Abstract
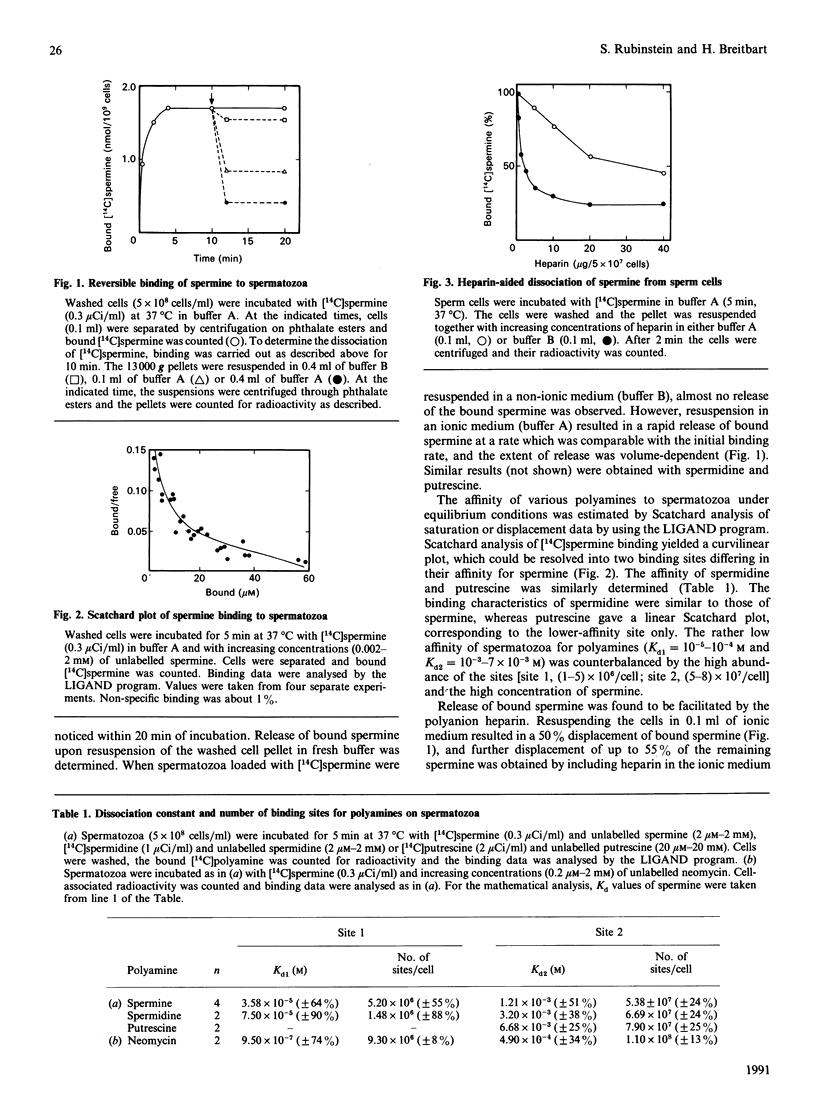
The binding properties of seminal polyamines to ram spermatozoa and their possible role in sperm capacitation and the acrosome reaction were studied. Binding and release of [14C]spermine from ram spermatozoa occurred at a rate faster than in somatic cells and were not energy-dependent. Release of bound spermine was further facilitated by heparin, a constituent of the female reproductive tract which was reported to induce capacitation and the acrosome reaction. High- and low-affinity polyamine-binding sites were identified, of which the high-affinity site was specific to polyamines with three or more amino groups. We also found that spermine inhibited the acrosome reaction and propose that it is the major seminal decapacitating factor. Since precise timing of capacitation and the acrosome reaction are critical for successful fertilization, it is suggested that the role of seminal spermine is to prevent premature capacitation and the acrosome reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R. The capacitation of the mammalian sperm. Nature. 1952 Aug 23;170(4321):326–326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Ahmed K., Goueli S. A., Williams-Ashman H. G. Polyamine-like effects of aminoglycosides on various messenger-independent protein kinase reactions. Biochim Biophys Acta. 1988 Sep 8;966(3):384–389. doi: 10.1016/0304-4165(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Ballas S. K., Mohandas N., Marton L. J., Shohet S. B. Stabilization of erythrocyte membranes by polyamines. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1942–1946. doi: 10.1073/pnas.80.7.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Av P., Rubinstein S., Breitbart H. Induction of acrosomal reaction and calcium uptake in ram spermatozoa by ionophores. Biochim Biophys Acta. 1988 Apr 7;939(2):214–222. doi: 10.1016/0005-2736(88)90065-x. [DOI] [PubMed] [Google Scholar]
- CHANG M. C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951 Oct 20;168(4277):697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Casillas E. R., Elder C. M., Hoskins D. D. Adenylate cyclase activity of bovine spermatozoa during maturation in the epididymis and the activation of sperm particulate adenylate cyclase by GTP and polyamines. J Reprod Fertil. 1980 Jul;59(2):297–302. doi: 10.1530/jrf.0.0590297. [DOI] [PubMed] [Google Scholar]
- Casslén B., Nilsson B. Human uterine fluid, examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin, glucose, and urea. Am J Obstet Gynecol. 1984 Dec 1;150(7):877–881. doi: 10.1016/0002-9378(84)90466-6. [DOI] [PubMed] [Google Scholar]
- Eichberg J., Zetusky W. J., Bell M. E., Cavanagh E. Effects of polyamines on calcium-dependent rat brain phosphatidylinositol-phosphodiesterase. J Neurochem. 1981 May;36(5):1868–1871. doi: 10.1111/j.1471-4159.1981.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Fleming A. D., Armstrong D. T. Effects of polyamines upon capacitation and fertilization in the guinea pig. J Exp Zool. 1985 Jan;233(1):93–100. doi: 10.1002/jez.1402330113. [DOI] [PubMed] [Google Scholar]
- HOMBURGER F., BERNFELD P., TREGIER A., GROSSMAN M. S., HARPEL P. Endometrial secretions. Ann N Y Acad Sci. 1963 Mar 30;106:683–691. [PubMed] [Google Scholar]
- Larsson L. I., Mørch-Jørgensen L., Hougaard D. M. Cellular and subcellular localization of polyamines cytochemical methods providing new clues to polyamine function in normal and neoplastic cells. Histochemistry. 1982;76(2):159–174. doi: 10.1007/BF00501919. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nahas N., Graff G. Inhibitory activity of polyamines on phospholipase C from human platelets. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1035–1040. doi: 10.1016/0006-291x(82)92043-5. [DOI] [PubMed] [Google Scholar]
- Parrish J. J., Susko-Parrish J., Winer M. A., First N. L. Capacitation of bovine sperm by heparin. Biol Reprod. 1988 Jun;38(5):1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- Pulkkinen P., Sinervirta R., Jänne J. Modification of the metabolism of rat epididymal spermatozoa by spermine. Biochem Biophys Res Commun. 1975 Nov 17;67(2):714–722. doi: 10.1016/0006-291x(75)90871-2. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Harrison R. A. Polyphosphoinositide breakdown and subsequent exocytosis in the Ca2+/ionophore-induced acrosome reaction of mammalian spermatozoa. Biochem J. 1989 Apr 15;259(2):397–406. doi: 10.1042/bj2590397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufo G. A., Jr, Schoff P. K., Lardy H. A. Regulation of calcium content in bovine spermatozoa. J Biol Chem. 1984 Feb 25;259(4):2547–2552. [PubMed] [Google Scholar]
- Russell L., Peterson R., Freund M. Direct evidence for formation of hybrid vesicles by fusion of plasma and outer acrosomal membranes during the acrosome reaction in boar spermatozoa. J Exp Zool. 1979 Apr;208(1):41–56. doi: 10.1002/jez.1402080106. [DOI] [PubMed] [Google Scholar]
- Saling P. M., Storey B. T., Wolf D. P. Calcium-dependent binding of mouse epididymal spermatozoa to the zona pellucida. Dev Biol. 1978 Aug;65(2):515–525. doi: 10.1016/0012-1606(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Dezeure F. Polyamine transport in mammalian cells. Int J Biochem. 1990;22(3):211–218. doi: 10.1016/0020-711x(90)90332-w. [DOI] [PubMed] [Google Scholar]
- Shah G. V., Sheth A. R. Inhibition of phosphodiesterase activity of human spermatozoa by spermine. Experientia. 1978 Aug 15;34(8):980–981. doi: 10.1007/BF01915298. [DOI] [PubMed] [Google Scholar]
- Sheth A. R., Gunjikar A. N., Shah G. V. Effect of LH, prolactin and spermine on ATPase activity of human spermatozoa. Andrologia. 1979 Jan;11(1):11–14. doi: 10.1111/j.1439-0272.1979.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G. Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem. 1984;58(1-2):51–61. doi: 10.1007/BF00240604. [DOI] [PubMed] [Google Scholar]
- Wilson W. L., Oliphant G. Isolation and biochemical characterization of the subunits of the rabbit sperm acrosome stabilizing factor. Biol Reprod. 1987 Aug;37(1):159–169. doi: 10.1095/biolreprod37.1.159. [DOI] [PubMed] [Google Scholar]


