Abstract
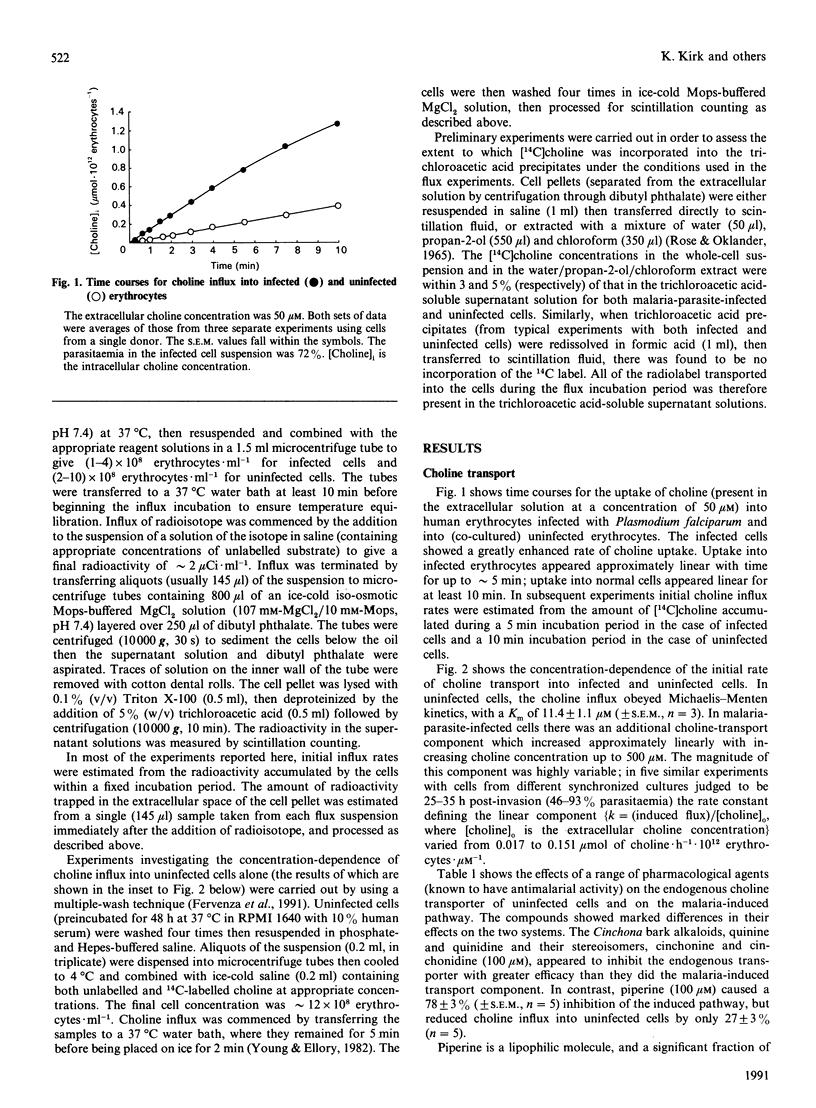
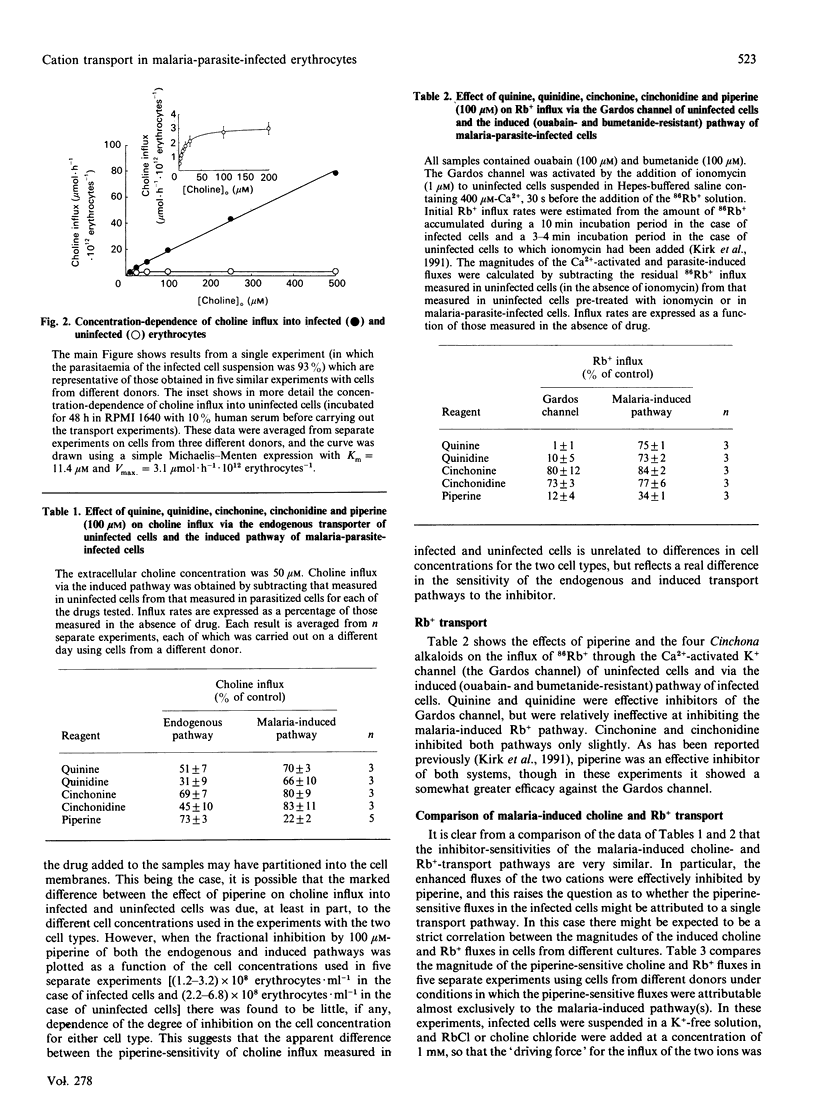
Human erythrocytes infected in vitro with the malaria parasite Plasmodium falciparum showed a markedly increased rate of choline influx compared with normal cells. Choline transport into uninfected cells (cultured in parallel with infected cells) obeyed Michaelis-Menten kinetics (Km approximately 11 microM). In malaria-parasite-infected cells there was an additional choline-transport component which failed to saturate at extracellular concentrations of up to 500 microM. This component was less sensitive than the endogenous transporter to inhibition by the Cinchona bark alkaloids quinine, quinidine, cinchonine and cinchonidine, but showed a much greater sensitivity than the native system to inhibition by piperine. The sensitivity of the induced choline transport to these reagents was similar to that of the malaria-induced (ouabain- and bumetanide-resistant) Rb(+)-transport pathway; however, the relative magnitudes of the piperine-sensitive choline and Rb+ fluxes in malaria-parasite-infected cells varied between cultures. This suggests either that the enhanced transport of the two cations was via functionally distinct (albeit pharmacologically similar) pathways, or that the transport was mediated by a pathway with variable substrate selectivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ancelin M. L., Vial H. J. Choline kinase activity in Plasmodium-infected erythrocytes: characterization and utilization as a parasite-specific marker in malarial fractionation studies. Biochim Biophys Acta. 1986 Jan 3;875(1):52–58. doi: 10.1016/0005-2760(86)90010-x. [DOI] [PubMed] [Google Scholar]
- Ancelin M. L., Vial H. J. Regulation of phosphatidylcholine biosynthesis in Plasmodium-infected erythrocytes. Biochim Biophys Acta. 1989 Jan 23;1001(1):82–89. doi: 10.1016/0005-2760(89)90310-x. [DOI] [PubMed] [Google Scholar]
- BLIGH J. The level of free choline in plasma. J Physiol. 1952 Jun;117(2):234–240. doi: 10.1113/jphysiol.1952.sp004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Dunn M. J. Alterations of red blood cell sodium transport during malarial infection. J Clin Invest. 1969 Apr;48(4):674–684. doi: 10.1172/JCI106025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford B. C., Haynes J. D., Chulay J. D., Wilson R. J. Selective stage-specific changes in the permeability to small hydrophilic solutes of human erythrocytes infected with Plasmodium falciparum. Mol Biochem Parasitol. 1985 Jun;16(1):43–60. doi: 10.1016/0166-6851(85)90048-9. [DOI] [PubMed] [Google Scholar]
- Elford B. C., Pinches R. A., Newbold C. I., Ellory J. C. Heterogeneous and substrate-specific membrane transport pathways induced in malaria-infected erythrocytes. Blood Cells. 1990;16(2-3):433–436. [PubMed] [Google Scholar]
- Ellory J. C., Elford B. C., Newbold C. I. Transport mechanisms across cell membranes. Parasitology. 1988;96 (Suppl):S5–S9. doi: 10.1017/s0031182000085942. [DOI] [PubMed] [Google Scholar]
- Fervenza F. C., Meredith D., Ellory J. C., Hendry B. M. Abnormal erythrocyte choline transport in patients with chronic renal failure. Clin Sci (Lond) 1991 Feb;80(2):137–141. doi: 10.1042/cs0800137. [DOI] [PubMed] [Google Scholar]
- Gati W. P., Lin A. N., Wang T. I., Young J. D., Paterson A. R. Parasite-induced processes for adenosine permeation in mouse erythrocytes infected with the malarial parasite Plasmodium yoelii. Biochem J. 1990 Nov 15;272(1):277–280. doi: 10.1042/bj2720277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Kutner S., Krugliak M., Cabantchik Z. I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985 Mar;14(3):313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Ginsburg H. Some reflections concerning host erythrocyte-malarial parasite interrelationships. Blood Cells. 1990;16(2-3):225–235. [PubMed] [Google Scholar]
- Ginsburg H., Stein W. D. New permeability pathways induced by the malarial parasite in the membrane of its host erythrocyte: potential routes for targeting of drugs into infected cells. Biosci Rep. 1987 Jun;7(6):455–463. doi: 10.1007/BF01116501. [DOI] [PubMed] [Google Scholar]
- Kirk K., Ashworth K. J., Elford B. C., Pinches R. A., Ellory J. C. Characteristics of 86Rb+ transport in human erythrocytes infected with Plasmodium falciparum. Biochim Biophys Acta. 1991 Jan 30;1061(2):305–308. doi: 10.1016/0005-2736(91)90296-k. [DOI] [PubMed] [Google Scholar]
- Krupka R. M., Devés R. The choline transport system of erythrocytes distribution of the free carrier in the membrane. Biochim Biophys Acta. 1980 Jul 16;600(1):228–232. doi: 10.1016/0005-2736(80)90427-7. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J., Smalley M. E., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978 Feb;72(1):87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Sherman I. W. The Wellcome Trust lecture. Mechanisms of molecular trafficking in malaria. Parasitology. 1988;96 (Suppl):S57–S81. doi: 10.1017/s003118200008598x. [DOI] [PubMed] [Google Scholar]


