Abstract
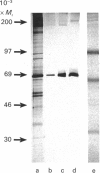
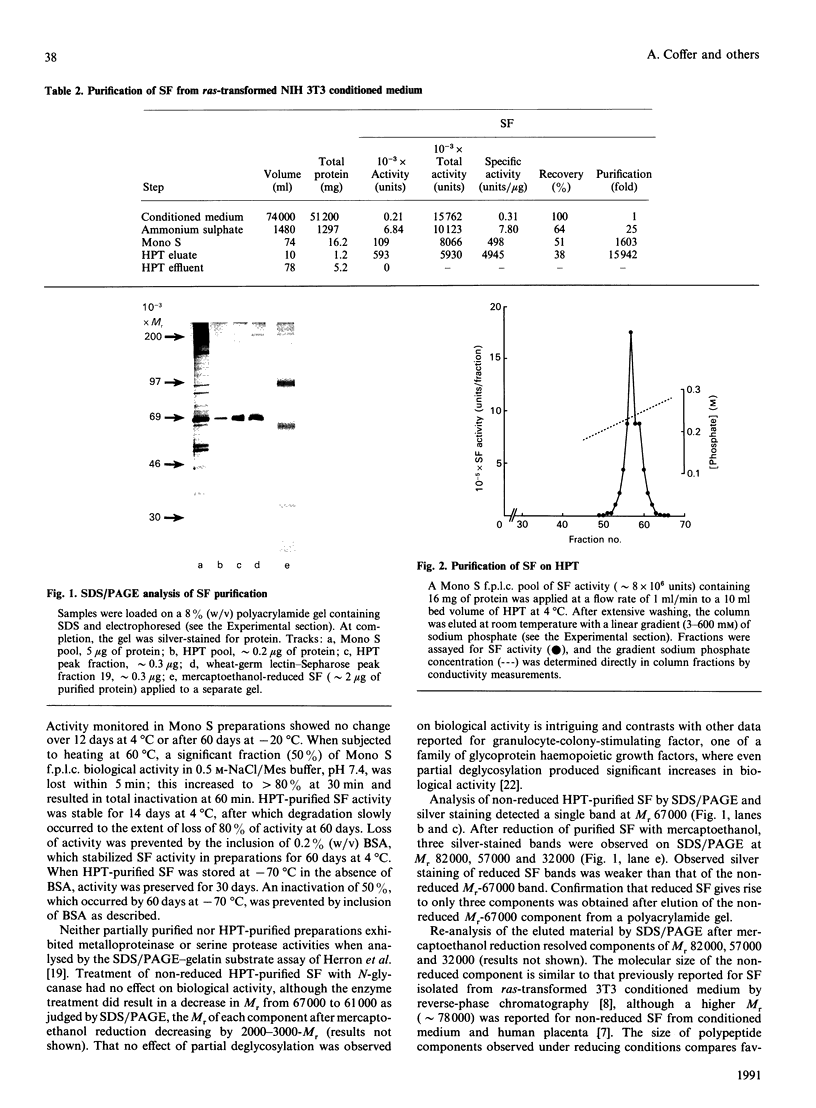
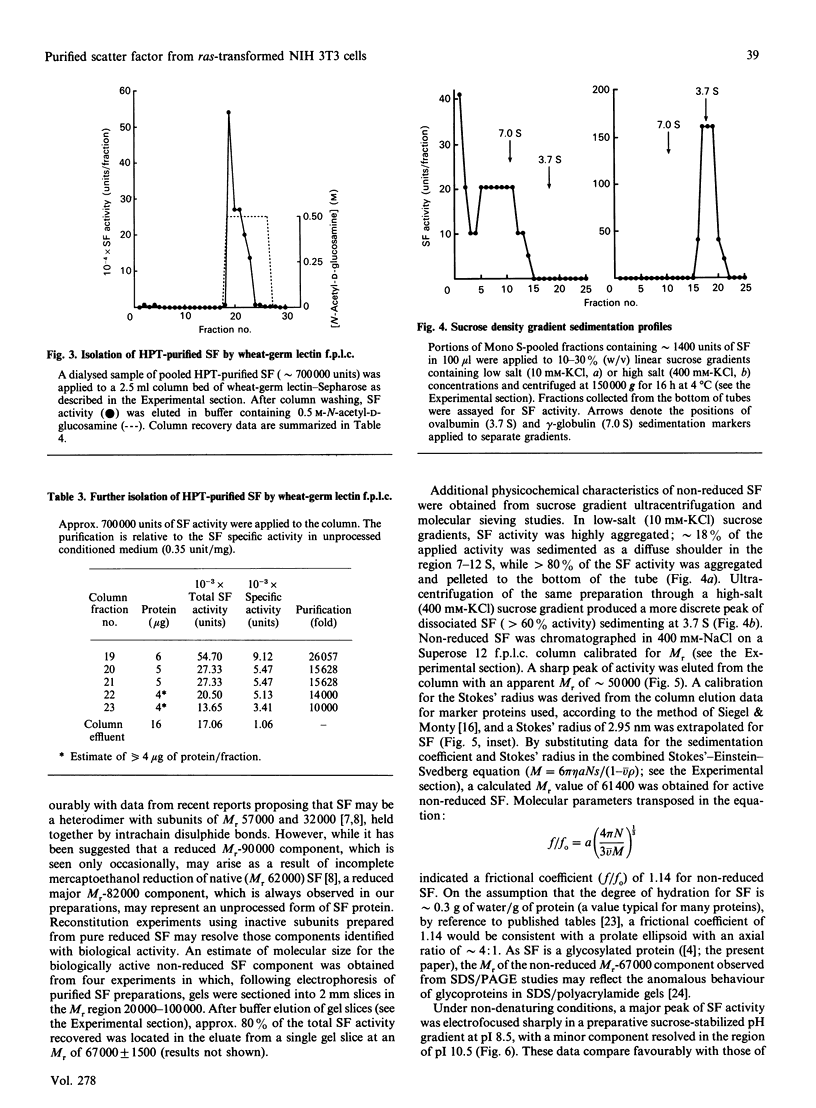
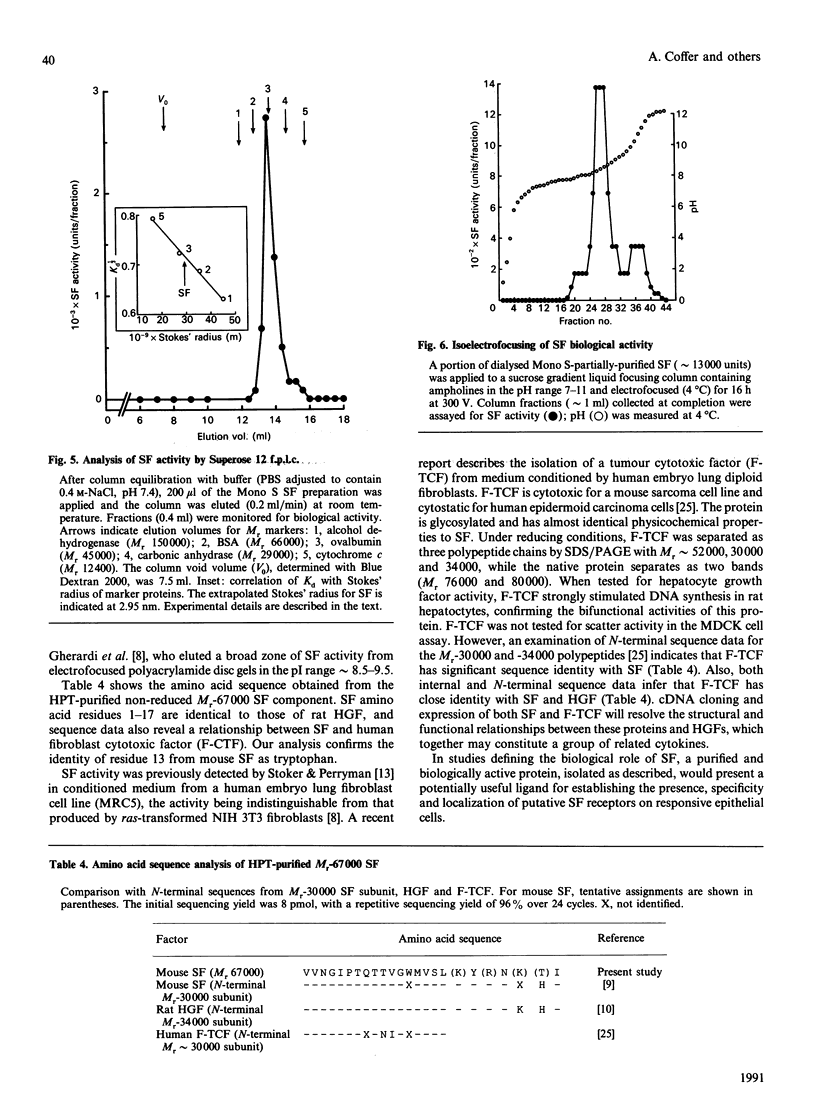
Scatter factor (SF), a glycoprotein produced by cultured fibroblasts, acts in vitro on epithelial cells causing separation and increased local motility. In this study, the polypeptide was purified to apparent homogeneity in high yields with conserved biological activity from medium conditioned by ras-transformed NIH 3T3 cells, by a three-step procedure involving ammonium sulphate fractionation, cation-exchange and hydroxyapatite chromatography. After purification, SF specific activity increased from approximately 0.3 units/microgram in unprocessed conditioned medium to approximately 5 units/ng, and cumulative recovery of biological activity was approximately 38%. Treatment of pure SF with N-glycanase resulted in a decreased Mr, but no concomitant effect was observed on biological activity. Proteolytic activity was absent from samples of both partially purified and pure SF. Our biochemical studies showed that SF, which is highly aggregated in low-ionic-strength media, is not aggregated in 0.4 M-salt. Under non-reducing conditions, pure SF migrated as a single stained band at Mr 67,000 on SDS/PAGE, and biological activity was eluted from unstained gels with an identical Mr. SF was electrofocused sharply at pI 8.5 with no degradation of activity. From ultracentrifugation studies (under non-aggregating conditions), the sedimentation coefficient of active SF was 3.7 S and f.p.l.c. molecular sieve chromatography indicated a Stokes' radius of 2.95 nm. The calculated Mr from these data was 61,400. The appearance of three stained polypeptides of Mr 82,000, 57,000 and 32,000 derived from the Mr-67,000 constituent after reduction with mercaptoethanol suggests that SF may be a heterodimer of Mr-57,000 and -32,000 subunits. Data from protein sequence analysis of the hydroxyapatite-purified protein confirms that SF has sequence identity with both rat hepatocyte growth factor and human fibroblast tumour cytotoxic factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Cebon J., Nicola N., Ward M., Gardner I., Dempsey P., Layton J., Dührsen U., Burgess A. W., Nice E., Morstyn G. Granulocyte-macrophage colony stimulating factor from human lymphocytes. The effect of glycosylation on receptor binding and biological activity. J Biol Chem. 1990 Mar 15;265(8):4483–4491. [PubMed] [Google Scholar]
- Coffer A. I., Lewis K. M., Brockas A. J., King R. J. Monoclonal antibodies against a component related to soluble estrogen receptor. Cancer Res. 1985 Aug;45(8):3686–3693. [PubMed] [Google Scholar]
- Coffer A. I., Milton P. J., Pryse-Davies J., King R. J. Purification of oestradiol receptor from human uterus by affinity chromotrgraphy. Mol Cell Endocrinol. 1977 Feb;6(4-5):231–246. doi: 10.1016/0303-7207(77)90098-3. [DOI] [PubMed] [Google Scholar]
- Gherardi E., Gray J., Stoker M., Perryman M., Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E., Stoker M. Hepatocytes and scatter factor. Nature. 1990 Jul 19;346(6281):228–228. doi: 10.1038/346228b0. [DOI] [PubMed] [Google Scholar]
- Gorbunoff M. J. The interaction of proteins with hydroxyapatite. I. Role of protein charge and structure. Anal Biochem. 1984 Feb;136(2):425–432. doi: 10.1016/0003-2697(84)90239-2. [DOI] [PubMed] [Google Scholar]
- Grey A. M., Schor A. M., Rushton G., Ellis I., Schor S. L. Purification of the migration stimulating factor produced by fetal and breast cancer patient fibroblasts. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2438–2442. doi: 10.1073/pnas.86.7.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Higashio K., Shima N., Goto M., Itagaki Y., Nagao M., Yasuda H., Morinaga T. Identity of a tumor cytotoxic factor from human fibroblasts and hepatocyte growth factor. Biochem Biophys Res Commun. 1990 Jul 16;170(1):397–404. doi: 10.1016/0006-291x(90)91287-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Teramoto H., Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappin D. J., Coull J. M., Köster H. Solid-phase sequence analysis of proteins electroblotted or spotted onto polyvinylidene difluoride membranes. Anal Biochem. 1990 May 15;187(1):10–19. doi: 10.1016/0003-2697(90)90410-b. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D., Kacinski B. M., Buckholz T., Vinter D. W. Smooth muscle releases an epithelial cell scatter factor which binds to heparin. In Vitro Cell Dev Biol. 1989 Feb;25(2):163–173. doi: 10.1007/BF02626174. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D. Protein factors which regulate cell motility. In Vitro Cell Dev Biol. 1989 Dec;25(12):1079–1087. doi: 10.1007/BF02621258. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Meromsky L., Romero R., Setter E., Goldberg I. Human placenta contains an epithelial scatter protein. Biochem Biophys Res Commun. 1990 May 16;168(3):1082–1088. doi: 10.1016/0006-291x(90)91140-n. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Stoker M. Effect of scatter factor on motility of epithelial cells and fibroblasts. J Cell Physiol. 1989 Jun;139(3):565–569. doi: 10.1002/jcp.1041390316. [DOI] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985 Aug;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Hagiya M., Nishizawa T., Seki T., Shimonishi M., Shimizu S., Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]