Abstract
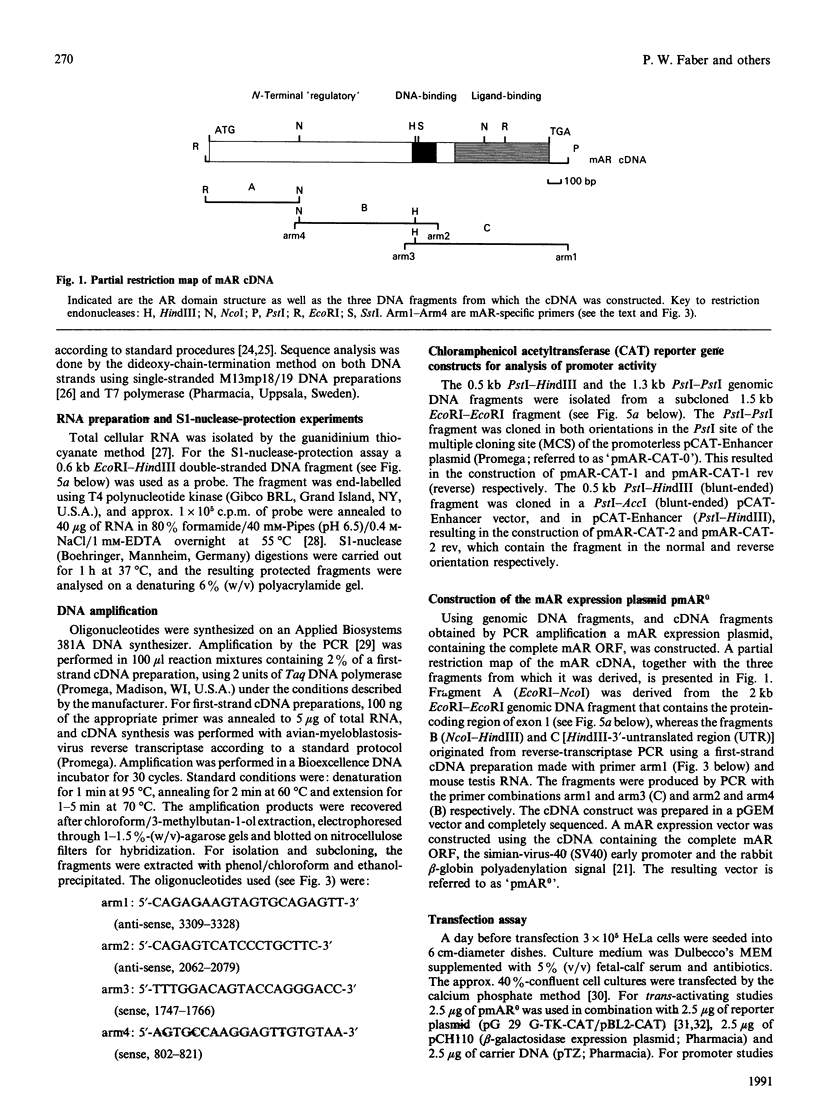
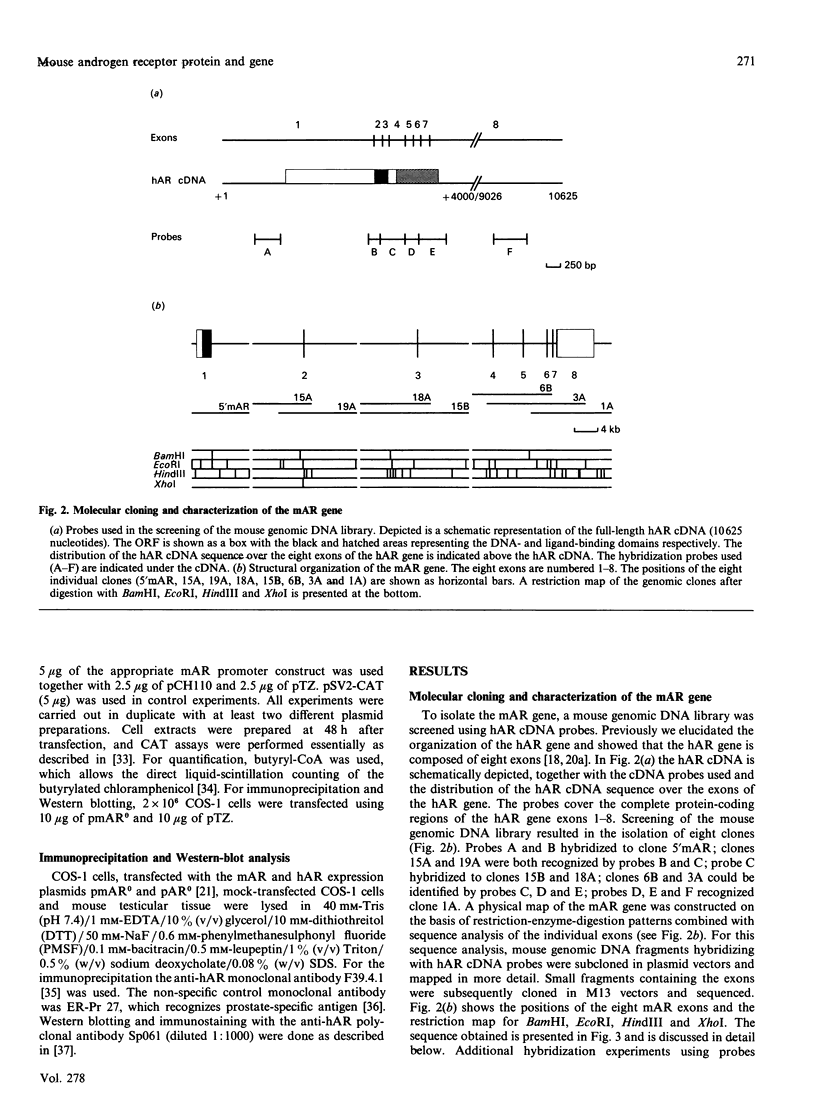
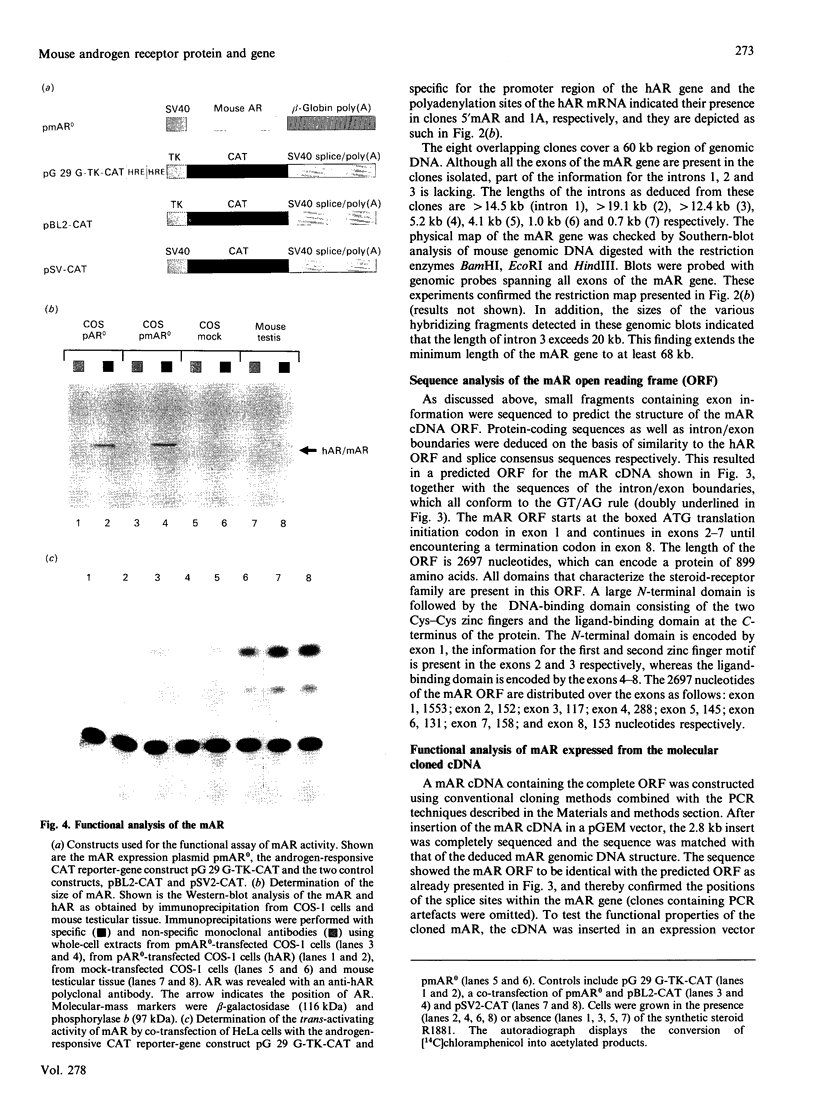
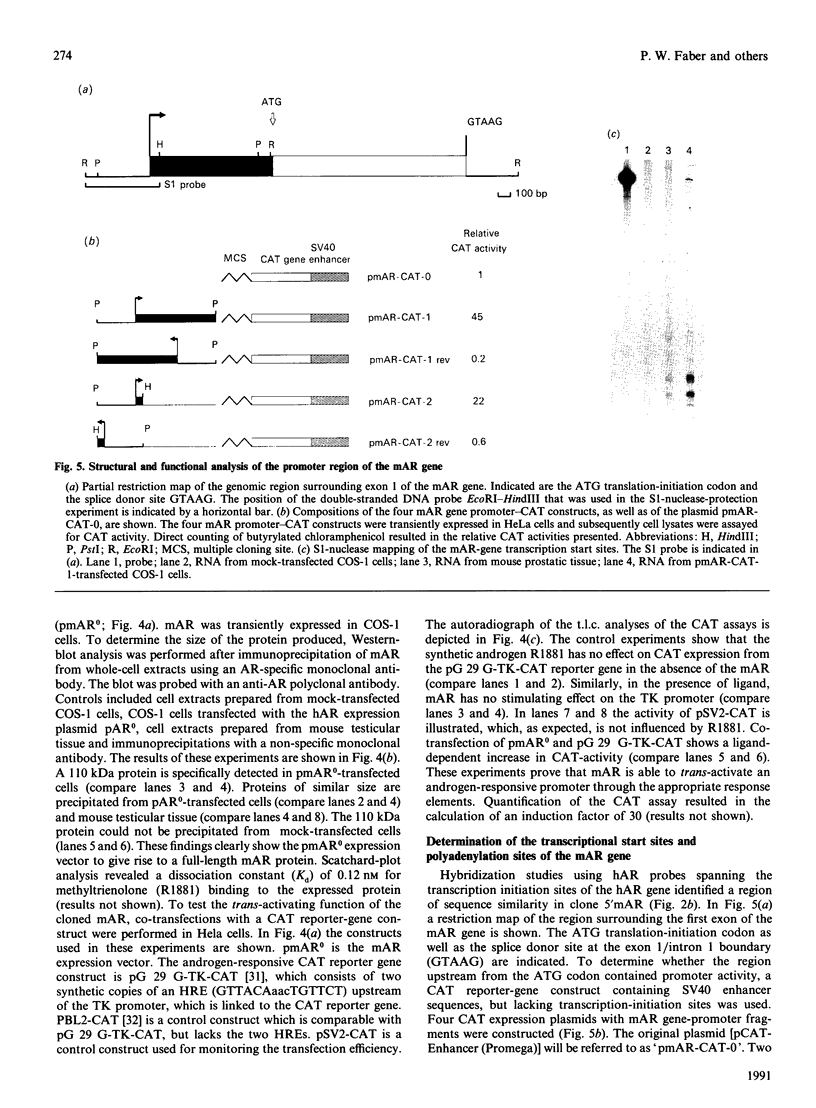
Screening a mouse genomic DNA library with human androgen-receptor (hAR) cDNA probes resulted in the isolation and characterization of eight genomic fragments that contain the eight exons of the mouse androgen-receptor (mAR) gene. On the basis of similarity to the hAR gene, the nucleotide sequences of the protein-coding parts of the exons as well as the sequences of the intron/exon boundaries were determined. An open reading frame (ORF) of 2697 nucleotides, which can encode an 899-amino-acid protein, could be predicted. The structure of the mAR ORF was confirmed by sequence analysis of mAR cDNA fragments, which were obtained by PCR amplification of mouse testis cDNA, using mAR specific primers. A eukaryotic mAR expression vector was constructed and mAR was transiently expressed in COS-1 cells. The expressed protein was shown by Western blotting to be identical in size with the native mAR. Co-transfection of HeLa cells with the mAR expression plasmid and an androgen-responsive chloramphenicol acetyltransferase (CAT) reporter-gene construct showed mAR to be able to trans-activate the androgen-responsive promoter in a ligand-dependent manner. Transcription-initiation sites of the mAR gene were identified by S1-nuclease protection experiments, and the functional activity of the promoter region was determined by transient expression of mAR promoter-CAT-reporter-gene constructs in HeLa cells. Structural analysis revealed the promoter of the mAR gene to be devoid of TATA/CCAAT elements. In addition, the promoter region is not remarkably (G + C)-rich. Potential promoter elements consist of a consensus Sp1 binding sequence and a homopurine stretch. The polyadenylation sites of mAR mRNA were identified by sequence similarity to the corresponding sites in the hAR mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baarends W. M., Themmen A. P., Blok L. J., Mackenbach P., Brinkmann A. O., Meijer D., Faber P. W., Trapman J., Grootegoed J. A. The rat androgen receptor gene promoter. Mol Cell Endocrinol. 1990 Nov 12;74(1):75–84. doi: 10.1016/0303-7207(90)90207-o. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brinkmann A. O., Faber P. W., van Rooij H. C., Kuiper G. G., Ris C., Klaassen P., van der Korput J. A., Voorhorst M. M., van Laar J. H., Mulder E. The human androgen receptor: domain structure, genomic organization and regulation of expression. J Steroid Biochem. 1989;34(1-6):307–310. doi: 10.1016/0022-4731(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Lubahn D. B., Wilson E. M., Joseph D. R., French F. S., Migeon C. J. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8151–8155. doi: 10.1073/pnas.85.21.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber P. W., Kuiper G. G., van Rooij H. C., van der Korput J. A., Brinkmann A. O., Trapman J. The N-terminal domain of the human androgen receptor is encoded by one, large exon. Mol Cell Endocrinol. 1989 Feb;61(2):257–262. doi: 10.1016/0303-7207(89)90137-8. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gallee M. P., Visser-de Jong E., van der Korput J. A., van der Kwast T. H., ten Kate F. J., Schroeder F. H., Trapman J. Variation of prostate-specific antigen expression in different tumour growth patterns present in prostatectomy specimens. Urol Res. 1990;18(3):181–187. doi: 10.1007/BF00295844. [DOI] [PubMed] [Google Scholar]
- Gaspar M. L., Meo T., Tosi M. Structure and size distribution of the androgen receptor mRNA in wild-type and Tfm/Y mutant mice. Mol Endocrinol. 1990 Oct;4(10):1600–1610. doi: 10.1210/mend-4-10-1600. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- He W. W., Fischer L. M., Sun S., Bilhartz D. L., Zhu X. P., Young C. Y., Kelley D. B., Tindall D. J. Molecular cloning of androgen receptors from divergent species with a polymerase chain reaction technique: complete cDNA sequence of the mouse androgen receptor and isolation of androgen receptor cDNA probes from dog, guinea pig and clawed frog. Biochem Biophys Res Commun. 1990 Sep 14;171(2):697–704. doi: 10.1016/0006-291x(90)91202-4. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Sinn E., Yamamoto T., Wang J., Roy A., Horikoshi M., Roeder R. G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor (TFIID). Nature. 1990 Jul 26;346(6282):387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Murphy M., George D. L. An S1 nuclease-sensitive homopurine/homopyrimidine domain in the c-Ki-ras promoter interacts with a nuclear factor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2705–2709. doi: 10.1073/pnas.87.7.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckaby C. S., Conneely O. M., Beattie W. G., Dobson A. D., Tsai M. J., O'Malley B. W. Structure of the chromosomal chicken progesterone receptor gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8380–8384. doi: 10.1073/pnas.84.23.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Jeltsch J. M., Turcotte B., Garnier J. M., Lerouge T., Krozowski Z., Gronemeyer H., Chambon P. Characterization of multiple mRNAs originating from the chicken progesterone receptor gene. Evidence for a specific transcript encoding form A. J Biol Chem. 1990 Mar 5;265(7):3967–3974. [PubMed] [Google Scholar]
- Johnson A. C., Jinno Y., Merlino G. T. Modulation of epidermal growth factor receptor proto-oncogene transcription by a promoter site sensitive to S1 nuclease. Mol Cell Biol. 1988 Oct;8(10):4174–4184. doi: 10.1128/mcb.8.10.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. C., Lieberman P. M., Schmidt M. C., Zhou Q., Pei R., Berk A. J. Cloning of a transcriptionally active human TATA binding factor. Science. 1990 Jun 29;248(4963):1646–1650. doi: 10.1126/science.2194289. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sar M., Tan J., Higgs H. N., Larson R. E., French F. S., Wilson E. M. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988 Dec;2(12):1265–1275. doi: 10.1210/mend-2-12-1265. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240(4850):327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Griffin J. E., Wilson J. D., McPhaul M. J. Definition of the human androgen receptor gene structure permits the identification of mutations that cause androgen resistance: premature termination of the receptor protein at amino acid residue 588 causes complete androgen resistance. Mol Endocrinol. 1990 Aug;4(8):1105–1116. doi: 10.1210/mend-4-8-1105. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Wilson J. D., Griffin J. E., McPhaul M. J. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J Clin Invest. 1990 May;85(5):1522–1528. doi: 10.1172/JCI114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Pinsky L., Kaufman M. Genetics of steroid receptors and their disorders. Adv Hum Genet. 1987;16:299–472. doi: 10.1007/978-1-4757-0620-8_5. [DOI] [PubMed] [Google Scholar]
- Ponglikitmongkol M., Green S., Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988 Nov;7(11):3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby V. E., Kemppainen J. A., Sar M., Lubahn D. B., French F. S., Wilson E. M. Expression of recombinant androgen receptor in cultured mammalian cells. Mol Endocrinol. 1990 Sep;4(9):1399–1407. doi: 10.1210/mend-4-9-1399. [DOI] [PubMed] [Google Scholar]
- Ris-Stalpers C., Kuiper G. G., Faber P. W., Schweikert H. U., van Rooij H. C., Zegers N. D., Hodgins M. B., Degenhart H. J., Trapman J., Brinkmann A. O. Aberrant splicing of androgen receptor mRNA results in synthesis of a nonfunctional receptor protein in a patient with androgen insensitivity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7866–7870. doi: 10.1073/pnas.87.20.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundlett S. E., Wu X. P., Miesfeld R. L. Functional characterizations of the androgen receptor confirm that the molecular basis of androgen action is transcriptional regulation. Mol Endocrinol. 1990 May;4(5):708–714. doi: 10.1210/mend-4-5-708. [DOI] [PubMed] [Google Scholar]
- Sai T. J., Seino S., Chang C. S., Trifiro M., Pinsky L., Mhatre A., Kaufman M., Lambert B., Trapman J., Brinkmann A. O. An exonic point mutation of the androgen receptor gene in a family with complete androgen insensitivity. Am J Hum Genet. 1990 Jun;46(6):1095–1100. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Ogg S. C., Wickens M. P. Point mutations in AAUAAA and the poly (A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acids Res. 1990 Oct 11;18(19):5799–5805. doi: 10.1093/nar/18.19.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. A., Joseph D. R., Quarmby V. E., Lubahn D. B., Sar M., French F. S., Wilson E. M. The rat androgen receptor: primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Mol Endocrinol. 1988 Dec;2(12):1276–1285. doi: 10.1210/mend-2-12-1276. [DOI] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., McPhaul M. J. Expression of the human androgen receptor gene utilizes a common promoter in diverse human tissues and cell lines. J Biol Chem. 1990 Aug 15;265(23):13776–13781. [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapman J., Klaassen P., Kuiper G. G., van der Korput J. A., Faber P. W., van Rooij H. C., Geurts van Kessel A., Voorhorst M. M., Mulder E., Brinkmann A. O. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988 May 31;153(1):241–248. doi: 10.1016/s0006-291x(88)81214-2. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Zahraoui A., Cuny G. Nucleotide sequence of the chicken proto-oncogene c-erbA corresponding to domain 1 of v-erbA. Eur J Biochem. 1987 Jul 1;166(1):63–69. doi: 10.1111/j.1432-1033.1987.tb13484.x. [DOI] [PubMed] [Google Scholar]
- Zegers N. D., Claassen E., Neelen C., Mulder E., van Laar J. H., Voorhorst M. M., Berrevoets C. A., Brinkmann A. O., van der Kwast T. H., Ruizeveld de Winter J. A. Epitope prediction and confirmation for the human androgen receptor: generation of monoclonal antibodies for multi-assay performance following the synthetic peptide strategy. Biochim Biophys Acta. 1991 Jan 23;1073(1):23–32. doi: 10.1016/0304-4165(91)90178-j. [DOI] [PubMed] [Google Scholar]
- van Laar J. H., Voorhorst-Ogink M. M., Zegers N. D., Boersma W. J., Claassen E., van der Korput J. A., Ruizeveld de Winter J. A., van der Kwast T. H., Mulder E., Trapman J. Characterization of polyclonal antibodies against the N-terminal domain of the human androgen receptor. Mol Cell Endocrinol. 1989 Nov;67(1):29–38. doi: 10.1016/0303-7207(89)90227-x. [DOI] [PubMed] [Google Scholar]




