.Crispe I N, Dao T, Klugewitz K, Mehal W Z, Metz D P. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 11.Doherty P C, Topham D J, Tripp R A, Cardin R D, Brooks J W, Stevenson P G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 12.Doherty P C, Zinkernagel R M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19:89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 13.Ebnet K, Hausmann M, Lehmann-Grube F, Müllbacher A, Kopf M, Lamers M, Simon M M. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995;14:4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenner F, Wittek R, Dumbell K R. The orthopoxviruses. San Diego, Calif: Academic Press; 1989. [Google Scholar]
- 15.Gilles P N, Guerrette D L, Ulevitch R J, Schreiber R D, Chisari F V. HBsAg retention sensitizes the hepatocyte to injury by physiological concentrations of interferon-gamma. Hepatology. 1992;16:655–663. doi: 10.1002/hep.1840160308. [DOI] [PubMed] [Google Scholar]
- 16.Gossmann J, Lohler J, Utermohlen O, Lehmann-Grube F. Murine hepatitis caused by lymphocytic choriomeningitis virus. II. Cells involved in pathogenesis. Lab Investig. 1995;72:559–570. [PubMed] [Google Scholar]
- 17.Grossman W J, Kimata J T, Wong F H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti L G, McClary H, Loudis J M, Chisari F V. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. J Exp Med. 2000;191:1247–1252. doi: 10.1084/jem.191.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayder H, Blanden R V, Korner H, Riminton D S, Sedgwick J D, Mullbacher A. Adenovirus-induced liver pathology is mediated through TNF receptors I and II but is independent of TNF or lymphotoxin. J Immunol. 1999;163:1516–1520. [PubMed] [Google Scholar]
- 23.Henkart P A. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 24.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 25.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 26.Kägi D, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. Lymphocyte-mediated cytotoxicity in vitro and in vivo: mechanisms and significance. Immunol Rev. 1995;146:95–115. doi: 10.1111/j.1600-065x.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 27.Kägi D, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 28.Kägi D, Seiler P, Pavlovic P, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. The roles of perforin- and fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 29.Kägi D, Vignaux F, Ledermann B E A. Fas and perforin pathways as major mechanisms of T-cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 30.Karupiah G R, Buller M L, van Rooijen N, Duarte C J, Chen J H. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataoka T, Shinohara N, Takayama, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 32.Kataoka T, Takaku K, Magae J, Shinohara N, Takayama H, Kondo S, Nagai K. Acidification is essential for maintaining the structure and function of lytic granules of CTL. Effect of concanamycin A, an inhibitor of vacuolar type H(+)-ATPase, on CTL-mediated cytotoxicity. J Immunol. 1994;153:3938–47. [PubMed] [Google Scholar]
- 33.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 34.Koszinowski U H, Del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 35.Krammer P H. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–209. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 36.Krammer P H, Dhein J, Walczak H, Behrmann I, Mariani S, Matiba B, Fath M, Daniel P T, Knipping E, Westendorp M O, et al. The role of APO-1-mediated apoptosis in the immune system. Immunol Rev. 1994;142:175–191. doi: 10.1111/j.1600-065x.1994.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann-Grube F, Assmann U, Loliger C, Moskophidis D, Lohler J. Mechanism of recovery from acute virus infection. I. Role of T lymphocytes in the clearance of lymphocytic choriomeningitis virus from spleens of mice. J Immunol. 1985;134:608–615. [PubMed] [Google Scholar]
- 38.Loehler J, Gossmann J, Kratzberg T, Lehmann-Grobe F. Murine hepatitis caused by lymphocytic choriomeningitis virus. I. The hepatic lesions. Lab Investig. 1994;70:263–278. [PubMed] [Google Scholar]
- 39.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 40.Mannweiler K, Lehmann-Grube F. Electron microscopy of LCM virus-infected L cells. Berlin, Germany: Springer-Verlag; 1973. [Google Scholar]
- 41.Mariani S M, Matiba B, Armandola E A, Krammer P H. The APO-1/Fas (CD95) receptor is expressed in homozygous MRL/lpr mice. Eur J Immunol. 1994;24:3119–3123. doi: 10.1002/eji.1830241231. [DOI] [PubMed] [Google Scholar]
- 42.Matloubian M, Suresh M, Glass A, Galvan M, Chow K, Whitmire J K, Walsh C M, Clark W R, Ahmed R. A role for perforin in downregulating T-cell responses during chronic viral infection. J Virol. 1999;73:2527–2536. doi: 10.1128/jvi.73.3.2527-2536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMichael A. Cytotoxic T lymphocytes specific for influenza virus. Curr Top Microbiol Immunol. 1994;189:75–91. doi: 10.1007/978-3-642-78530-6_5. [DOI] [PubMed] [Google Scholar]
- 44.Moskophidis D, Battegay M, Bruendler M A, Laine E, Gresser I, Zinkernagel R M. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–1955. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müllbacher A, Ebnet K, Blanden R V, Stehle T, Museteanu C, Simon M M. Granzyme A is essential for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müllbacher A, Tha Hla R, Museteanu C, Simon M M. Perforin is essential for the control of ectromelia virus but not related poxviruses in mice. J Virol. 1999;73:1665–1667. doi: 10.1128/jvi.73.2.1665-1667.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müllbacher A, Waring P, Tha Hla R, Tran T, Chin S, Stehle T, Museteanu C, Simon M M. Granzymes are the essential downstream effector molecules for the control of primary infections by cytolytic leukocytes. Proc Natl Acad Sci USA. 1999;96:13950–13955. doi: 10.1073/pnas.96.24.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 49.Murray L J, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990;20:163–170. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 50.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 51.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 52.Nakamoto Y, Guidotti L G, Pasquetto V, Schreiber R D, Chisari F V. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J Immunol. 1997;158:5692–5697. . (Erratum, 163:1092, 1999.) [PubMed] [Google Scholar]
- 53.Nansen A, Jensen T, Christensen J P, Andreasen S O, Ropke C, Marker O, Thomsen A R. Compromised virus control and augmented perforin-mediated immunopathology in IFN-gamma-deficient mice infected with lymphocytic choriomeningitis virus. J Immunol. 1999;163:6114–6122. [PubMed] [Google Scholar]
- 54.Pircher H, Moskophidis D, Rohrer U, Burki K, Hengartner H, Zinkernagel R M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 55.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Hengartner H, Zinkernagel R M. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Podack E R, Hengartner H, Lichtenheld M G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 57.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 58.Riera L, Gariglio M, Valente G, Mullbacher A, Museteanu C, Landolfo S, Simon M M. Murine cytomegalovirus replication in salivary glands is controlled by both perforin and granzymes during acute infection. Eur J Immunol. 2000;30:1350–1355. doi: 10.1002/(SICI)1521-4141(200005)30:5<1350::AID-IMMU1350>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 59.Rouvier R, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T-cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seglen P O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973;82:391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- 61.Shresta S, Graubert T A, Thomas D A, Raptis S Z, Ley T J. Granzyme A initiates an alternative pathway for granule-mediated apoptosis. Immunity. 1999;10:595–605. doi: 10.1016/s1074-7613(00)80059-x. [DOI] [PubMed] [Google Scholar]
- 62.Simmons A, Tscharke D, Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 63.Simon M M, Hausmann M, Tran T, Ebnet K, Tschopp J, ThaHla R, Mullbacher A. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A × B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J Exp Med. 1997;186:1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon M M, Kramer M D. Granzyme A. Methods Enzymol. 1994;244:68–79. doi: 10.1016/0076-6879(94)44006-9. [DOI] [PubMed] [Google Scholar]
- 65.Simon M M, Waring P, Lobigs M, Nil A, Tran T, Hla R T, Chin S, Mullbacher A. Cytotoxic T cells specifically induce Fas on target cells, thereby facilitating exocytosis-independent induction of apoptosis. J Immunol. 2000;165:3663–3672. doi: 10.4049/jimmunol.165.7.3663. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 67.Tiegs G. Experimental hepatitis and role of cytokines. Acta Gastroenterol Belg. 1997;60:176–179. [PubMed] [Google Scholar]
- 68.Topham D J, Tripp R A, Doherty P C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 69.Tschopp J. Granzyme B. Methods Enzymol. 1994;244:80–87. doi: 10.1016/0076-6879(94)44007-7. [DOI] [PubMed] [Google Scholar]
- 70.Vignaux F, Goldstein P. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur J Immunol. 1994;24:923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- 71.Walsh C M, Matloubian M, Liu C C, et al. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe H, Ohtsuka K, Kimura M, Ikarashi Y, Ohmori K, Kusumi A, Ohteki T, Seki S, Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992;146:145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- 73.Zimmermann C, Rawiel M, Blaser C, Kaufmann M, Pircher H. Homeostatic regulation of CD8+ T cells after antigen challenge in the absence of Fas (CD95) Eur J Immunol. 1996;26:2903–2910. doi: 10.1002/eji.1830261215. [DOI] [PubMed] [Google Scholar]
- 74.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 75.Zinkernagel R M, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]