Abstract
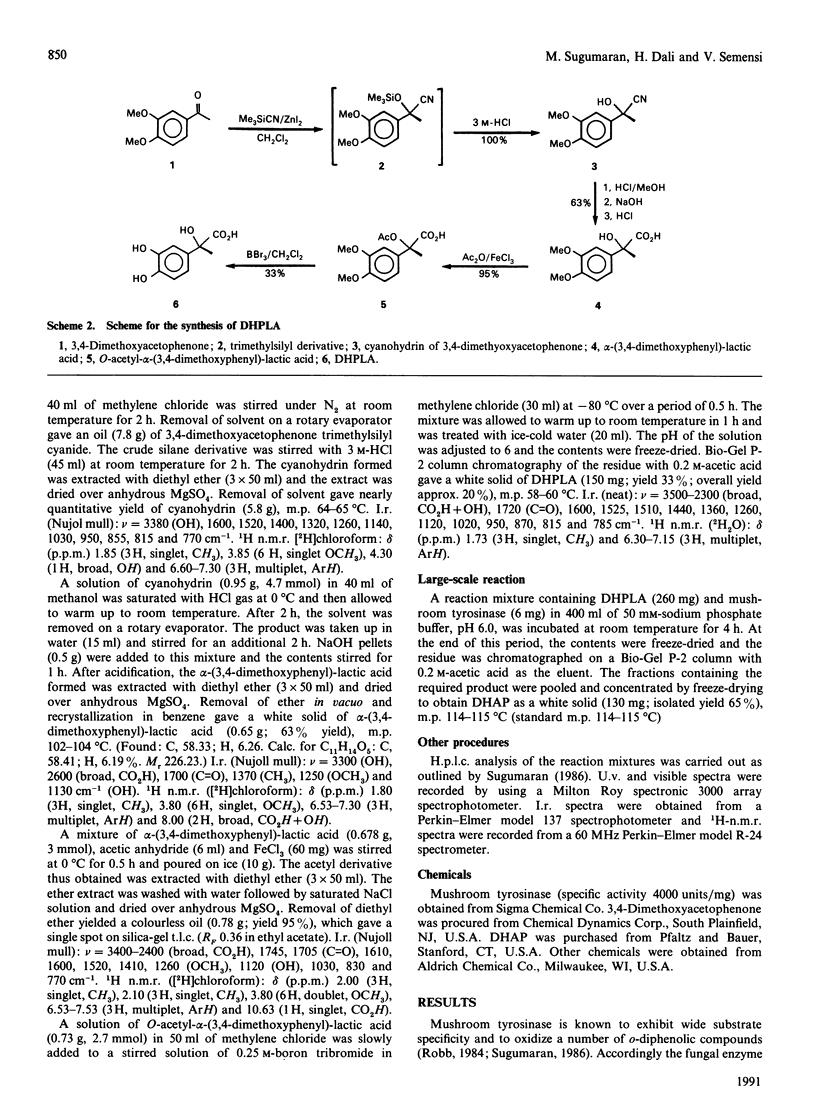
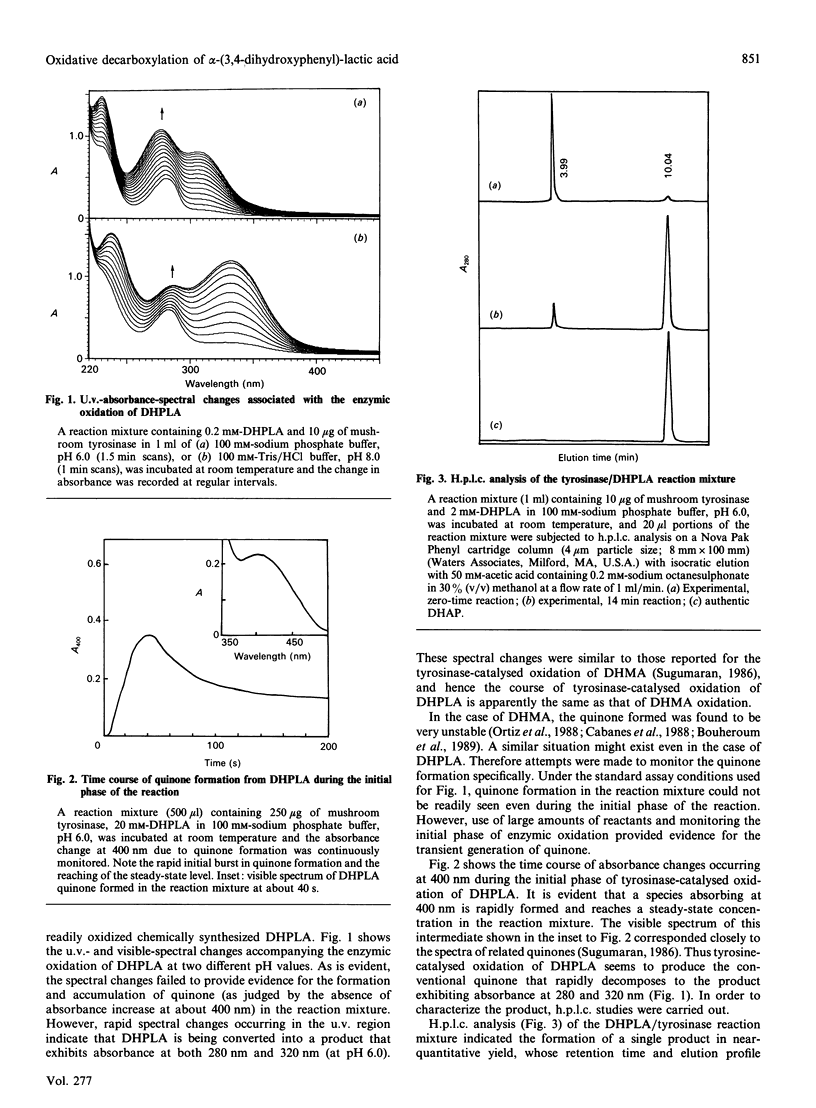
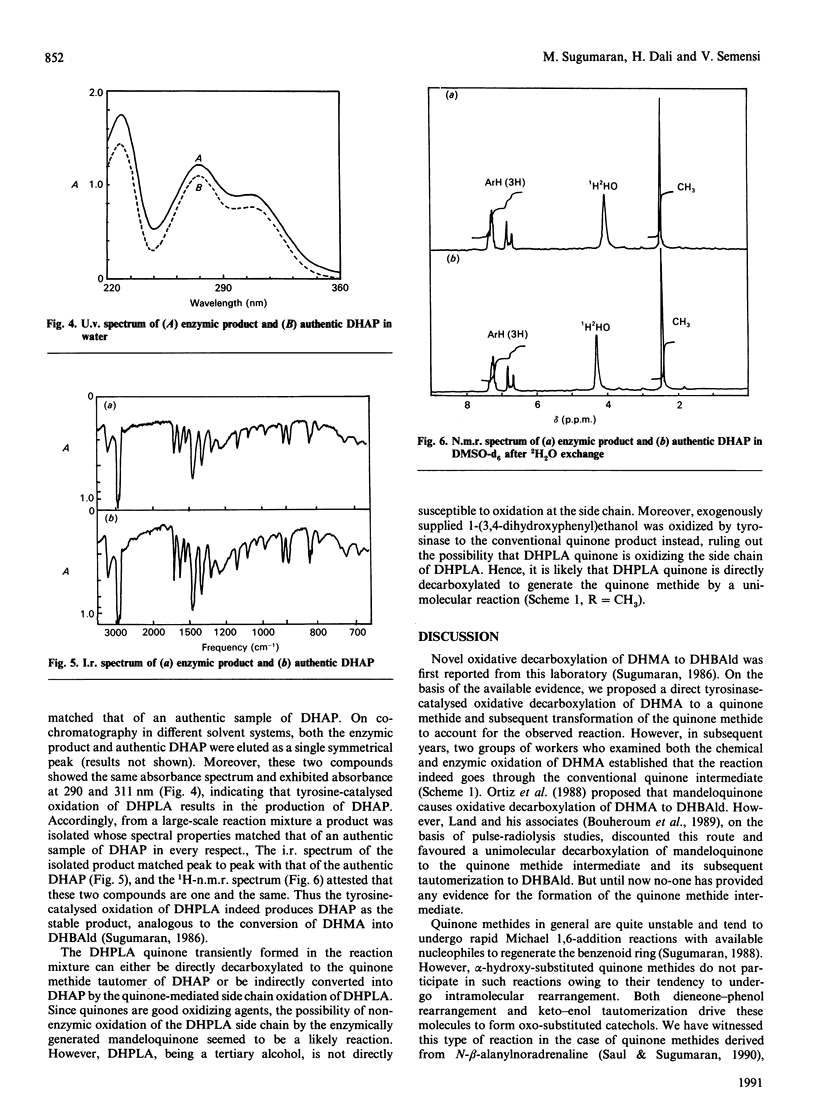
Mushroom tyrosinase, which is known to catalyse the conversion of o-diphenols into o-benzoquinones, has been shown to catalyse the oxidative decarboxylation of 3,4-dihydroxymandelic acid [Sugumaran (1986) Biochemistry 25, 4489-4492]. To account for this unusual reaction, a quinone methide intermediate has been proposed. Since all attempts to trap this intermediate ended in vain, mechanistic studies were designed to support the formation of this transient product. Replacement of the alpha-proton in 3,4-dihydroxymandelic acid with a methyl group generates alpha-(3,4-dihydroxyphenyl)-lactic acid, the enzymic oxidation of which should produce 3,4-dihydroxyacetophenone as the end product if the oxidative decarboxylation proceeds through the quinone methide intermediate. Accordingly, chemically synthesized alpha-(3,4-dihydroxyphenyl)-lactic acid on enzymic oxidation produced 3,4-dihydroxyacetophenone as the major isolatable product. Non-steady-state kinetic analysis of the enzyme reaction attested to the transient formation of the conventional quinone product. Thus the enzymic oxidation of alpha-(3,4-dihydroxyphenyl)-lactic acid seems to generate the conventional quinone, which, owing to its instability, is rapidly decarboxylated to yield the transient quinone methide. The coupled dieneonephenol re-arrangement and ketol-enol tautomerism transforms the quinone methide into 3,4-dihydroxyacetophenone.
Full text
PDF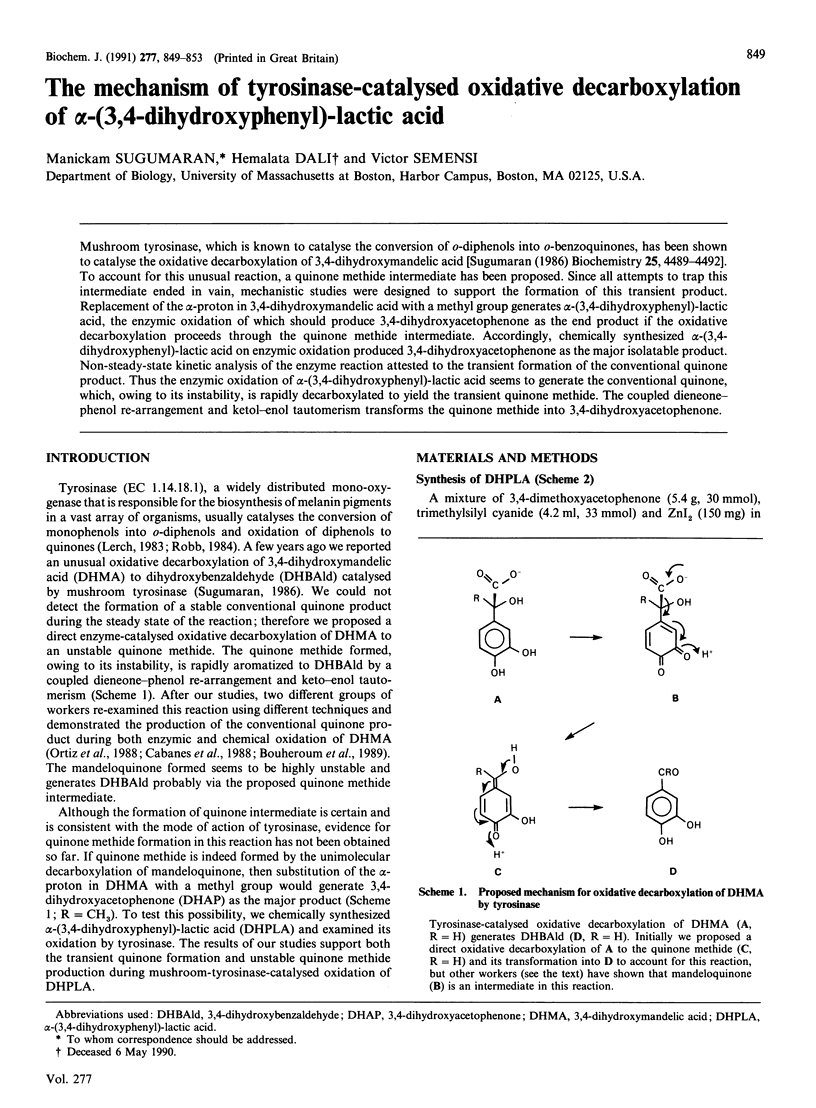

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabanes J., Sanchez-Ferrer A., Bru R., García-Carmona F. Chemical and enzymic oxidation by tyrosinase of 3,4-dihydroxymandelate. Biochem J. 1988 Dec 1;256(2):681–684. doi: 10.1042/bj2560681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN S., BRIDGERS W. F., EISENBERG F., FRIEDMAN S. The source of oxygen in the phenylalanine hydroxylase and the copamine-beta-hydroxylase catalyzed rections. Biochem Biophys Res Commun. 1962 Dec 19;9:497–502. doi: 10.1016/0006-291x(62)90115-8. [DOI] [PubMed] [Google Scholar]
- Lerch K. Neurospora tyrosinase: structural, spectroscopic and catalytic properties. Mol Cell Biochem. 1983;52(2):125–138. doi: 10.1007/BF00224921. [DOI] [PubMed] [Google Scholar]
- Martínez Ortiz F., Tudela Serrano J., Rodríguez López J. N., Varón Castellanos R., Lozano Teruel J. A., García-Cánovas F. Oxidation of 3,4-dihydroxymandelic acid catalyzed by tyrosinase. Biochim Biophys Acta. 1988 Nov 2;957(1):158–163. doi: 10.1016/0167-4838(88)90169-0. [DOI] [PubMed] [Google Scholar]
- Prota G. Progress in the chemistry of melanins and related metabolites. Med Res Rev. 1988 Oct-Dec;8(4):525–556. doi: 10.1002/med.2610080405. [DOI] [PubMed] [Google Scholar]
- Saul S. J., Sugumaran M. 4-alkyl-o-quinone/2-hydroxy-p-quinone methide isomerase from the larval hemolymph of Sarcophaga bullata. I. Purification and characterization of enzyme-catalyzed reaction. J Biol Chem. 1990 Oct 5;265(28):16992–16999. [PubMed] [Google Scholar]
- Saul S., Sugumaran M. A novel quinone: quinone methide isomerase generates quinone methides in insect cuticle. FEBS Lett. 1988 Sep 12;237(1-2):155–158. doi: 10.1016/0014-5793(88)80191-1. [DOI] [PubMed] [Google Scholar]
- Sugumaran M., Schinkmann K., Dali H. Mechanism of activation of 1,2-dehydro-N-acetyldopamine for cuticular sclerotization. Arch Insect Biochem Physiol. 1990;14(2):93–109. doi: 10.1002/arch.940140205. [DOI] [PubMed] [Google Scholar]
- Sugumaran M., Semensi V., Dali H., Nellaiappan K. Oxidation of 3,4-dihydroxybenzyl alcohol: a sclerotizing precursor for cockroach ootheca. Arch Insect Biochem Physiol. 1991;16(1):31–44. doi: 10.1002/arch.940160105. [DOI] [PubMed] [Google Scholar]
- Sugumaran M., Semensi V., Dali H., Saul S. Nonenzymatic transformations of enzymatically generated N-acetyldopamine quinone and isomeric dihydrocaffeiyl methyl amide quinone. FEBS Lett. 1989 Sep 25;255(2):345–349. doi: 10.1016/0014-5793(89)81118-4. [DOI] [PubMed] [Google Scholar]
- Sugumaran M. Tyrosinase catalyzes an unusual oxidative decarboxylation of 3,4-dihydroxymandelate. Biochemistry. 1986 Aug 12;25(16):4489–4492. doi: 10.1021/bi00364a005. [DOI] [PubMed] [Google Scholar]


