Abstract
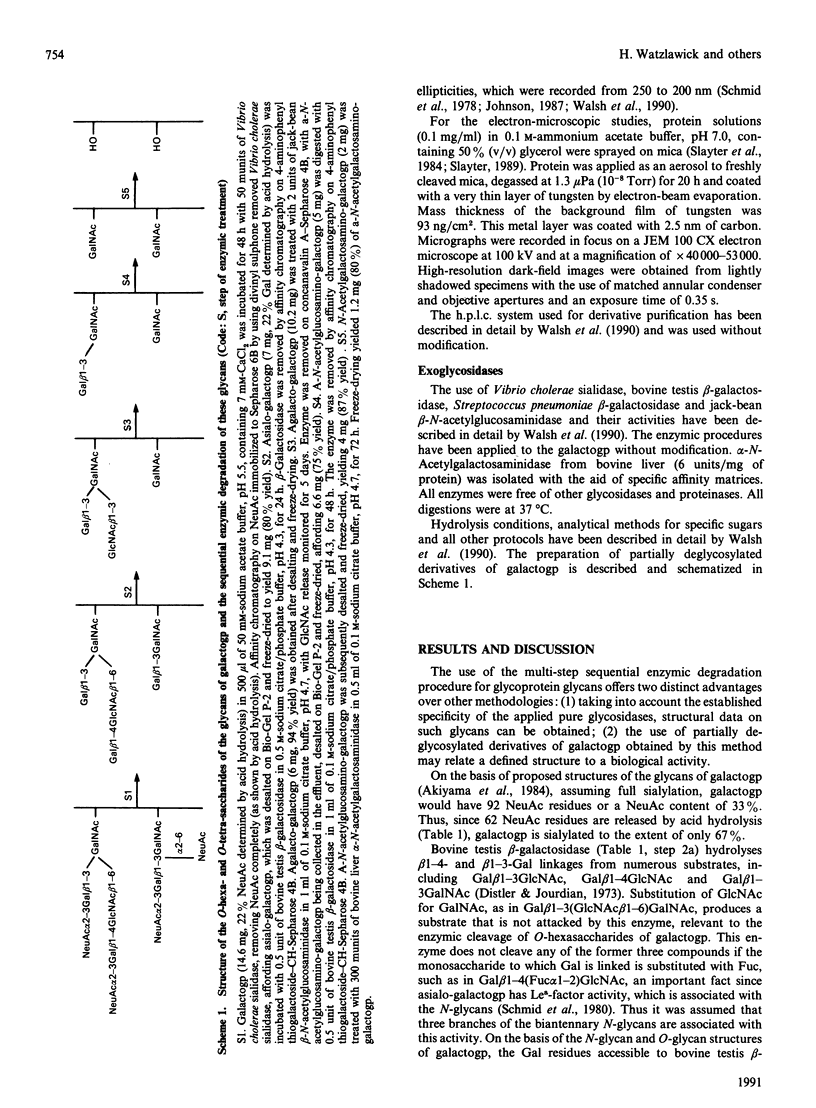
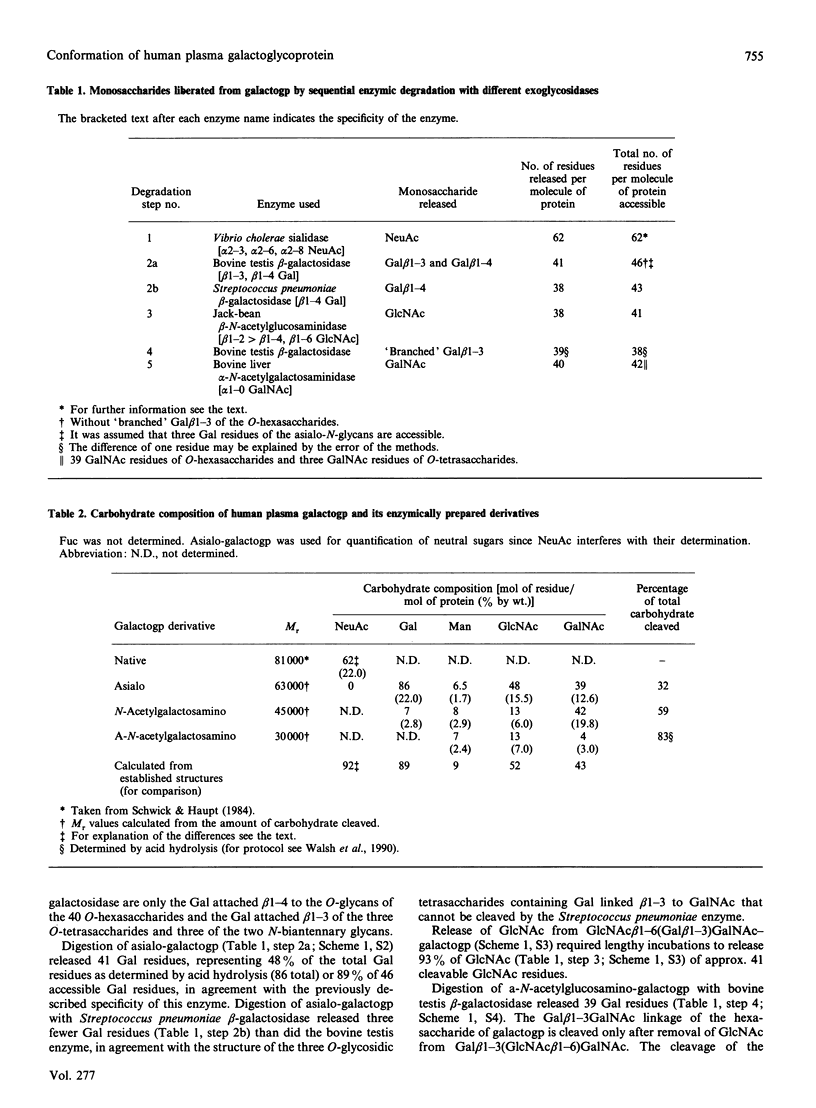
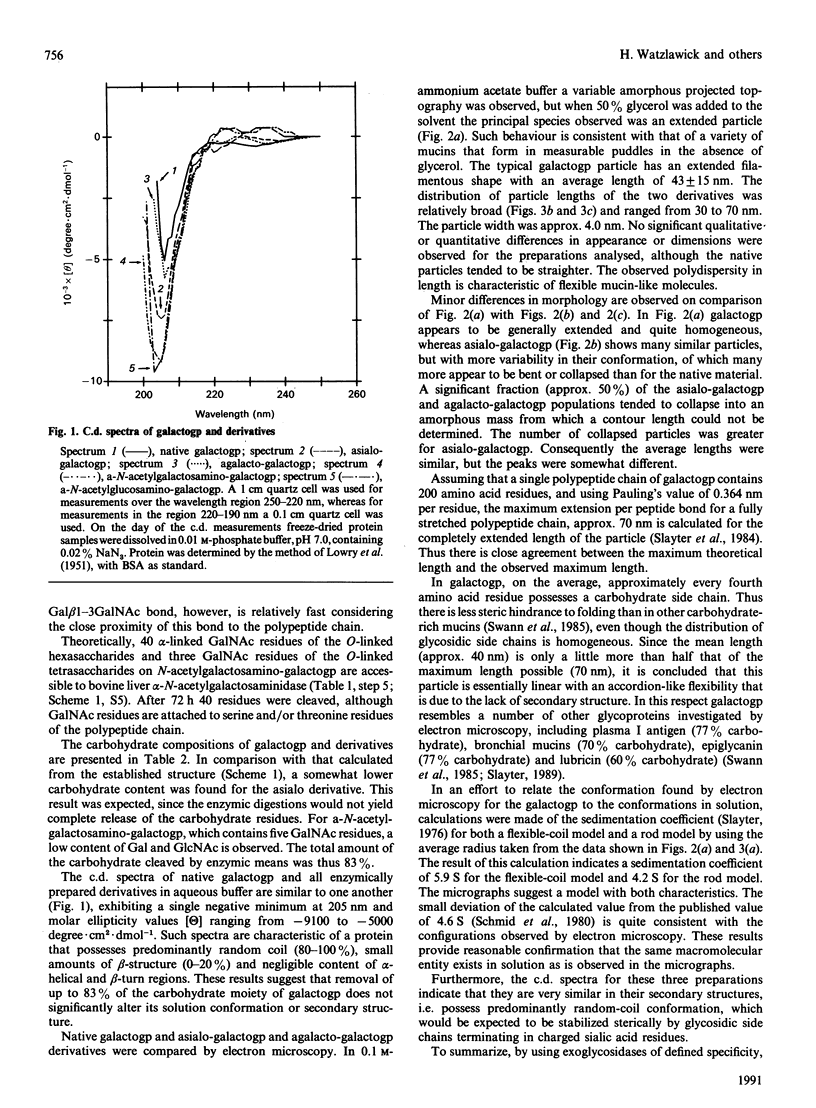
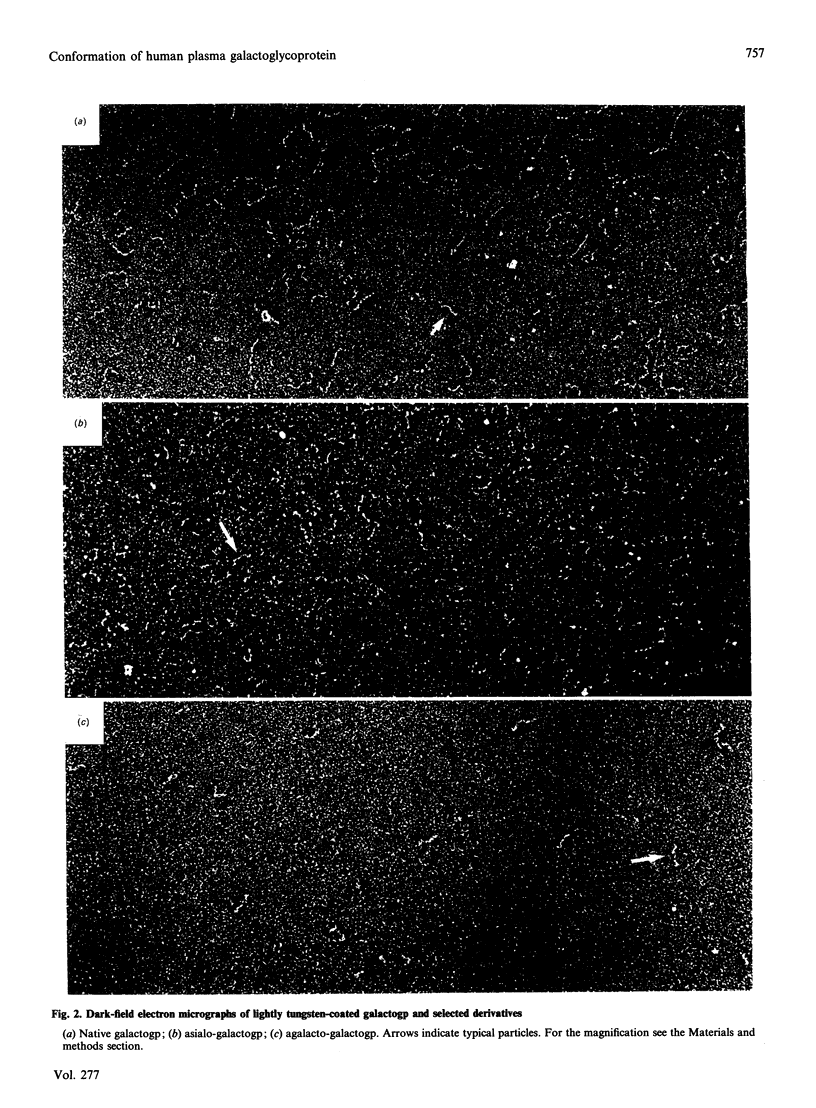
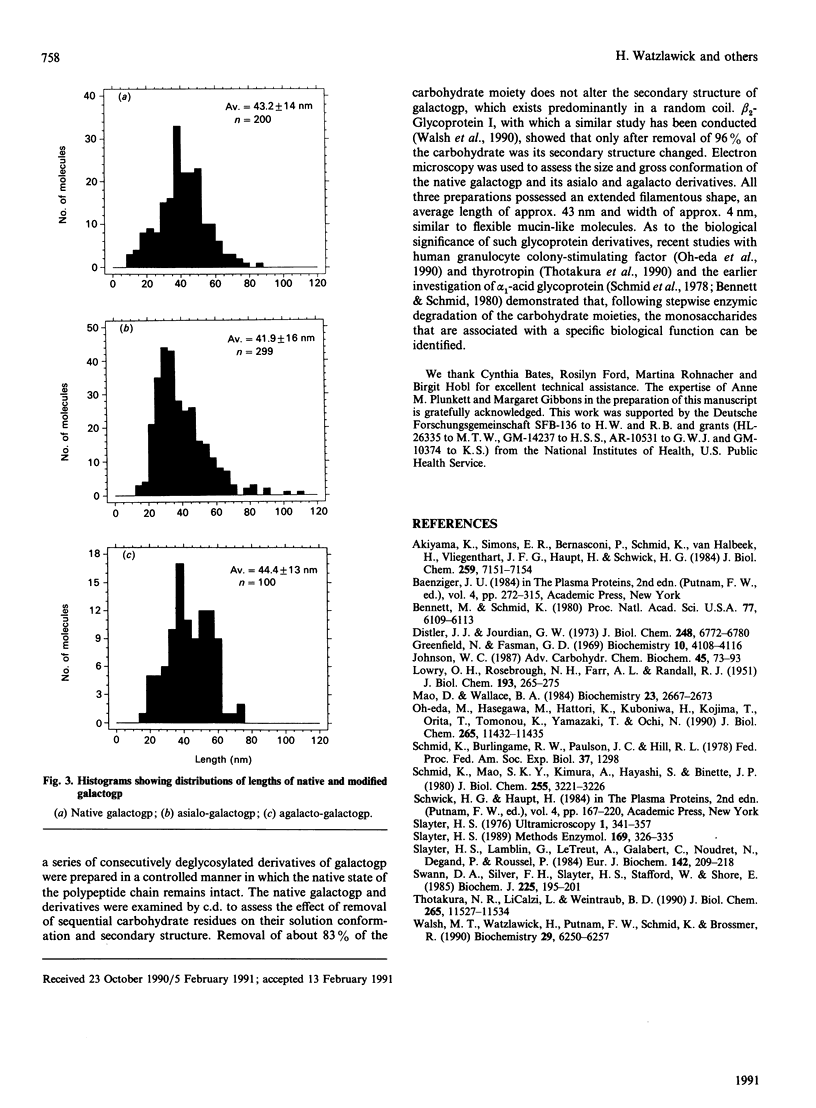
Galactoglycoprotein is a unique human plasma protein [76% carbohydrate (23% N-acetylneuraminic acid, 20% galactose, 3% mannose, 1% fucose and 29% N-acetylgalactosamine plus N-acetylglucosamine) and 24% polypeptide, a single polypeptide chain of about 200 amino acid residues that is high in serine and threonine content] [Schmid, Mao, Kimura, Hayashi & Binette (1980) J. Biol. Chem. 255, 3221-3226]. Highly purified exoglycosidases with well-defined specificities were used to prepare five derivatives of galactoglycoprotein in which sequential residues of N-acetylneuraminic acid, galactose, N-acetylglucosamine, a second galactose and N-acetylgalactosamine were removed with 83% of the total carbohydrate cleaved. C.d. shows that native galactoglycoprotein and all derivatives in aqueous buffer are predominantly random coil, suggesting that removal of a large number of electrostatic net charges, as well as the major portion of the carbohydrate moiety, does not alter the secondary structure of the polypeptide chain. Examination of the size and conformation of tungsten-shadowed galactoglycoprotein and asialo and agalacto derivatives by electron microscopy shows the size and conformation of all three preparations to be similar, with only minor differences in particle length and width.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama K., Simons E. R., Bernasconi P., Schmid K., van Halbeek H., Vliegenthart J. F., Haupt H., Schwick H. G. The structure of the carbohydrate units of human plasma galactoglycoprotein determined by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1984 Jun 10;259(11):7151–7154. [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J. J., Jourdian G. W. The purification and properties of beta-galactosidase from bovine testes. J Biol Chem. 1973 Oct 10;248(19):6772–6780. [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr The circular dichroism of carbohydrates. Adv Carbohydr Chem Biochem. 1987;45:73–124. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mao D., Wallace B. A. Differential light scattering and absorption flattening optical effects are minimal in the circular dichroism spectra of small unilamellar vesicles. Biochemistry. 1984 Jun 5;23(12):2667–2673. doi: 10.1021/bi00307a020. [DOI] [PubMed] [Google Scholar]
- Oh-eda M., Hasegawa M., Hattori K., Kuboniwa H., Kojima T., Orita T., Tomonou K., Yamazaki T., Ochi N. O-linked sugar chain of human granulocyte colony-stimulating factor protects it against polymerization and denaturation allowing it to retain its biological activity. J Biol Chem. 1990 Jul 15;265(20):11432–11435. [PubMed] [Google Scholar]
- Schmid K., Mao S. K., Kimura A., Hayashi S., Binette J. P. Isolation and characterization of a serine-threonine-rich galactoglycoprotein from normal human plasma. J Biol Chem. 1980 Apr 10;255(7):3221–3226. [PubMed] [Google Scholar]
- Slayter H. S. Dark-field electron microscopy of platelet adhesive macromolecules. Methods Enzymol. 1989;169:326–335. doi: 10.1016/0076-6879(89)69072-6. [DOI] [PubMed] [Google Scholar]
- Slayter H. S. High-resolution metal replication of macromolecules. Ultramicroscopy. 1976 Sep-Oct;1(4):341–357. doi: 10.1016/0304-3991(76)90050-4. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Silver F. H., Slayter H. S., Stafford W., Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985 Jan 1;225(1):195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thotakura N. R., LiCalzi L., Weintraub B. D. The role of carbohydrate in thyrotropin action assessed by a novel approach using enzymatic deglycosylation. J Biol Chem. 1990 Jul 15;265(20):11527–11534. [PubMed] [Google Scholar]
- Walsh M. T., Watzlawick H., Putnam F. W., Schmid K., Brossmer R. Effect of the carbohydrate moiety on the secondary structure of beta 2-glycoprotein. I. Implications for the biosynthesis and folding of glycoproteins. Biochemistry. 1990 Jul 3;29(26):6250–6257. doi: 10.1021/bi00478a020. [DOI] [PubMed] [Google Scholar]



