Abstract
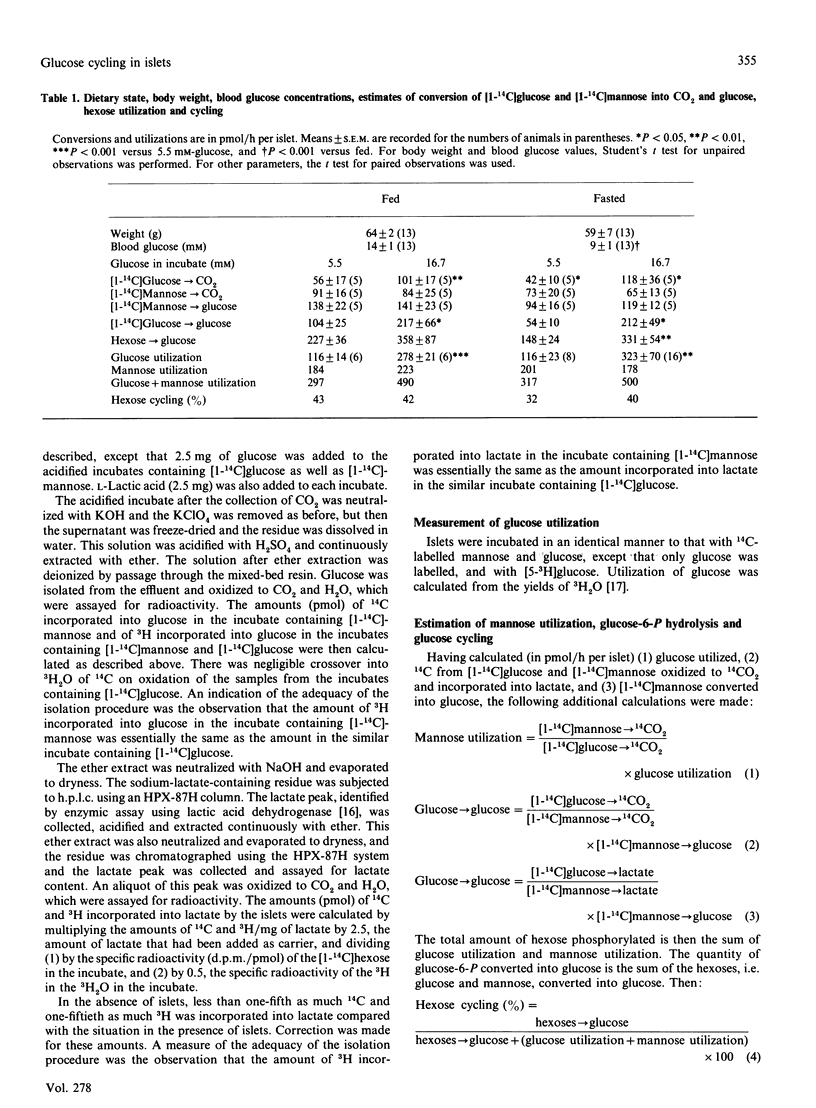
Pancreatic islets from fed and fasted obese hyperglycaemic (ob/ob) mice were incubated with [1-14C]glucose at 5.5 mM and 16.7 mM, [1-14C]mannose at 16.7 mM, and 3H2O. Yields of 14CO2 and 14C-labelled lactate, and amounts of 14C from [1-14C]mannose incorporated into glucose and of 3H bound to C-2 of glucose, were measured. Glucose utilization was determined from yields of 3H2O from [5-3H]glucose. From the results using 14C, 32-43% of the hexoses phosphorylated to hexose 6-phosphate were estimated to have been dephosphorylated, i.e. cycled. Estimates of hexose cycling from 3H incorporation into glucose were similar. Differences in the ratios of 14C yields in CO2 and lactate indicated incomplete isotopic equilibration between glucose 6-phosphate and fructose 6-phosphate, making the estimates of cycling semi-quantitative. In the fasted state, a larger percentage of the hexose utilized went to lactate than in the fed state. Thus conversion of mannose into glucose in islets indicates the occurrence of glucose cycling in islets. Yields of 14C from [1-14C]mannose, compared with from [1-14C]glucose, provide an approach for quantifying the extent of this cycling. These data provide further evidence for extensive glucose cycling occurring in ob/ob islets in both the fed and the fasted state.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anjaneyulu R., Anjaneyulu K., Carpinelli A. R., Sener A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release: enzymes of mannose metabolism in pancreatic islets. Arch Biochem Biophys. 1981 Nov;212(1):54–62. doi: 10.1016/0003-9861(81)90342-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Glucose-6-phosphatase activity of mouse pancreatic islets. Nature. 1968 Aug 24;219(5156):857–858. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- Giroix M. H., Sener A., Pipeleers D. G., Malaisse W. J. Hexose metabolism in pancreatic islets. Inhibition of hexokinase. Biochem J. 1984 Oct 15;223(2):447–453. doi: 10.1042/bj2230447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965 Oct 8;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- Herman R. H. Mannose metabolism. I. Am J Clin Nutr. 1971 Apr;24(4):488–498. doi: 10.1093/ajcn/24.4.488. [DOI] [PubMed] [Google Scholar]
- Hostetler K., Cooperstein S. J., Landau B. R., Lazarow A. Pathways of glucose metabolism in the isolated islet of the goosefish in vitro. Am J Physiol. 1966 Nov;211(5):1057–1062. doi: 10.1152/ajplegacy.1966.211.5.1057. [DOI] [PubMed] [Google Scholar]
- Khan A., Chandramouli V., Ostenson C. G., Ahrén B., Schumann W. C., Löw H., Landau B. R., Efendić S. Evidence for the presence of glucose cycling in pancreatic islets of the ob/ob mouse. J Biol Chem. 1989 Jun 15;264(17):9732–9733. [PubMed] [Google Scholar]
- Khan A., Chandramouli V., Ostenson C. G., Berggren P. O., Löw H., Landau B. R., Efendic S. Glucose cycling is markedly enhanced in pancreatic islets of obese hyperglycemic mice. Endocrinology. 1990 May;126(5):2413–2416. doi: 10.1210/endo-126-5-2413. [DOI] [PubMed] [Google Scholar]
- Khan A., Chandramouli V., Ostenson C. G., Löw H., Landau B. R., Efendić S. Glucose cycling in islets from healthy and diabetic rats. Diabetes. 1990 Apr;39(4):456–459. doi: 10.2337/diab.39.4.456. [DOI] [PubMed] [Google Scholar]
- MERLEVEDE W., WEAVER G., LANDAU B. R. Effects of thyrotropic hormone on carbohydrate metabolism in thyroid slices. J Clin Invest. 1963 Jul;42:1160–1171. doi: 10.1172/JCI104801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Sener A. The stimulus-secretion coupling of glucose-induced insulin release: effect of aminooxyacetate upon nutrient-stimulated insulin secretion. Endocrinology. 1982 Aug;111(2):392–397. doi: 10.1210/endo-111-2-392. [DOI] [PubMed] [Google Scholar]
- Marynissen G., Leclercq-Meyer V., Sener A., Malaisse W. J. Perturbation of pancreatic islet function in glucose-infused rats. Metabolism. 1990 Jan;39(1):87–95. doi: 10.1016/0026-0495(90)90153-4. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Ostenson C. G., Grill V. Differences in long-term effects of L-glutamine and D-glucose on insulin release from rat pancreatic islets. Mol Cell Endocrinol. 1986 May;45(2-3):215–221. doi: 10.1016/0303-7207(86)90150-4. [DOI] [PubMed] [Google Scholar]
- Ostenson C. G. Regulation of glucagon release: effects of insulin on the pancreatic A2-cell of the guinea pig. Diabetologia. 1979 Nov;17(5):325–330. doi: 10.1007/BF01235889. [DOI] [PubMed] [Google Scholar]
- Postle A. D., Bloxham D. P. The use of tritiated water to measure absolute rates of hepatic glycogen synthesis. Biochem J. 1980 Oct 15;192(1):65–73. doi: 10.1042/bj1920065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M., Golden S., Katz J. Lactate metabolism in the perfused rat hindlimb. Biochem J. 1984 Sep 1;222(2):281–292. doi: 10.1042/bj2220281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder P. J., Kashket S., O'Sullivan J. B. Pentose cycle in isolated islets during glucose-stimulated insulin release. Am J Physiol. 1970 Oct;219(4):876–880. doi: 10.1152/ajplegacy.1970.219.4.876. [DOI] [PubMed] [Google Scholar]
- TäljedalIB Presence, induction and possible role of glucose 6-phosphatase in mammalian pancreatic islets. Biochem J. 1969 Sep;114(2):387–394. doi: 10.1042/bj1140387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspohl E. J., Händel M., Ammon H. P. Pentosephosphate shunt activity of rat pancreatic islets: its dependence on glucose concentration. Endocrinology. 1979 Nov;105(5):1269–1274. doi: 10.1210/endo-105-5-1269. [DOI] [PubMed] [Google Scholar]
- Waddell I. D., Burchell A. The microsomal glucose-6-phosphatase enzyme of pancreatic islets. Biochem J. 1988 Oct 15;255(2):471–476. doi: 10.1042/bj2550471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajngot A., Chandramouli V., Schumann W. C., Kumaran K., Efendić S., Landau B. R. Testing of the assumptions made in estimating the extent of futile cycling. Am J Physiol. 1989 May;256(5 Pt 1):E668–E675. doi: 10.1152/ajpendo.1989.256.5.E668. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Colca J. R., Turk J., Florholmen J., McDaniel M. L. Regulation of Ca2+ homeostasis by islet endoplasmic reticulum and its role in insulin secretion. Am J Physiol. 1988 Feb;254(2 Pt 1):E121–E136. doi: 10.1152/ajpendo.1988.254.2.E121. [DOI] [PubMed] [Google Scholar]
- Zawalich W. S., Rognstad R., Pagliara A. S., Matschinsky F. M. A comparison of the utilization rates and hormone-releasing actions of glucose, mannose, and fructose in isolated pancreatic islets. J Biol Chem. 1977 Dec 10;252(23):8519–8523. [PubMed] [Google Scholar]


