Abstract
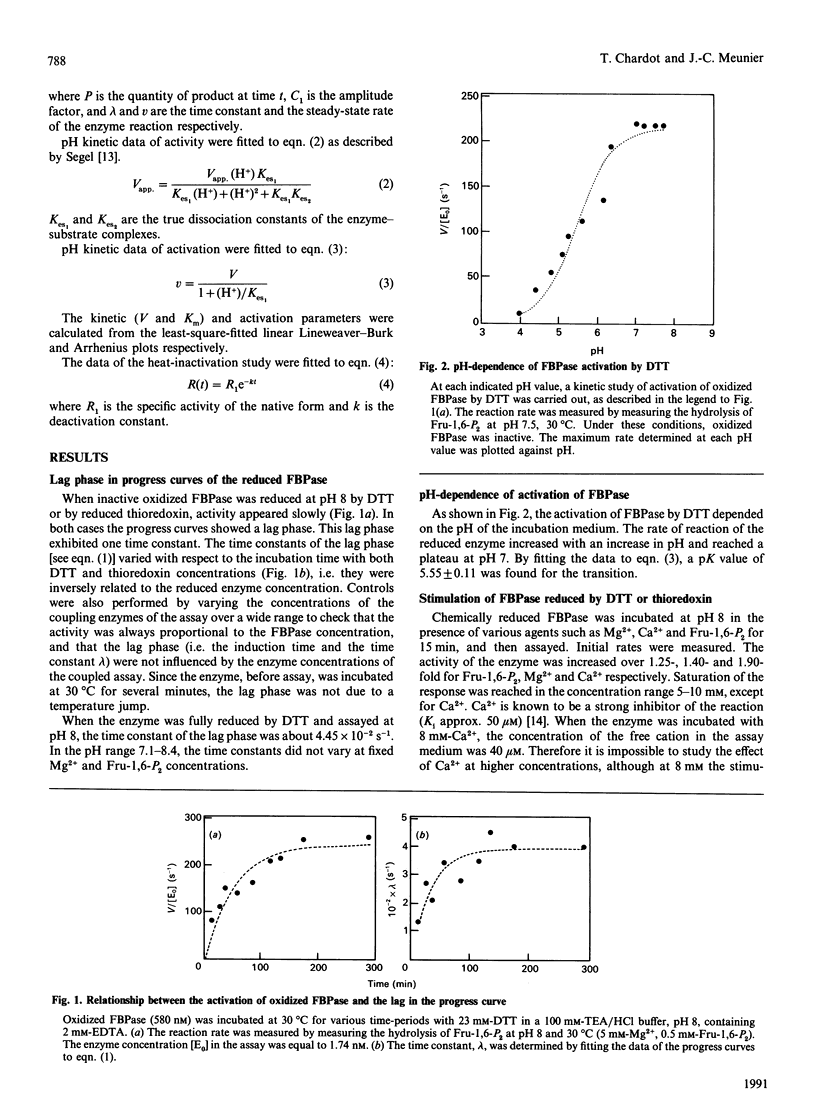
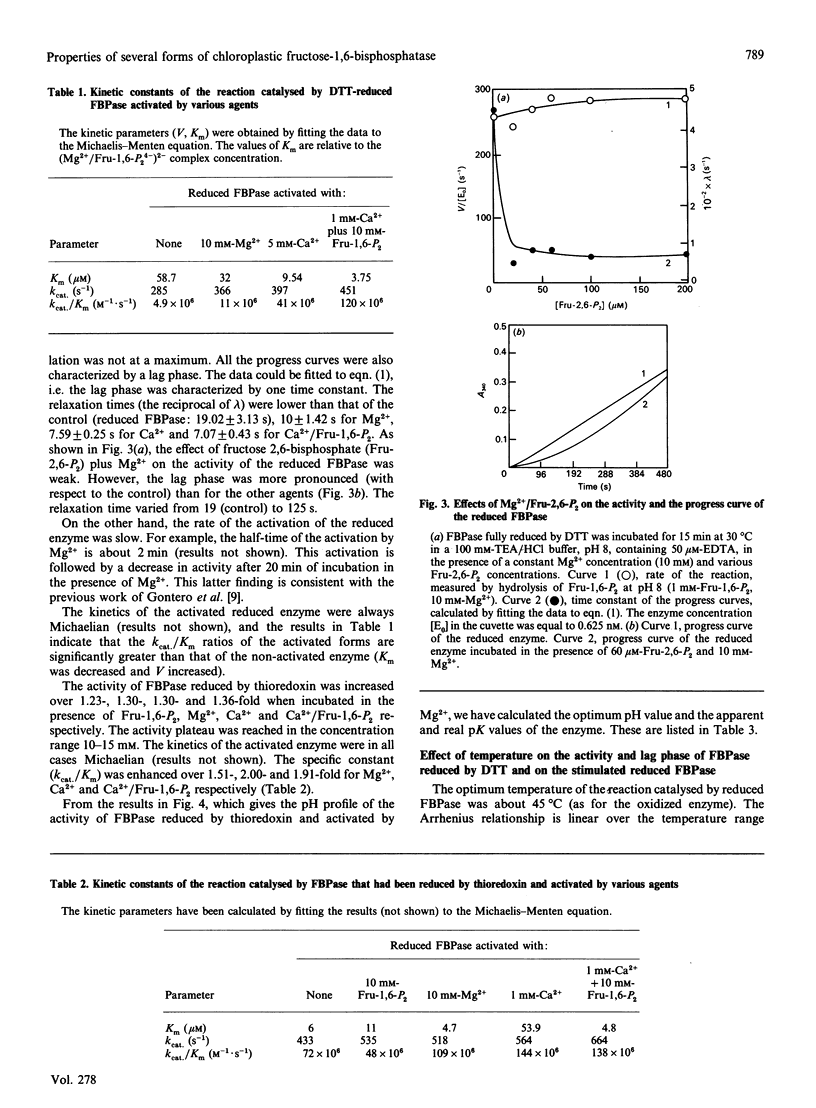
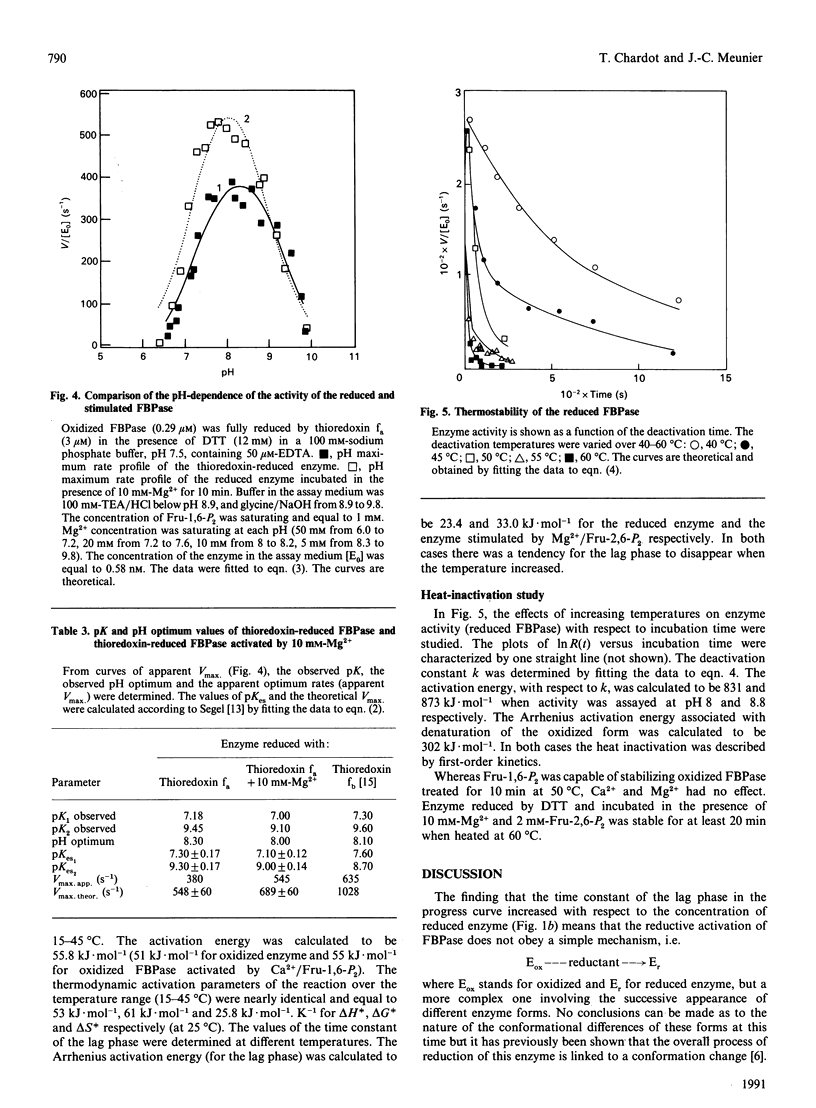
Fructose-1,6-bisphosphatase (FBPase) can be reduced and activated by either dithiothreitol or reduced thioredoxin. This activation is pH-dependent. An amino acid group with a pK value of 5.55 is involved in this process. Both enzyme forms can also be stimulated by agents such as fructose 1,6-bisphosphate, Mg2+, Ca2+ and Ca2+/fructose 1,6-bisphosphate. FBPase reduced by dithiothreitol is more strongly activated than the enzyme reduced by thioredoxin. The specificity constant (kcat./Km) is enhanced over 2.5-25-fold and 1.5-2-fold (depending on the agent used) for FBPase reduced by dithiothreitol and thioredoxin respectively. In both cases, no new kinetic properties appeared. The pH-activity profile of the stimulated enzyme is slightly shifted towards the acidic side with respect to the reduced enzyme. A lag phase is observed in the progress curve of both enzymic forms, treated or untreated. Each agent used to stimulate must induce a new conformation of the enzyme, more active than the initial one, characterized by a specificity constant and a relaxation time. This lag phase tends to disappear when the assay temperature is increased. Temperature has the same effect on the activity of oxidized, reduced and stimulated FBPase, but different effects on the stability of the different forms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buc J., Rivière M., Gontero B., Sauve P., Meunier J. C., Ricard J. Affinity chromatography, on fructose-bisphosphatase-Sepharose, of two chloroplastic thioredoxins F. Purification and comparative molecular properties. Eur J Biochem. 1984 Apr 2;140(1):199–202. doi: 10.1111/j.1432-1033.1984.tb08086.x. [DOI] [PubMed] [Google Scholar]
- Cadet F., Meunier J. C., Ferté N. Effects of pH and fructose 2,6-bisphosphate on oxidized and reduced spinach chloroplastic fructose-1,6-bisphosphatase. Eur J Biochem. 1987 Jan 15;162(2):393–398. doi: 10.1111/j.1432-1033.1987.tb10614.x. [DOI] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other enzymes of the Calvin cycle. Biochem J. 1980 Jun 15;188(3):775–779. doi: 10.1042/bj1880775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. A., Droux M., Kosower N. S., Buchanan B. B. Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys. 1989 May 15;271(1):223–239. doi: 10.1016/0003-9861(89)90273-7. [DOI] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Hertig C. M., Wolosiuk R. A. Studies on the hysteretic properties of chloroplast fructose-1,6-bisphosphatase. J Biol Chem. 1983 Jan 25;258(2):984–989. [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Nury S., Meunier J. C., Mouranche A. The kinetics of the thermal deactivation of transglutaminase from guinea-pig liver. Eur J Biochem. 1989 Mar 1;180(1):161–166. doi: 10.1111/j.1432-1033.1989.tb14627.x. [DOI] [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Wolosiuk R. A. Studies on the regulatory properties of chloroplast fructose-1,6-bisphosphatase. Biochim Biophys Acta. 1978 Jan 12;522(1):130–138. doi: 10.1016/0005-2744(78)90329-7. [DOI] [PubMed] [Google Scholar]
- Violet M., Meunier J. C. Kinetic study of the irreversible thermal denaturation of Bacillus licheniformis alpha-amylase. Biochem J. 1989 Nov 1;263(3):665–670. doi: 10.1042/bj2630665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]


