Abstract
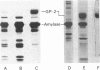
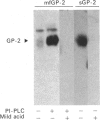
GP-2 is the major membrane protein of the exocrine pancreatic secretory granule. It is an integral protein which is anchored by a phosphatidylinositolglycan. In addition to being present in the soluble contents of the granule, GP-2 is also actively secreted by the pancreas. Although 93% of the GP-2 in the resting secretions of anaesthetized rats could be pelleted, Triton X-114 phase extraction showed that 70% of this GP-2 had lost its hydrophobic properties. Proteases have been postulated to release GP-2 from the membrane, but phospholipases also have the capacity to release the protein from the membrane by hydrolysis of its peculiar glycosylphosphatidylinositol membrane anchor. These studies show the presence of inositol 1,2-(cyclic)monophosphate on the secreted hydrophilic GP-2, confirming the involvement of an endogenous phospholipase C in the solubilization of GP-2 by the exocrine pancreas. It is therefore concluded that most of the GP-2 secreted by the pancreas of anaesthetized rats under resting conditions is released from the membrane by a phospholipase C which hydrolyses the phosphodiester bond linking GP-2 to its diradylglycerol anchor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcaraz G., Kinet J. P., Kumar N., Wank S. A., Metzger H. Phase separation of the receptor for immunoglobulin E and its subunits in Triton X-114. J Biol Chem. 1984 Dec 10;259(23):14922–14927. [PubMed] [Google Scholar]
- Arvan P., Castle J. D. Phasic release of newly synthesized secretory proteins in the unstimulated rat exocrine pancreas. J Cell Biol. 1987 Feb;104(2):243–252. doi: 10.1083/jcb.104.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin A. R., Grondin G., Vachereau A., St-Jean P., Cabana C. Detection and characterization of microvesicles in the acinar lumen and in juice of unstimulated rat pancreas. J Histochem Cytochem. 1986 Aug;34(8):1079–1084. doi: 10.1177/34.8.3734418. [DOI] [PubMed] [Google Scholar]
- Birk H. W., Koepsell H. Reaction of monoclonal antibodies with plasma membrane proteins after binding on nitrocellulose: renaturation of antigenic sites and reduction of nonspecific antibody binding. Anal Biochem. 1987 Jul;164(1):12–22. doi: 10.1016/0003-2697(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havinga J. R., Slot J. W., Strous G. J. Membrane detachment and release of the major membrane glycoprotein of secretory granules in rat pancreatic exocrine cells. Eur J Cell Biol. 1985 Nov;39(1):70–76. [PubMed] [Google Scholar]
- Havinga J. R., Strous G. J., Poort C. Intracellular transport of the major glycoprotein of zymogen granule membranes in the rat pancreas. Demonstration of high turnover at the plasma membrane. Eur J Biochem. 1984 Oct 1;144(1):177–183. doi: 10.1111/j.1432-1033.1984.tb08446.x. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Broomfield S. J., Turner A. J. Characterization of antibodies to the glycosyl-phosphatidylinositol membrane anchors of mammalian proteins. Biochem J. 1991 Jan 15;273(Pt 2):301–306. doi: 10.1042/bj2730301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The integral and peripheral proteins of the zymogen granule membrane. Biochim Biophys Acta. 1984 Feb 15;769(3):611–621. doi: 10.1016/0005-2736(84)90060-9. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The major protein of pancreatic zymogen granule membranes (GP-2) is anchored via covalent bonds to phosphatidylinositol. Biochem Biophys Res Commun. 1988 Jul 29;154(2):818–823. doi: 10.1016/0006-291x(88)90213-6. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beaudoin A. R. Different patterns of proteins are secreted by the pig pancreas when stimulated by secretin alone or in combination with caerulein. Biochim Biophys Acta. 1985 Oct 30;847(1):132–135. doi: 10.1016/0167-4889(85)90164-8. [DOI] [PubMed] [Google Scholar]
- Leblond F. A., Talbot B. G., Lauzon I., LeBel D. A competition enzyme-linked immunosorbent assay (ELISA) for the measurement of pancreatic GP-2 glycoprotein. J Immunol Methods. 1989 Nov 13;124(1):71–75. doi: 10.1016/0022-1759(89)90187-7. [DOI] [PubMed] [Google Scholar]
- Paquette J., Leblond F. A., Beattie M., LeBel D. Reducing conditions induce a total degradation of the major zymogen granule membrane protein in both its membranous and its soluble form. Immunochemical quantitation of the two forms. Biochem Cell Biol. 1986 May;64(5):456–462. doi: 10.1139/o86-064. [DOI] [PubMed] [Google Scholar]
- Scheffer R. C., Poort C., Slot J. W. Fate of the major zymogen granule membrane-associated glycoproteins from rat pancreas. A biochemical and immunocytochemical study. Eur J Cell Biol. 1980 Dec;23(1):122–128. [PubMed] [Google Scholar]