Abstract
Objective
This study aims to explore the effect of Internet + E-Coach chronic disease system intervention on fasting blood glucose (FPG), 2-hour postprandial blood glucose(2hPG), fasting serum insulin (FINS), triglyceride (TG), alanine transaminase (ALT) and quality of life in patients with chronic diabetic retinopathy.
Methods
208 patients with chronic diabetic retinopathy who were treated in the hospital from March 2021 to March 2023 are chosen and separated into two groups by random number Table 104 patients in the control group received routine continuous intervention, and the research group received Internet + E-Coach chronic disease system intervention. The cognition of disease related knowledge, blood related indicators inflammatory factor levels and improvement of life quality between the two groups before intervention, 6 and 12 months after intervention were compared.
Results
Before the intervention, the comparison between the two groups in disease related knowledge scores, blood glucose, blood lipid, liver function indicators, inflammatory factor, and low vision quality of life scale (CLVQOL) scores was with P > 0.05. After 6 and 12 months of intervention, the research group had significantly higher scores for basic blood glucose intervention, healthy diet, reasonable exercise, and correct medication use compared to the control group (P < 0.05). FPG, 2hPG, TG and ALT in the research group were lower than those in the control group. FINS were higher in the control group, with P < 0.05. Interleukin-6 (IL-6), tumor necrosis factor (TNF-α), and hypersensitive C-reactive protein (Hs-CRP) in the research group were obviously lower than those in the control group (P < 0.05). The scores of far vision, movement and light perception, adjustment ability, reading and fine work, and daily living ability in the research group were higher than those in the control group, with P < 0.05.
Conclusion
The intervention of Internet + E-Coach chronic disease system can improve the knowledge of chronic diabetic retinopathy patients about their own condition, stabilize the levels of blood sugar, blood lipid and liver function indicators, reduce the inflammatory reaction of the body, and improve the quality of life.
Keywords: Internet + E-Coach chronic disease system; Chronic diabetic retinopathy; Blood glucose, blood lipid, alanine transaminase; Quality of life
Background
Chronic diabetic retinopathy (DR) is a critical complication of diabetes patients. Foreign data survey showed that the average incidence rate of disease can reach more than 50%, and disease is an important cause of blindness [1]. At present, DR treatment is mainly aimed at delaying the progress of the disease and protecting vision. It can improve the condition through the management of diabetes, drug treatment, laser treatment or surgery. However, the course of the disease is long, and it is necessary to maintain the stable state of blood sugar level after the implementation of treatment to maintain the condition. However, the majority of patients are elderly and have a poor level of knowledge cognition, which leads to a decrease in compliance with rehabilitation, abnormal changes in disease-related blood indicators, increased body inflammatory response, worsened condition, and decreased quality of life.
Research of Dorna showed that most elderly patients with diabetes have poor knowledge of their own condition, low drug compliance during treatment, and large fluctuations in their condition [2]. Therefore, in the rehabilitation of DR patients, it needs to provide scientific and effective intervention measures, which are very important for maintaining the condition and promoting prognosis. Mounirou et al. [3] conducted research to provide comprehensive and targeted continuous intervention measures for DR patients, which can ensure their daily medication and maintain good habits.
The Internet + E-Coach chronic disease system is a new form of clinical care that combines health coach technology with the Internet, transforming patients’ own behavior into a theoretical basis, following the treatment and recovery process of chronic diseases. Researcher ensures that patients can receive the same level of intervention guidance at different locations to improve patients’ self-management ability and compliance in home rehabilitation [4]. Sahar [5] applied the E-Coach chronic disease system intervention to hypertension patients, enhancing their understanding of the condition, improving their self-management ability, and maintaining stable blood pressure levels. This topic applied Internet + E-Coach chronic disease system intervention in patients with chronic DR, and analyzed the nursing effect. Now it is reported as follows. For this reason, the main objective of this study was to evaluate the effects of the Internet + E-Coach chronic disease system intervention on glucose, lipids, liver function and quality of life in patients with DR. Meanwhile, to further clarify the effectiveness of the system, this study asked a Population-Intervention-Comparison-Outcome (PICO) question, i.e., among patients suffering from chronic DR, whether the Internet + E-Coach chronic disease system intervention is more effective in improving blood glucose, liver function, and quality of life compared with conventional continuous intervention, is it more effective in improving patients’ blood glucose, lipid, liver function levels and quality of life? It is reported as follows. This study hypothesizes that the Internet + E-Coach system will significantly improve these health indicators and improve patients’ quality of life compared to conventional continuous intervention.
Methods
Research materials
This study was designed as a randomized controlled trial (RCT) with the expectation of recruiting approximately 220 patients with chronic DR, and 208 patients with chronic DR (DR) who met the criteria and were treated at hospital between March 2021 and March 2023 were actually included. Patients were randomly assigned to either the research group or the control group in a 1:1 ratio of 104 patients per group by a computer-generated random number table to ensure that there were no systematic differences between groups. To minimize selection bias, the inclusion and exclusion criteria of the sample were strictly controlled to ensure that the two groups were comparable in terms of baseline characteristics. Intervention implementers were aware of the patient groups, and data analysts remained blinded. There were 56 males and 48 females in the control group. Age ranged from 45 to 58 years old, with an average age of (52.48 ± 5.38) years. The disease course was 1–8 months, with an average course of (4.15 ± 2.01) months. Education background: 22 cases of junior high school or below, 61 cases of high school or technical secondary school, and 21 cases of college or above. Body mass index (BMI): 19–26 kg/m2, with an average BMI of (24.18 ± 1.26) kg/m2,. There were 59 males and 41 females in the research group. Age ranged from 47 to 60 years old, with an average age of (52.93 ± 5.14) years. The course of the disease was 2–9 months, with an average course of (4.42 ± 2.11) months. Education background: 24 cases of junior high school or below, 65 cases of high school or technical secondary school, and 15 cases of college or above. BMI: 20–27 kg/m2, with an average BMI of (24.52 ± 1.66) kg/m2. The comparison in gender, age, course of disease, cultural background, and BMI was with P > 0.05. At the beginning of the study, all included patients were assessed at baseline, covering disease duration, degree of retinopathy, and general health status, and potential confounders such as age, gender, duration of diabetes, and baseline health status (e.g., hypertension, coronary artery disease, etc.) were recorded. Based on the previous studies and literature review, it was assumed that the mean difference between the intervention and control groups in the main outcome indicators, such as FPG, 2hPG, TG, etc., was 0.5 standard deviations, and a two-sided test was used, with the level set at 0.05 and the target statistical validity set at 0.80. The sample size was calculated as shown in Eq. (1).
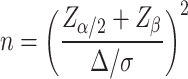 |
1 |
In Eq. (1), 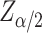 denotes the critical value of the standard normal distribution, and if
denotes the critical value of the standard normal distribution, and if 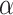 is 0.05, then the value of Z is 1.96;
is 0.05, then the value of Z is 1.96; 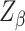 denotes the test efficacy;
denotes the test efficacy; 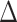 denotes the effect size between the two groups; and
denotes the effect size between the two groups; and 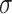 denotes the sample standard deviation. Considering the effect size and expected between-group differences, the minimum sample size required for this study was estimated to be 95 patients per group. To ensure adequate statistical validity and to avoid bias, it ultimately increased the sample size to 104 cases per group, for a total sample size of 208 cases.
denotes the sample standard deviation. Considering the effect size and expected between-group differences, the minimum sample size required for this study was estimated to be 95 patients per group. To ensure adequate statistical validity and to avoid bias, it ultimately increased the sample size to 104 cases per group, for a total sample size of 208 cases.
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria of this study were strictly based on the 2014 China DR Clinical Diagnosis and Treatment Guidelines [6] to ensure the diagnostic accuracy and consistency of the sample. (A) Patients should to meet the diagnostic criteria for chronic DR in the 2014 China DR Clinical Diagnosis and Treatment Guidelines. (B) Patients should have normal cognitive function and audio-visual ability, and be able to understand and participate in the study. (C) The patient’s condition should be stable and all vital signs should be within normal range. (D) The patient and his/her legal representative should give informed consent and be willing to comply with the study requirements.
Exclusion criteria
(A) Allergic reactions, patients with a history of severe allergic reactions to commonly used hypoglycemic agents or any of the drugs involved in this study. (B) Disease stage, patients in the proliferative phase of DR, which may affect the effectiveness of the intervention due to complication. (C) Serious complications, patients with severe diabetic complications such as diabetic foot, diabetic nephropathy, diabetic ketoacidosis, etc. (D) Dysfunction, patients with severe hepatic or renal dysfunction or coagulopathy.
Selection process
After initial screening, all patients who meet the inclusion criteria and do not fulfill the exclusion criteria will undergo further evaluation. In contrast to the general DR patient population, this assessment process was conducted by an independent researcher who is not involved in the implementation of the intervention, ensuring a fair and consistent inclusion and exclusion process for all patients. During this process, the researcher was not involved in the decision of subgroups to maintain randomization and blinding.
Research method
Patients in both groups received individualized medication with gliclazide extended-release tablets, metformin hydrochloride, and acarbose during the period of care, with regimens developed and maintained by the attending physician. Data collection took place before the intervention (1 day before discharge), and at 6 and 12 months post-intervention, and measures included FPG, 2hPG, FINS, TG, ALT, IL-6, TNF-α, Hs-CRP, and quality of life (CLVQOL scale). The CLVQOL scale was in self-report form and had been validated in a similar patient population. All data were collected by trained and qualified researchers using standardized instruments to minimize measurement error. All blood samples were tested by the same model of fully automated biochemistry analyzer (Beckman Coulter AU5800) in the same ISO 15,189 accredited hospital laboratory to ensure data consistency and reliability. Specific drug use: the selected drugs included Gliclazide sustained-release tablets (manufacturer: Anhui Lianyi Pharmaceutical Co., Ltd.; National medicine permission number (NMPN): H20067657; Specification: 30 mg×2 plate × 15 tablets/box). Drug application method: the first dose was 30 mg/day, and if the blood sugar was stable, this dose should be maintained continuously. If blood sugar was not maintained well, the drug dose could be gradually increased to 60 mg, 90 mg, or 120 mg/day, with an interval of at least 1 month. If blood sugar was still high after 2 weeks of treatment, the drug dose could be increased after 2 weeks of treatment at this dose, and the max drug dose could be controlled below 120 mg/day. Researcher suggested that patients take medication at breakfast. Metformin hydrochloride (manufacturer: Baiyunshan Dongtai Shangqiu Pharmaceutical Co., Ltd.; NMPN: H20043292; Specification: 0.25 g × 48 tablets/bottle). Drug application method: the first medication was 0.25 g, 2–3 times/day, and the blood sugar was stable to maintain the drug dosage; If blood sugar was still not controlled, the drug dosage could be gradually increased to 1–1.5 g/day, and the daily medication should be less than 2 g. Patients were advised to take medication immediately during or after meals. Acarbose (manufacturer: Bayer Healthcare Co., Ltd.; NMPN: H20010716; specification: 50 mg × 30 tablets) Drug application method: the starting dose of medication could 50 mg/time, 3 times/day. If blood sugar control could poor, the drug dosage should be gradually increased to 0.1 g/day, 3 times per day, or according to individual medical advice, it could be increased to 0.2 g/day. Medications should be swallowed immediately before meals or chewed together with the first few bites of food. All participants were assessed for information technology prior to the intervention. For patients with low levels of information technology and no family support, the research team provided one-on-one training on platform login, data entry, and online communication. After training, participants were required to pass a competency test to ensure they could use the platform independently. To accommodate the working hours of most participants, the intervention design focused on flexibility, allowing them to record and interact in the morning, during lunch breaks, or in the evening. For participants with limited mobility or special needs, the platform provided customized advice and a reminder function to help them comply with the plan and adjust the intensity of the intervention to ensure that the intervention was practical and not burdensome.
Control group
Patients were assigned to the control group and continued to receive routine care, including daily diabetes management education and monthly telephone follow-ups, without experimental interventions or sham treatments. The control group visited the hospital every two months for comprehensive checkups, but there was no real-time data monitoring or automatic reminders. Patients carried self-monitoring records (e.g., blood glucose logbooks) at follow-up visits, and physicians adjusted treatment based on the records and test results. All data were maintained through the hospital’s electronic system and reviewed regularly. Follow-up visits were weekly before discharge and twice a month after discharge for 12 months.
Research group
The Internet + E-Coach chronic disease system intervention were Implemented, including daily medication reminders, diet and exercise advice, health education push, and regular online counseling. Patients recorded health data (e.g., blood glucose, blood lipids, liver function) through the APP, and the healthcare team adjusted intervention strategies accordingly. Patients used the platform for at least 30 min a day, and the platform generated personalized recommendations and pushed health education content, including diabetes management and diet control. Knowledge recommendations were delivered in the form of sub-modules containing videos, text materials and interactive quizzes, with a quiz at the end of each module. The platform monitored data in real time, detected abnormalities and notified patients and doctors, while reminding patients to take medication and record blood glucose on time. Every two months patients were required to go to the hospital for a comprehensive checkup, with weekly follow-ups before intervention and bimonthly follow-ups after discharge for 12 months to monitor the effects.
Observation indicators and evaluation
At the end of the 6-month follow-up, the number of remaining follow-ups was 98 in the research group and 96 in the control group. At the end of the 12-month follow-up, the number of remaining follow-ups was 95 in the research group and 93 in the control group. Some patients were unable to complete all follow-up visits due to personal reasons (e.g., relocation, change in health status, etc.), but overall the completion rate of follow-up visits was high in both groups, with no significant loss-of-follow-up bias. The following indicators were evaluated before intervention (1 day before discharge), 6 and 12 months after intervention.
Cognition of disease related knowledge: the department assisted the investigator to prepare a questionnaire of disease related knowledge based on the questionnaire of diet management knowledge for type 2 diabetes patients [7] and the actual situation of this study. During the development process, the study invited a number of experts in the field for content review and validated the reliability and validity of the questionnaire through a pre-test with 50 similar patients to ensure its applicability and accuracy. The questionnaire included 25 items, including basic intervention of blood sugar, healthy diet, reasonable exercise, and correct drug use. The total score of 0 ~ 5 grade scoring method was 100. The higher the score, the higher the patient’s awareness of various knowledge. A pre-test sample of 50 patients with DR was selected, who were similar to the study target population in terms of age, gender, and disease duration. The questionnaire data were analyzed using SPSS software, and Cronbach’s alpha coefficient was achieved by calculating the mean correlation between the items in the questionnaire, which took a value of 0.868, showing good internal consistency. Content validity was achieved by reviewing the questionnaire by five diabetes management experts, when the CVI was 0.895, indicating good content validity of the questionnaire.
Blood indicators: Outcomes assessed by the attending physician assisting the investigators prior to discharge and at review included standardized indicators for patients with diabetes, such as blood glucose levels (FPG, 2hPG), insulin levels, and lipid levels (TG). Attention was also given to outcomes specific to patients with DR, such as indicators of liver function (ALT) and inflammation (IL-6, TNF-α, Hs-CRP).
Inflammatory factor indicators: The attending physician assisted the investigator in conducting laboratory tests on IL-6, TNF-α, and Hs-CRP before discharge and during follow-up. Venous blood was taken from the patient on an empty stomach in the morning and enzyme-linked immunosorbent assay was utilized to monitor the above indicators.
Quality of life: The investigator was assisted by a responsible nurse to evaluate the CLVQOL using a total of 4 dimensions and 25 items [8]. The scale included far vision, movement and light perception (12 items), regulatory ability (4 items), reading and fine work (5 items), and daily living ability (4 items), with a score of 0–5 levels. The total score was 0-125 points. The higher the score, the better the quality of life. The Cronbach’s α coefficient of the scale was 0.891, and the content coefficient was 0.867, indicating good internal reliability and validity.
Participants in the intervention group acquired systematic health management knowledge through the Internet + E-Coach system, and the study predicted that their knowledge, blood sample values (insulin levels, glucose, lipids, liver function, and inflammatory markers), and quality of life using CLVQOL would be significantly higher than those of the control group at 6 and 12 months of the intervention.
Ethical considerations and informed consent
The study was proposed to first pass the ethical review of the hospital. The purpose and significance of the study were explained to the study subjects and after obtaining consent. The patients were assured that the privacy of the patients would not be disclosed. It was promised to the research subjects that the results of this study would be limited to the use of this study, would not involve their own rights and interests, and would not affect their normal treatment, in accordance with the principle of voluntary participation. The information of all research subjects would be replaced by the code, and the patient’s information would not be disclosed throughout the study and when the results were published.
Statistical data analysis
Data entered into statistical software SPSS25.0, [n (%)] was used to describe counting data, and inter group chi square χ2 inspection was utilized. The measurement data conforming to normal distribution were defined by mean ± standard deviation (x ± s), and independent t test was performed. It had statistically significant difference (P < 0.05). Meanwhile, to ensure the accuracy of the results, the study conducted multivariate regression analysis and analysis of variance (ANOVA) during the analysis of extraneous variables (such as patient’s age, gender, duration of the disease, cultural background, body mass index, etc.) that may affect the results to control the disturbance of the results of the study caused by these extraneous variables, and the difference was statistically significant (P < 0.05).
Results
Comparison of disease related knowledge cognition between two groups before intervention, 6 and 12 months after intervention
Before the intervention, there was no significant difference between the two groups in the cognitive scores of basic glycemic intervention, healthy diet, rational exercise and proper medication (P > 0.05), which indicated that the baseline levels of the two groups were similar. After 6 and 12 months of intervention, the research group showed significant improvement in all cognitive scores, and the scores were significantly higher than those of the control group (P < 0.05). Especially at 12 months, the research group showed a more significant increase in all scores, indicating that the Internet + E-Coach chronic disease system intervention had a significant advantage in promoting patients’ understanding and application of disease management knowledge. Through the chi-square test and independent samples t-test, it was found that the difference between the research group and the control group in several key clinical indicators was statistically significant (P < 0.05). The result was consistent with the study of Ding P et al. [10], both of which demonstrated that applying a similar E-Coach system to patients with bronchial asthma also significantly improved the level of awareness of disease knowledge, as denoted in Table 1.
Table 1.
Comparison of knowledge cognition between two groups before intervention, 6 and 12 months of intervention (‾x ± s, points)
| Norm | Period of intervention | Groups | n | x ± s, points | t | P |
|---|---|---|---|---|---|---|
| Basic blood glucose intervention | Before intervention | Control group | 104 | 10.08 ± 1.26 | 0.471 | 0.638 |
| Research group | 104 | 10.16 ± 1.19 | ||||
| Intervention for 6 months | Control group | 104 | 16.55 ± 2.48* | 2.884 | 0.004 | |
| Research group | 104 | 17.61 ± 2.81* | ||||
| Intervention for 12 months | Control group | 104 | 18.45 ± 3.00*# | 3.992 | 0.000 | |
| Research group | 104 | 20.15 ± 3.14*# | ||||
| Healthy diet | Before intervention | Control group | 104 | 8.02 ± 1.68 | 0.372 | 0.710 |
| Research group | 104 | 8.15 ± 1.32 | ||||
| Intervention for 6 months | Control group | 104 | 15.86 ± 3.18* | 2.670 | 0.008 | |
| Research group | 104 | 17.10 ± 3.51* | ||||
| Intervention for 12 months | Control group | 104 | 17.41 ± 3.67*# | 4.099 | 0.000 | |
| Research group | 104 | 19.67 ± 4.26*# | ||||
| Reasonable exercise | Before intervention | Control group | 104 | 12.15 ± 1.02 | 1.254 | 0.211 |
| Research group | 104 | 12.34 ± 1.16 | ||||
| Intervention for 6 months | Control group | 104 | 17.11 ± 2.02* | 3.260 | 0.001 | |
| Research group | 104 | 18.06 ± 2.18* | ||||
| Intervention for 12 months | Control group | 104 | 19.97 ± 2.26*# | 2.527 | 0.012 | |
| Research group | 104 | 20.82 ± 2.58*# | ||||
| Proper medication | Before intervention | Control group | 104 | 10.08 ± 1.68 | 0.404 | 0.687 |
| Research group | 104 | 10.17 ± 1.53 | ||||
| Intervention for 6 months | Control group | 104 | 16.51 ± 2.53* | 2.516 | 0.013 | |
| Research group | 104 | 17.43 ± 2.74* | ||||
| Intervention for 12 months | Control group | 104 | 19.57 ± 2.98*# | 2.647 | 0.009 | |
| Research group | 104 | 20.69 ± 3.12*# |
Note: * Compared with before intervention in this group, P < 0.05; # Compared with this group after 6 months of intervention, P < 0.05. A 95% CI indicates that there is a 95% probability that the estimate will fall within that interval in repeated sampling
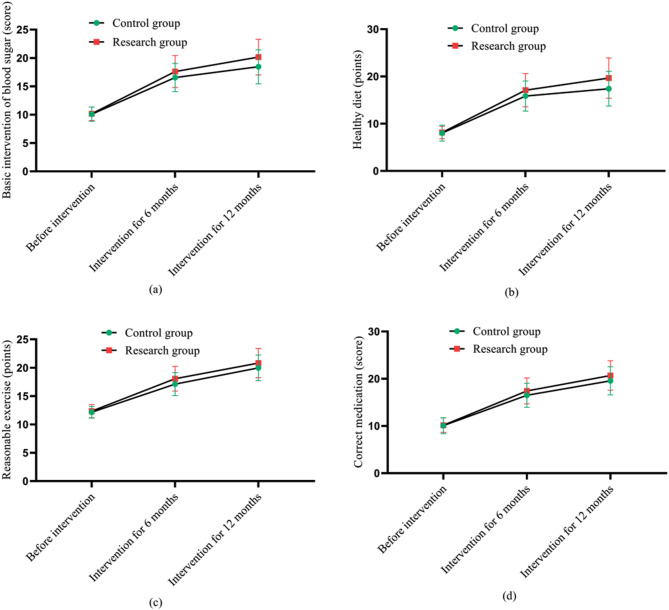
Compared with the control group, which received routine ongoing interventions, the research group, after 6 and 12 months of intervention with the Internet + E-Coach chronic disease system, showed an improvement of 7% and 3% in glycemic baseline interventions, 4% and 3% in healthy eating scores, 5% and 3% in sensible exercise scores, and 6% and 4% in correct medication scores, respectively. From the figure, the comparison in the scores of various indicators before intervention was with P > 0.05. The various indicators showed an upward trend after 6 and 12 months of intervention, and the research group was higher than the control group (P < 0.05) (See Fig. 1).
Fig. 1.
Cognitive score of disease related knowledge before intervention, 6 and 12 months of intervention. Note: (a) shows the basic intervention indicators for blood sugar; Fig. 1 (b) shows the indicators for healthy diet; Fig. 1 (c) shows the indicators for reasonable exercise; Fig. 1 (d) shows the indicators for correct medication; Among them, the pink hexagon is the research group and the green quadrilateral is the control group; The ups and downs of the curve indicate the changes in the levels of various indicators before and after intervention
Comparison of blood glucose, blood lipids, and liver function levels between two groups before intervention, 6 and 12 months after intervention
Before the intervention, there was no significant difference in the blood indices of FPG, 2hPG, TG, ALT and FINS between the two groups (P > 0.05), indicating that the physiological status of the two groups was consistent at baseline. After 6 and 12 months of intervention, the FPG, 2hPG, TG, and ALT indexes of the research group decreased significantly and were lower than those of the control group (P < 0.05), showing the significant effect of the research group in controlling blood glucose and blood lipids. In addition, FINS levels were significantly higher in the research group, reflecting better islet function. This result is similar to the study of Verma et al. [13] who noted that internet-assisted intervention methods can help patients to better control their blood glucose and inflammation levels, as shown in Table 2.
Table 2.
Comparison of blood glucose, blood lipids, and liver function levels between the two groups before intervention, 6 and 12 months after intervention (‾x ± s)
| Norm | Period of intervention | Groups | n | x ± s, points(95% CI) | t | P |
|---|---|---|---|---|---|---|
| FPG(mmol/L) | Before intervention | Control group | 104 | 10.86 ± 2.16 | 0.950 | 0.343 |
| Research group | 104 | 10.58 ± 2.09 | ||||
| Intervention for 6 months | Control group | 104 | 8.75 ± 1.68* | 5.911 | 0.000 | |
| Research group | 104 | 7.46 ± 1.46* | ||||
| Intervention for 12 months | Control group | 104 | 7.00 ± 1.32*# | 12.127 | 0.000 | |
| Research group | 104 | 5.12 ± 0.87*# | ||||
| 2hPG(mmol/L) | Before intervention | Control group | 104 | 12.68 ± 2.15 | 0.442 | 0.659 |
| Research group | 104 | 12.81 ± 2.09 | ||||
| Intervention for 6 months | Control group | 104 | 10.21 ± 1.72* | 9.214 | 0.000 | |
| Research group | 104 | 8.24 ± 1.34* | ||||
| Intervention for 12 months | Control group | 104 | 8.74 ± 1.32*# | 6.726 | 0.000 | |
| Research group | 104 | 7.55 ± 1.23*# | ||||
| FINS (mmol/L) | Before intervention | Control group | 104 | 12.84 ± 2.16 | 0.292 | 0.771 |
| Research group | 104 | 12.75 ± 2.29 | ||||
| Intervention for 12 months | Control group | 104 | 15.24 ± 3.91* | 4.323 | 0.000 | |
| Research group | 104 | 17.66 ± 4.16* | ||||
| Intervention for 12 months | Control group | 104 | 17.06 ± 4.56*# | 3.965 | 0.000 | |
| Research group | 104 | 19.46 ± 5.29*# | ||||
| TG (mmol/L) | Before intervention | Control group | 104 | 3.99 ± 1.52 | 0.540 | 0.590 |
| Research group | 104 | 3.87 ± 1.68 | ||||
| Intervention for 6 months | Control group | 104 | 3.02 ± 1.28* | 3.319 | 0.001 | |
| Research group | 104 | 2.46 ± 1.15* | ||||
| Intervention for 12 months | Control group | 104 | 2.62 ± 1.12*# | 5.139 | 0.000 | |
| Research group | 104 | 1.86 ± 1.01*# | ||||
| ALT(U/L) | Before intervention | Control group | 104 | 66.58 ± 12.48 | 0.130 | 0.896 |
| Research group | 104 | 66.74 ± 12.06 | ||||
| Intervention for 6 months | Control group | 104 | 59.19 ± 10.16* | 2.276 | 0.024 | |
| Research group | 104 | 56.09 ± 9.47* | ||||
| Intervention for 12 months | Control group | 104 | 56.45 ± 8.16*# | 2.035 | 0.043 | |
| Research group | 104 | 54.26 ± 7.56*# |
Note:# Compared with this group after 6 months of intervention, P < 0.05; * Compared with before intervention in this group, P < 0.05; FPG: Fasting blood glucose; 2hPG: 2 h postprandial blood glucose; FINS: Fasting serum insulin; TG: Triglyceride; ALT: Alanine transaminase. A 95% CI indicates that there is a 95% probability that the estimate will fall within that interval in repeated sampling
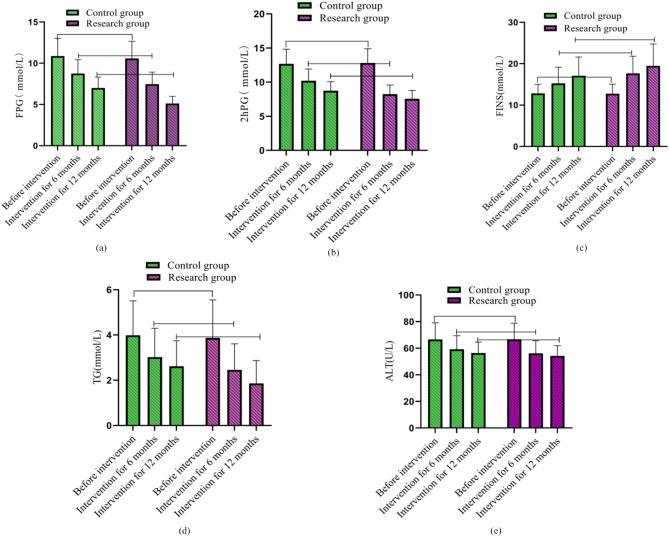
Compared with the control group that received conventional continuous intervention, FPG levels decreased by 3 mmol/L and 4 mmol/L, 2hPG levels decreased by 3 mmol/L and 5 mmol/L, and TG levels decreased by 1 mmol/L and 1.6 mmol/L in the research group after 6 and 12 months of intervention with the Internet + E-Coach chronic disease system, respectively. ALT levels decreased by 6 U/L and 10 U/L, respectively, and FINS levels were elevated by 3 mmol/L and 5 mmol/L, respectively. The comparison of changes in the levels of various indicators is shown in Fig. 2 (a), (b), (c), (d) and (e). As shown in the figure, the comparison in the scores of various indicators before intervention was with P > 0.05. The FPG, 2hPG, TG, and ALT showed a decreasing trend at 6 and 12 months of intervention, while the levels of FINS showed an increasing trend, and the control group was lower than the research group (P < 0.05).
Fig. 2.
Comparison of changes in blood glucose, blood lipids, and liver function levels before intervention, 6 and 12 months after intervention. Note: (a), (b), (c) with (d) and (e) shows the FPG indicator, 2hPG indicator, TG indicator, and ALT indicator, respectively. Among them, the green column combined with a circular shape is used as the control group, while the green cylinder combination is the control group and the purple square column combination is the research group. The columnar fluctuation indicates the changes in the levels of various indicators before and after intervention
Comparison of inflammatory factor levels between two groups before intervention, 6 and 12 months after intervention
Before the intervention, there was no significant difference in the levels of inflammatory factors such as IL-6, TNF-α, and Hs-CRP between the two groups (P > 0.05), indicating a consistent baseline status. After 6 and 12 months of intervention, the levels of inflammatory factors in the research group decreased significantly and were lower than those in the control group (P < 0.05), showing a significant anti-inflammatory effect. This result suggested that the Internet + E-Coach chronic disease system intervention not only helped to improve metabolic indicators, but also effectively reduced the inflammatory response of the body, which may improve the general health status of patients by reducing the release of systemic inflammatory mediators. This result is consistent with the findings of Najm I et al. [14]. Both emphasized that systematic and continuous care could significantly improve the inflammatory state of patients, thereby slowing the progression of chronic disease, as expressed in Table 3.
Table 3.
Comparison of inflammatory factor levels between two groups before intervention, 6 and 12 months after intervention
| Norm | Period of intervention | Groups | n | x ± s, points(95% CI) | t | P |
|---|---|---|---|---|---|---|
| IL-6(pg/mL) | Before intervention | Control group | 104 | 18.55 ± 5.12 | 0.233 | 0.816 |
| Research group | 104 | 18.72 ± 5.40 | ||||
| Intervention for 6 months | Control group | 104 | 14.82 ± 3.01* | 2.826 | 0.005 | |
| Research group | 104 | 13.69 ± 2.75* | ||||
| Intervention for 12 months | Control group | 104 | 13.06 ± 2.56*# | 3.027 | 0.003 | |
| Research group | 104 | 12.06 ± 2.19*# | ||||
| TNF-α(ng/L) | Before intervention | Control group | 104 | 157.59 ± 12.37 | 0.346 | 0.730 |
| Research group | 104 | 158.19 ± 12.62 | ||||
| Intervention for 6 months | Control group | 104 | 86.44 ± 9.68* | 8.858 | 0.000 | |
| Research group | 104 | 75.46 ± 8.13* | ||||
| Intervention for 12 months | Control group | 104 | 46.38 ± 8.12*# | 10.064 | 0.000 | |
| Research group | 104 | 36.28 ± 6.23*# | ||||
| Hs-CRP(mg/L) | Before intervention | Control group | 104 | 8.59 ± 0.92 | 0.676 | 0.500 |
| Research group | 104 | 8.67 ± 0.78 | ||||
| Intervention for 6 months | Control group | 104 | 6.71 ± 0.59* | 10.814 | 0.000 | |
| Research group | 104 | 5.96 ± 0.39* | ||||
| Intervention for 12 months | Control group | 104 | 4.62 ± 0.32*# | 5.276 | 0.000 | |
| Research group | 104 | 3.48 ± 0.18*# |
Note:# Compared with this group after 6 months of intervention, P < 0.05; IL-6: Interleukin-6; TNF- α: Tumor necrosis factor; * Compared with before intervention in this group, P < 0.05; Hs-CRP: Hypersensitivity C-reactive protein. A 95% CI indicates that there is a 95% probability that the estimate will fall within that interval in repeated sampling
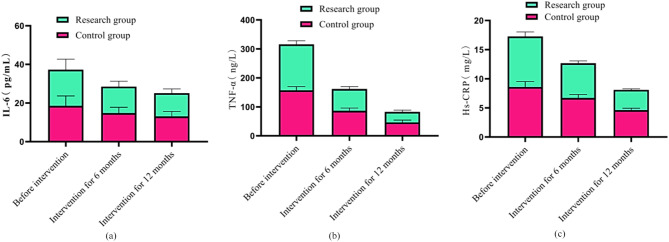
Compared with the control group that received routine ongoing interventions, the research group that received the Internet + E-Coach chronic disease system interventions for 6 and 12 months experienced a decrease in the index levels of IL-6 by 7 pg/mL and 10% pg/mL, respectively, a decrease in the index level of TNF-alpha by 157 bg/L, respectively, and a decrease in the index level of Hs-CRP by 4 g/mL and 10 g/mL, respectively. The comparison of changes in the levels of various indicators is shown in Fig. 3 (a), (b) and (c). From the figure, before the intervention, the comparison in the scores of various indicators between the two groups was with P > 0.05. The IL-6, TNF-α, Hs-CRP levels showed a decreasing trend at 6 and 12 months of intervention, and the control group was lower than the research group (P < 0.05).
Fig. 3.
Comparison of inflammatory factor levels between two groups before intervention, 6 and 12 months after intervention. Note: (a), (b), (c) show the IL-6, TNF- α and Hs-CRP indicators, respectively; Green and red bars represent the control and research groups, respectively; The columnar fluctuation indicates the changes in the levels of various indicators before and after intervention
Comparison of CLVQOL scale scores between two groups before intervention, 6 and 12 months after intervention
Before the intervention, there was no significant difference between the two groups in all scores of the CLVQOL scale (P > 0.05), showing that the baseline levels of the two groups were similar in the areas of distance visual acuity, motor and light perception, accommodation, reading and fine work, and daily living ability. After 6 and 12 months of intervention, all quality-of-life scores of the research group were significantly higher than those of the control group (P < 0.05), especially the improvement in daily living ability and reading and fine work was more significant. In addition, no serious adverse effects related to the intervention were observed during the study, and all participants were able to tolerate the intervention and reported no significant negative health effects, as shown in Table 4.
Table 4.
CLVQOL scale scores before intervention, 6 and 12 months after intervention in two groups (‾x ± s, points)
| Norm | Period of intervention | Groups | n | x ± s, points (95% CI) | t | P |
|---|---|---|---|---|---|---|
| Distant vision, movement and light perception | Before intervention | Control group | 104 | 25.68 ± 2.13 | 0.285 | 0.776 |
| Research group | 104 | 25.59 ± 2.42 | ||||
| Intervention for 6 months | Control group | 104 | 29.45 ± 3.81* | 3.187 | 0.002 | |
| Research group | 104 | 31.19 ± 4.06* | ||||
| Intervention for 12 months | Control group | 104 | 32.18 ± 4.16*# | 5.391 | 0 | |
| Research group | 104 | 35.59 ± 4.93*# | ||||
| Regulatory ability | Before intervention | Control group | 104 | 5.19 ± 1.33 | 1.25 | 0.213 |
| Research group | 104 | 5.40 ± 1.08 | ||||
| Intervention for 6 months | Control group | 104 | 8.10 ± 2.10* | 3.276 | 0.001 | |
| Research group | 104 | 9.11 ± 2.34* | ||||
| Intervention for 12 months | Control group | 104 | 9.96 ± 2.67*# | 3.185 | 0.002 | |
| Research group | 104 | 11.24 ± 3.11*# | ||||
| Reading and fine work | Before intervention | Control group | 104 | 7.68 ± 2.11 | 0.778 | 0.438 |
| Research group | 104 | 7.93 ± 2.51 | ||||
| Intervention for 6 months | Control group | 104 | 10.67 ± 3.59* | 3.131 | 0.002 | |
| Research group | 104 | 12.28 ± 3.82* | ||||
| Intervention for 12 months | Control group | 104 | 12.44 ± 4.16*# | 2.257 | 0.025 | |
| Research group | 104 | 13.89 ± 5.06*# | ||||
| Daily living ability | Before intervention | Control group | 104 | 6.18 ± 1.36 | 0.490 | 0.625 |
| Research group | 104 | 6.27 ± 1.29 | ||||
| Intervention for 6 months | Control group | 104 | 9.67 ± 2.16* | 4.837 | 0 | |
| Research group | 104 | 11.37 ± 2.86* | ||||
| Intervention for 12 months | Control group | 104 | 13.48 ± 3.25*# | 2.327 | 0.021 | |
| Research group | 104 | 14.64 ± 3.91*# |
Note:# Compared with this group after 6 months of intervention, P < 0.05; * Compared with before intervention in this group, P < 0.05; CLVQOL: Low vision quality of life scale. A 95% CI indicates that there is a 95% probability that the estimate will fall within that interval in repeated sampling
Discussion
Diabetes as a potentially devastating disease that has increased with the number of deaths attributed to it and its incidence in the last few decades and constitutes a major public health challenge of the 21st century, therefore the guidelines for the management of diabetic patients has become vital [9–15].
The significance of implementing Internet + E-Coach chronic disease system intervention for DR patients
DR is a common complication of diabetes and one of the leading causes of blindness [16]. Due to the vascular and neurological damage caused by the hyperglycemic state of DR patients, stabilizing blood glucose and improving self-management are key. This study showed that the Internet + E-Coach chronic disease system intervention significantly improved patients’ disease awareness, glycemic control, and quality of life. This system provided personalized health education and dynamic monitoring through the Internet platform, which enhanced patients’ self-management ability and adherence, thus effectively improving clinical outcomes. The results of the study showed that the application of the Internet + E-Coach system in the management of chronic complications of diabetes had obvious advantages compared with traditional intervention methods.
The impact of Internet + E-Coach chronic disease system intervention on the cognition of disease related knowledge in DR patients
It was found that after 6 and 12 months of intervention, the cognitive scores of patients in the research group were significantly higher than those of the control group in the areas of basic glycemic intervention, healthy diet, rational exercise, and proper medication (P < 0.05). This result suggested that the Internet + E-coach system could effectively improve the disease-related knowledge of DR patients. Compared with traditional care methods, this system enabled patients to understand the core content of disease management more deeply through personalized health education and continuous remote guidance.The study by Ding et al. showed that the application of a similar E-Coach system in bronchial asthma patients also significantly improved the cognitive level of disease knowledge [17], which further verified the reliability and generalizability of the results of this study. In addition, a study by Wilson et al. also showed that Internet-based nursing interventions could enhance patients’ health knowledge and promote the stability of long-term care outcomes [18].
The effect of Internet + E-Coach chronic disease system intervention on blood glucose, blood lipids, liver function, and inflammatory factors in DR patients
IL-6 is a cytokine, belonging to the biological class, which has an effect on the inflammatory response, tissue repair and immunoregulation of the human body. TNF-α is also a cytokine, produced by lymphocytes, macrophages and immune cells in the human body, which can participate in the inflammatory response, immunoregulation and apoptosis. Hs-CRP is a serum biomarker synthesized by the liver, which is mainly used to evaluate the inflammatory state of the body, and has a great effect on the inflammatory response and can reflect the level of inflammation in the body [19]. The results of the study showed that after 6 and 12 months of intervention, the FPG, 2hPG, TG and ALT of the research group were significantly reduced and lower than those of the control group (P < 0.05), while the level of FINS was significantly increased (P < 0.05), which showed the significant effect of the Internet + E-Coach system in stabilizing blood glucose, improving blood lipids and liver function. In addition, the levels of inflammatory factors such as IL-6, TNF-α, and Hs-CRP decreased significantly in the research group, indicating that the system intervention was effective in reducing the body’s inflammatory response. These findings were supported by the study of Verma et al. who pointed out that the Internet-assisted intervention approach could help patients better control their blood glucose and inflammation levels [20]. Najm et al. further emphasized that systematic and continuous care could significantly improve the inflammatory state of patients, thereby slowing the progression of chronic disease [21]. The present study is consistent with these literatures, suggesting that the Internet + E-coach system has a wide range of applications in chronic disease management for DR patients.
The impact of Internet + E-Coach chronic disease system intervention on the life quality of DR patients
This study expressed that compared to the control group receiving routine continuous care, the life quality in the research group was improved. DR patients could obtain comprehensive educational information on drug treatment, disease management, and healthy living through systematic intervention, which could enhance their self-management ability in disease rehabilitation on the basis of improving their cognition. Daily treatment according to the health plan of nursing staff could effectively control the condition and reduce the occurrence of complications. Through dynamic monitoring and guidance through the APP, abnormal patient conditions could be detected in the early stage, early disease control could be carried out in a timely manner, further development of the disease could be prevented, life ability could be improved, and quality of life could be improved. Zhou et al., applied E-Coach chronic disease system intervention in patients with chronic heart failure to improve their health literacy, daily life ability and life quality [22]. This study is consistent with their research.
Conclusion
In summary, the Internet + E-Coach chronic disease system intervention was statistically significant in improving glycemic control, decreasing inflammatory markers, and enhancing quality of life in DR patients (P < 0.05). It can be said that the results of the study have important clinical implications for healthcare providers and patients. Patients made significant progress in glycemic control, reduction in inflammation, and improvement in quality of life through rationally designed interventions. Statistical significance further supported the reliability of these findings and enhanced their clinical application. The results of this study were valid in specific clinical settings, but generalizability to different regions, settings with limited healthcare resources, or other populations has not been validated. Further studies in diverse settings are needed in the future to assess the broad applicability of the intervention.
Acknowledgements
Not applicable.
Author contributions
XY, XC, ZY, XQ, DQ and ZL done the screening and data extraction, did the analysis, designed and conceived the study, analyzed, wrote and drafted the manuscript and provided feedback; all the authors approved the final version of the article to be published.
Funding
The research is supported by: Wuxi Municipal Health Commission, Construction and application of E-Coach management model for diabetes retinopathy based on Internet Plus (No. Q202026).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Nanjing Medical University. All the experiments of this study were conducted in accordance to the relevant guidelines and regulations or in accordance to the Declaration of Helsinki. Written informed consent was obtained from the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fung TH, Patel B, Wilmot EG, Amoaku WM. Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond). 2022;22(2):112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorna MS. Food Intake among the Diabetic and non-diabetic Elderly Population in Brazil. Arq Bras Cardiol. 2022;118(2):398–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mounirou BAM, Adam ND, Yakoura AKH et al. Diabetic Retinopathy: an overview of treatments. Indian J Endocrinol Metab 2022 Mar-Apr;26(2):111–8. [DOI] [PMC free article] [PubMed]
- 4.Knoll K, Leiter SM, Rosner S. Etal. Impact of Tele-Coaching during the COVID-19 pandemic on risk-reduction behavior of patients with heart failure. Telemed J E Health. 2022;28(6):823–31. [DOI] [PubMed] [Google Scholar]
- 5.Sahar PJ. Community-based intervention of chronic disease management program in rural areas of Indonesia. Front Nursing, 2022, 9(2):187–195.
- 6.Fang A, Abdelgadir D, Gopalan A, Ross T, Uratsu CS, Sterling SA, Grant RW, Iturralde E. Engaging patients in population-based chronic disease management: A qualitative study of barriers and intervention opportunities. Patient Educ Couns. 2022;105(1):182–189. [DOI] [PMC free article] [PubMed]
- 7.Brock I, Prendergast W, Maitland A. Mast cell activation disease and immunoglobulin deficiency in patients with hypermobile Ehlers-Danlos syndrome/hypermobility spectrum disorder. Am J Med Genet C Semin Med Genet. 2021;187(4):473–481. [DOI] [PubMed]
- 8.Cao S, Wen J, Song G, et al. Systemic lupus erythematosus complicated with right eyelid ptosis caused by intracranial aneurysm: a case report. J New Med. 2023;54(1):75–9. [Google Scholar]
- 9.Mi W, Xia Y, Bian Y. Meta-analysis of the association between aldose reductase gene (CA)n microsatellite variants and risk of diabetic retinopathy. Exp Ther Med. 2019;18(6):4499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou Y, Xiang J, Wang B, Duan S, Song R, Zhou W, Tan S, He B. Pathogenesis and comprehensive treatment strategies of Sarcopenia in elderly patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2024;14:1263650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Zhang Y, Yang Y, Pan J. Diabetes-related avoidable hospitalisations and its relationship with primary healthcare resourcing in China: a cross-sectional study from Sichuan Province. Health Soc Care Community. 2022;30(4):e1143–56. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Xu S, Ding Y, Li L, Huang C, Bao M, Li S, Wang Q. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: a mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1307468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Zhang P, Chen Y, Jiang J, Zhou Z, Zhu H. Comparing SARC-CalF with SARC-F for Screening Sarcopenia in adults with type 2 diabetes Mellitus. Front Nutr. 2022;9:803924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo M, Cao Q, Wang D, Tan R, Shi Y, Chen J, Chen R, Tang G, Chen L, Mei Z, Xiao Z. The impact of diabetes on postoperative outcomes following spine surgery: a meta-analysis of 40 cohort studies with 2.9 million participants. Int J Surg. 2022;104:106789. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Zhang Z, Li L, Liang X, Wu Y, Wang X, Ma H, Cheng J, Zhang A, Tang P, Wang CZ, Wan JY, Yao H, Yuan CS. Gut microbiota induces DNA methylation via SCFAs predisposing obesity-prone individuals to diabetes. Pharmacol Res. 2022;182:106355. [DOI] [PubMed] [Google Scholar]
- 16.Little K, Llorián-Salvador M, Scullion S, RECOGNISED consortium (GA 847749). Common pathways in dementia and diabetic retinopathy: understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol Metab. 2022;33(1):50–71. [DOI] [PubMed] [Google Scholar]
- 17.Ding P, Zhou Y, Long KL, Zhang S, Gao PY. Acute mesenteric ischemia due to percutaneous coronary intervention: a case report. World J Clin Cases. 2022;10(28):10244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RL, Higgins O, Editorial. The continued importance of mental health nurses engaging with social media and related emerging technologies. Int J Ment Health Nurs. 2023;32(2):349–51. [DOI] [PubMed] [Google Scholar]
- 19.Verma I, Gopaldasani V, Jain V, et al. The impact of peer coach-led type 2 diabetes mellitus interventions on glycaemic control and self-management outcomes: a systematic review and meta-analysis. Prim Care Diabetes. 2022;16(6):719–35. [DOI] [PubMed] [Google Scholar]
- 20.Yi M, Zhao W, Tan Y, et al. The causal relationships between obstructive sleep apnea and elevated CRP and TNF-α protein levels. Ann Med. 2022;54(1):1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najm I, Lal D, Alonso Vanegas M, et al. The ILAE consensus classification of focal cortical dysplasia: an update proposed by an ad hoc task force of the ILAE diagnostic methods commission. Epilepsia. 2022;63(8):1899–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou YY, Zhou TC, Chen N, et al. Risk factor analysis and clinical decision tree model construction for diabetic retinopathy in Western China. World J Diabetes. 2022;13(11):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.