Abstract
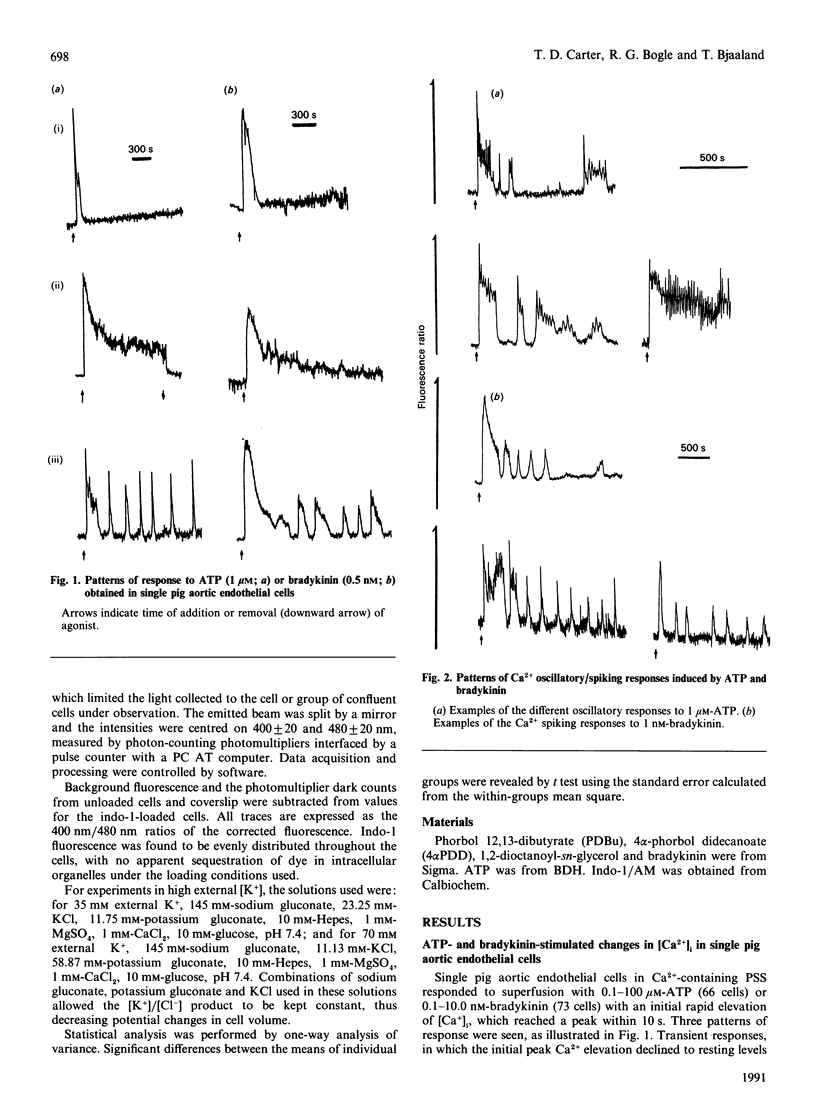
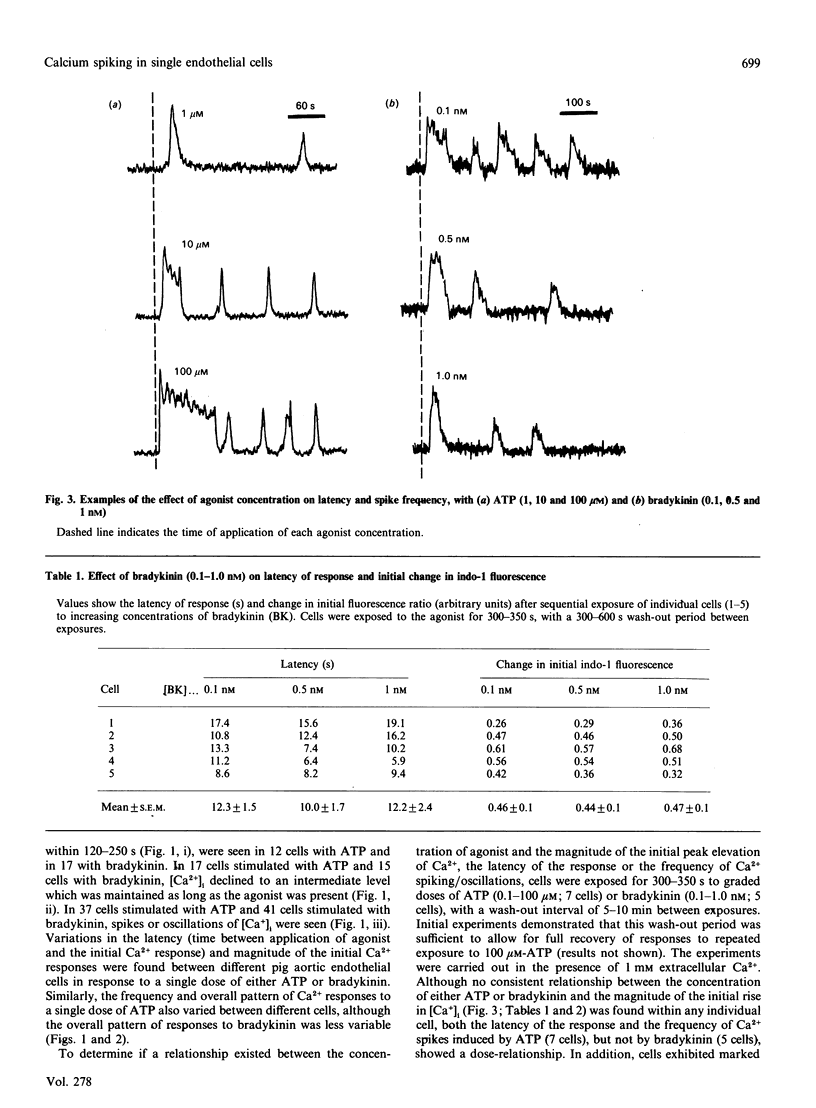
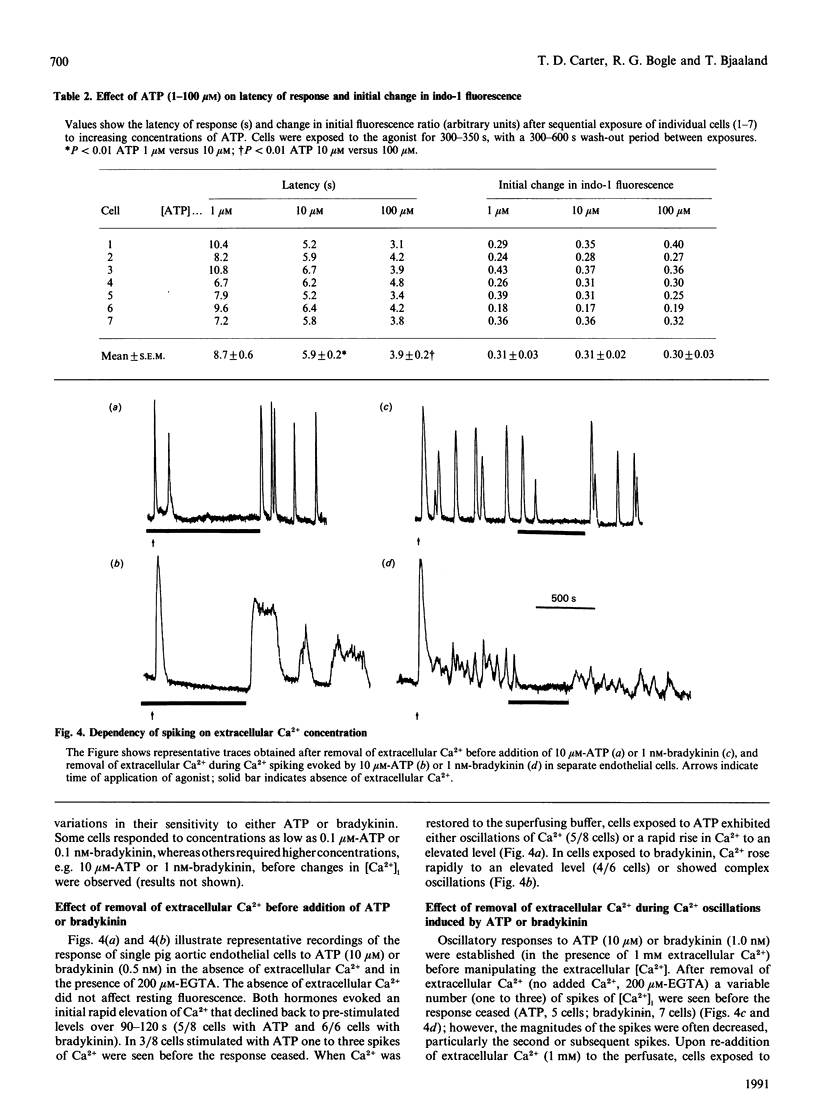
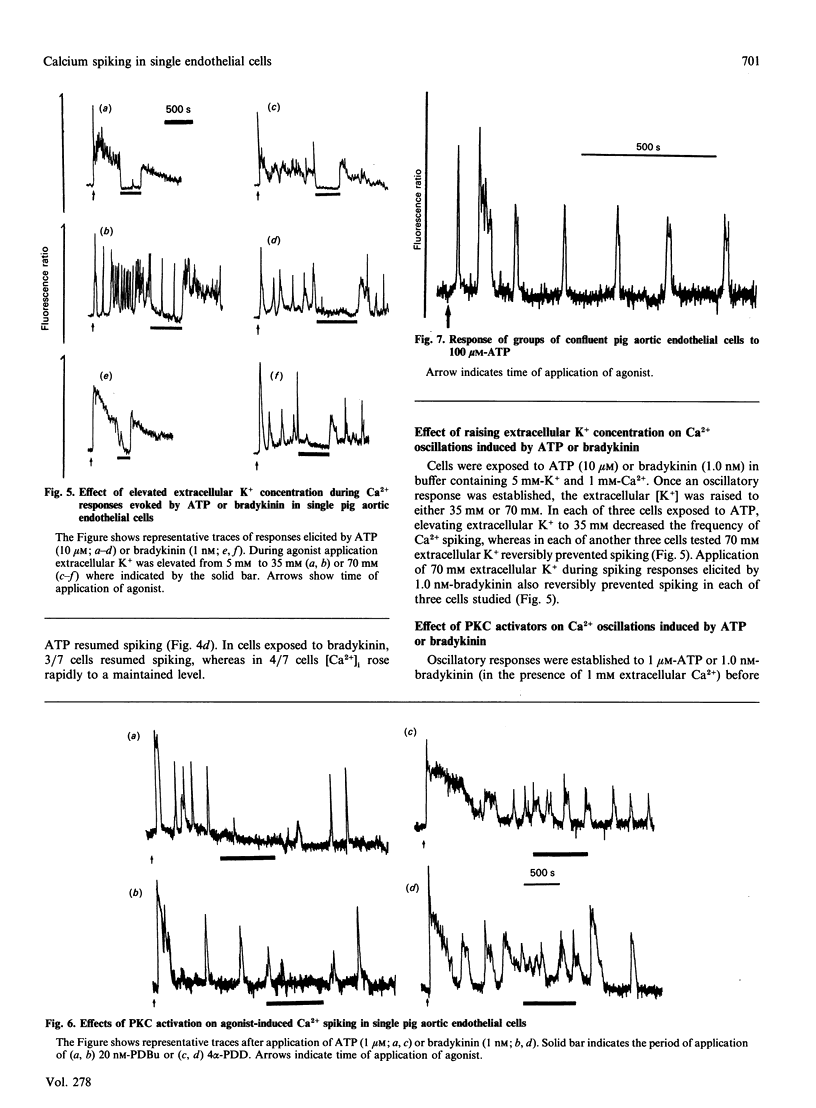
Single pig aortic endothelial cells in culture loaded with the Ca(2+)-sensitive fluorescent dye Indo-1 were stimulated with ATP (0.1-100 microM) or bradykinin (0.1-5.0 nM). Spiking or oscillations of [Ca2+]i were seen in approx. 50% of cells stimulated with either agonist. Non-spiking or transient responses in which [Ca2+]i returned to pre-stimulation levels rapidly 9120-250 s), or sustained responses in which [Ca2+]i remained elevated for many minutes, were seen in a further 20% of cells in each case, stimulated with either agonist. There was a marked variation between individual cells in the latency, magnitude, frequency and overall pattern of oscillations induced by ATP and bradykinin, although the patterns of response to bradykinin were less variable. In cells where repetitive spikes were seen, a relation between concentration of ATP and the latency of the response and the frequency of spiking was evident. Effects of removal of extracellular Ca2+, elevation of extracellular K+ concentration (35 or 70 mM) or exposure to phorbol 12,13-dibutyrate or 1,2-dioctanoyl-sn-glycerol were tested on the spiking Ca2+ responses. Each of these procedures reversibly slowed or prevented Ca2+ spiking evoked by ATP or bradykinin. In contrast, the inactive phorbol ester 4 alpha-phorbol didecanoate had no effect on Ca2+ spiking evoked by these hormones. Our results thus indicate that the responses of single cells to ATP or bradykinin exhibit marked heterogeneity, and suggest that secretory events driven by extracellular Ca2+ may be regulated by repetitive spikes or oscillations of Ca2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990 Jun 4;265(1-2):133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Pearson J. D. Protein kinase C activation alters the sensitivity of agonist-stimulated endothelial-cell prostacyclin production to intracellular Ca2+. Biochem J. 1989 Sep 1;262(2):431–437. doi: 10.1042/bj2620431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Newton J. S., Jacob R., Pearson J. D. Homologous desensitization of ATP-mediated elevations in cytoplasmic calcium and prostacyclin release in human endothelial cells does not involve protein kinase C. Biochem J. 1990 Nov 15;272(1):217–221. doi: 10.1042/bj2720217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Cybulsky M. I., Brock T. A. ACh-induced calcium transients in primary cultures of rabbit aortic endothelial cells. Am J Physiol. 1988 Dec;255(6 Pt 2):H1549–H1553. doi: 10.1152/ajpheart.1988.255.6.H1549. [DOI] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. K., Sims P. J. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987 Feb;79(2):600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. Calcium oscillations in electrically non-excitable cells. Biochim Biophys Acta. 1990 May 22;1052(3):427–438. doi: 10.1016/0167-4889(90)90152-4. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F., Procopio J. Comparative roles of voltage and Cl ions upon activation of a Cl conductive pathway in toad skin. Pflugers Arch. 1988 Oct;412(6):634–640. doi: 10.1007/BF00583765. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Lewis M. J., Henderson A. H. A phorbol ester inhibits the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1987 Jun 4;137(2-3):167–171. doi: 10.1016/0014-2999(87)90218-4. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Irvine R. F. Synchronized repetitive spikes in cytoplasmic calcium in confluent monolayers of human umbilical vein endothelial cells. FEBS Lett. 1990 Nov 26;275(1-2):173–176. doi: 10.1016/0014-5793(90)81465-z. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sakai T. Acetylcholine induces Ca-dependent K currents in rabbit endothelial cells. Jpn J Pharmacol. 1990 Jun;53(2):235–246. doi: 10.1254/jjp.53.235. [DOI] [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Tsunoda Y., Stuenkel E. L., Williams J. A. Oscillatory mode of calcium signaling in rat pancreatic acinar cells. Am J Physiol. 1990 Jan;258(1 Pt 1):C147–C155. doi: 10.1152/ajpcell.1990.258.1.C147. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J. 1987 Sep 15;246(3):619–623. doi: 10.1042/bj2460619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


