Abstract
Background
Vegan and vegetarian dietary patterns are known to beneficially modulate risk factors for cardiovascular disease; however, the current literature does not differentiate between various plant-based diets. This study aimed to examine the association between various plant-based diets and plasma lipids and glycaemic indices compared to a regular meat-eating diet.
Methods
A cross-sectional study of Australian adults (n = 230) aged 30-75yrs habitually consuming the following were recruited: vegan, lacto-vegetarian, pesco-vegetarian, semi-vegetarian, or regular meat-eater. Multivariable regression analysis was used to adjust for covariates.
Results
Compared to regular meat-eaters, vegans had significantly lower total cholesterol (-0.77mmol/L,95% CI -1.15, -0.39, P < 0.001), low-density lipoprotein cholesterol (LDL-C, -0.71mmol/L, 95% CI -1.05, -0.38, P < 0.001), non-high-density lipoprotein cholesterol (non-HDL-C, -0.75mmol/L, 95% CI -1.11, -0.39, P < 0.001), total cholesterol/HDL-C-ratio (-0.49mmol/L, 95% CI -0.87, -0.11, P = 0.012), fasting blood glucose (FBG, -0.29mmol/L, 95% CI -0.53, -0.06, P = 0.014), haemoglobin A1C (-1.85mmol/mol, 95% CI -3.00, -0.71, P = 0.002) and insulin (-1.76mU/L, 95% CI -3.26, -0.26, P = 0.021) concentrations. Semi-vegetarians had significantly lower LDL-C (-0.41mmol/L, 95% CI -0.74, -0.08, P = 0.041) and non-HDL-C (-0.40mmol/L, 95% CI -0.76, -0.05, P = 0.026) and lacto-ovo vegetarians had significantly lower FBG (-0.34mmol/L, 95% CI -0.56, -0.11, P = 0.003) compared to regular meat-eaters. There were no differences in HDL-C and triglycerides between plant-based and regular-meat diets.
Conclusions
Plasma lipaemic and glycaemic measures as a collective were more favourable among vegans, whereas among lacto-ovo vegetarians and semi-vegetarians, only some measures were favourable.
Trial registration
ACTRN12621000743864. Date 6/11/2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02340-5.
Keywords: Lipids, Insulin, Glucose, Cardio-vascular disease, Plant-based, Vegan, Vegetarian, T2D; dyslipidaemia; pesco-vegetarian; semi-vegetarian
Background
In 2022, it was estimated that over 1.3 million Australians were diagnosed with type 2 diabetes mellitus (T2D) [1] and over 2.1 million reported hyperlipidaemia [2]. These chronic, non-communicable, progressive conditions are independent contributors to the onset of cardiovascular disease (CVD), which represented close to a third of all deaths globally in 2021 [3]. Lipids play a vital role in the development of atherosclerosis, a leading cause of CVD [4]. A lipid profile characterised by elevated total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL-C) and reduced high-density lipoprotein (HDL-C), is widely recognised in the assessment of atherosclerosis and management of CVD [4]. Sustained dyslipidaemia leads to lipids and fibrous elements to build up in arteries resulting in the formation of atheroma plaques which causes narrowing of the arteries thus increasing risk of various CVDs and cardio-metabolic related conditions [5]. Individuals with T2D also have considerably higher risk of CVD morbidity and mortality [6]. Haemoglobin A1C (HbA1c) is the most used biomarker for diagnosis of T2D and pre-diabetes, although fasting blood glucose (FBG) and insulin also play an important role in marking disease progression and monitoring disease management. T2D and elevated glycaemic indices predisposes individuals to atherosclerotic CVD through pathogenic mechanisms mainly linked to hyperglycaemic and sustained chronic hyperinsulinemia which disrupts metabolic profiles via intracellular signalling pathways [7].
Elevated plasma lipids and glycaemic indices are caused by a complex interaction of environmental, genetic and lifestyle factors [8]. Key management strategies include modifications to lifestyle factors such as dietary pattern and physical activity as they influence numerous metabolic pathways involved in disease prognosis and progression including lipid metabolism, insulin utilisation, blood pressure and body composition [8]. Glucose and lipid metabolism are closely linked to each other given their important roles in energy metabolism. Studies have shown hypertriglyceridemia and low concentrations of HDL-C may not only be the consequence but also the cause of disturbed glucose metabolism [9]. Therefore, it is essential to uncover dietary interventions that target both these metabolic pathways to reduce risk of CVD and other cardiometabolic related morbidities.
The adoption of plant-based diets (PBDs) has become a global movement with recent market research indicating 12% of the Australian and United Kingdom populations are following a vegetarian or minimal meat diet [10, 11]. PBDs may be linked to favourable nutrient compositions with lower reported dietary intakes of saturated fat and cholesterol and higher intakes of dietary fibre, unsaturated fats, and polyphenols [12, 13]. If planned carefully to provide adequate nutrition they have been shown to be superior in comparison to a regular meat-eating diet for various health outcomes such as reduced risk of CVD [14], T2D [15] weight loss [16, 17]. A broad definition of PBDs within the scientific community is a dietary pattern high in vegetables, fruit, whole grains, legumes, nuts and unsaturated oil, and nil or low intakes of meat, poultry and/or seafood [14]. In order to create standardised definitions of PBDs, this study will use definitions previously implemented in Australian cohorts [18–21] which was originally adapted from Mihrshahi et al. [22] and aligned with the World Health Organisation [23]. These definitions focus on PBDs that typically exclude meat, dairy, and seafood consumption to various degrees (vegan, lacto-ovo vegetarian, pesco-vegetarian and semi-vegetarian).
PBDs have been offered as a potential strategy for managing glycaemic control [24] and plasma lipids [25]. A systematic review of five randomised controlled trials (RCTs), reported a significant improvement in markers of glycaemic control from baseline to end-point after at least four weeks or more of PBD intervention. In this review, PBD interventions were not defined in detail and vegan and vegetarian dietary patterns were pooled together in the analyses [26]. Another systematic review of 71 studies which were mostly cross-sectional, found vegetarian dietary patterns (including vegans) were associated with lower FBG compared to a omnivorous diet [27]. Studies included in this review also vaguely defined vegan and vegetarian diets or included no definition, and all studies reported a high risk of bias, therefore, differences between variations of plant-based eating were not established. Very few studies have investigated the effects of PBDs, inclusive of pesco-vegetarian and semi-vegetarian on glycaemic indices and risk of T2D. The Adventist Health-2 study (AHS-2) found the vegans, lacto-ovo vegetarians, semi-vegetarians but not pesco-vegetarians were associated with a substantial and independent reduced risk of T2D after a two year follow up period [28]. There has only been one analysis on an Australian cohort that evaluated glycaemic indices and risk of T2D among individuals following various PBDs, which was a secondary analysis among older women [15]. Prevalence of impaired glucose tolerance was reported to be lower in vegans, vegetarians, semi-vegetarians, and pesco-vegetarians compared to regular meat-eaters [15].
The effect of PBDs has also proved to be advantageous in the control of plasma lipids. Multiple pooled meta-analyses of both RCTs and observational studies have reported that compared to RMEs, individuals following vegan and vegetarian diets demonstrate lower concentrations of TC, LDL-C and HDL-C [25, 29–31]. Despite lower concentrations of HDL-C reported in observational studies, vegetarian diets have not been associated with poor cardiovascular health [32] and genetic variants that raise HDL-C do not necessarily reduce risk of coronary heart disease [33]. The AHS-2 study included analyses of additional categories of plant-based eating and found the risk of elevated TC and LDL-C beyond the recommended ranges were lower in lacto-ovo, pesco-vegetarians and vegans, although semi-vegetarians were not examined in this sample [34]. There has not been a previous Australian-based study which had the primary aim to evaluate the association between plasma lipids and various types of PBDs. Given the growing popularity of PBDs, an Australian-based study specifically designed to investigate individuals habitually following PBDs is warranted to understand the potential nutritional implications of PBDs and their influence on important cardiometabolic parameters.
Vegetarian diets (including vegans) have been associated with lower glycaemic and plasma lipids; however, previous literature does not explore various types of commonly adhered to PBDs in society and lacks cohesive categorisation. Therefore, the aim of this cross-sectional study is to examine the association between individuals following various PBDs and plasma lipids and glycaemic indices compared to a regular meat-eating diet.
Materials and methods
Study design and participants
The research protocol detailing the study design has been published elsewhere [20]. This cross-sectional study of 230 aged 30–75 years, involved data collection at one time point and was conducted at the Nutraceuticals Research Program, School of Biomedical Sciences & Pharmacy, University of Newcastle, Callaghan NSW, Australia. Those aged 30–75 following a dietary pattern for ≥ 6 months were eligible to participate. Participants that were ineligible were pregnant or breast feeding, had current or previous diagnosis of CVD, and/or significantly changed their usual physical activity levels or dietary pattern within the last 6 months. Additional exclusions applied to those taking lipid medications and/or glycaemic agents. The assessment of habitual weekly intake of meat, seafood, eggs, and dairy was used as an screening criteria to categorise participants into dietary patterns and has been published elsewhere [20]. The dietary patterns in which participants were recruited into include, vegan (nil animal-based foods), lacto-ovo vegetarian (LOV, nil meat, ± eggs, ± dairy), pesco-vegetarian (PV, nil meat, seafood consumption ≥ 1 per week, ± dairy, ± eggs), semi-vegetarian (SV, meat consumption ≤ 2 per week) or regular meat-eaters (RMEs) (meat consumption ≥ 7 per week). Lead investigator collected two-day diet histories and fasted blood samples via venepuncture after an overnight fast (~ 10–12 h) from enrolled participants who provided written informed consent. A self-reported questionnaire was completed by participants to collect information regarding demographic and medical history, prescribed or over-the-counter medication(s), habitual supplement use, smoking status, duration of following dietary pattern, level of education, age, and sex. Definitions for overweight and obese were; BMI ≥ 25 kg/m2 and ≥ 30 kg/m2, respectively [35].
Assessment of dietary patterns
The Food Frequency Questionnaire (FFQ) employed to evaluate food and nutrient intake over the previous 3–6 months was the online self-administered 120-question Australian Eating Food Survey (AES®) FFQ which has been validated in the Australian population [36, 37]. Qualitative intake of food groups and food categories was derived from the total sum of all food frequency questions relating to the food group. An Accredited Practicing Dietitian conducted a comprehensive in-person interview to collect diet history across two habitual days and quantitatively evaluate the usual eating patterns of participants. Data was presented as average means consumed (mg/g) per day from micronutrients and macronutrients. Intake of plant-based dairy and meat alternatives and food fortification were included in the data collection. Assessment of micronutrient and macronutrients dietary intake is more accurately obtained through dietitian administered diet histories than FFQs [38]. FoodWorks (version 10, Xyris®, Brisbane, Australia, sourced online) was used to assess data.
Assessment of plasma lipids and glycaemic indices
Blood lipids collected included TC, TG, LDL-C, HDL-C, non-HDL-C and TC/HDL-C ratio. Glycaemic indices collected included FBG, HbA1c and insulin. All samples were collected by a trained phlebotomist via venepuncture after an overnight fast (~ 10–12 h) on the same day as the study appointment which were measured using an auto-analyser by the commercial pathology service provider, NSW Health Pathology. Additional analyses were also conducted to evaluate prevalence of metabolic syndrome (MetS), metabolic components and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). The definition of MetS used was devised by the National Cholesterol Education Program Adult Treatment Panel III in 2001 and updated by the American Heart Association and the National Heart Lung and Blood Institute in 2005 [39]. This criteria of MetS includes having at least three of the following: abdominal obesity (> 102 cm for men and > 88 cm for women), hypertriglyceridemia (≥ 1.69 mmol/L), low HDL-C (< 1.03 mmol/L for men and < 1.29 mmol/L for women), hypertension (≥ 130/≥85mmHg), and elevated FBG (≥ 5.6 mmol/L). Participants taking antihypertensive, oral blood glucose and/or lipid lowering medications were considered to indicate the presence of the respective risk factor. HOMA-IR was used to quantify insulin resistance and beta-cell function. It was calculated by multiplying insulin FBG, then dividing by the constant 22.5, i.e. HOMA-IR = (insulin×FBG)/22.5 [40]. A HOMA-IR of ≥ 1.7 was used as the cut off for insulin resistance [41–43].
Covariate analyses
Self-reported questionnaires were used to collect demographic characteristics (age, sex, education, duration of dietary pattern) and lifestyle factors (smoking status, physical activity, supplement, and medication use). The remaining variables were collected from a two-day dietitian administered diet history (total energy intake and alcohol consumption). The validated International Physical Activity Questionnaire (IPAQ, Long Version October 2002) was used to evaluate daily physical activity levels which was expressed as metabolic equivalent of task minutes per week (METs/week) [44]. Smoking status was categorised as ‘non-smoker’ or ‘current smoker’. Height was collected to the nearest 0.5 cm and weight to the nearest 0.1 kg by a qualified and certified clinician which was used to calculate BMI (kg/m2). Models were adjusted for age, sex, physical activity, energy intake, duration of dietary pattern, alcohol intake, smoking status, level of education, BMI as a mediator and the addition of eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) supplement use in the analyses of plasma lipids.
Statistical analyses
Normality of the participant characteristics data was assessed for normal distribution via inspection of histograms and quantile plot and reported as mean ± SD and categorical data as counts (n) and frequencies (%). Participant characteristics and nutrient intake levels were compared across dietary pattern groups via one-way ANOVA and Tukey post-hoc test or Fisher’s Exact tests. Under the assumption that a mean difference in LDL/HDL ratio between RMEs and vegans is 0.6mmol/L [45] (a significant and clinically relevant difference) and between subject standard deviation is 0.9mmol/L, it was estimated that a sample size of 36 per group was required to elicit a power of 80% to detect a difference (Cohens D 0.6 SD) at the confidence interval of 95%. A total of 44–48 participants were recruited into each dietary group. To identify the association between various PBDs and lipaemic and glycaemic biomarkers compared to a regular meat-eating diet, a Seemingly unrelated regression model was employed on each outcome to appropriately adjust for the mediating effect of BMI as well as other important confounders. Variables included in the adjusted models included age, sex, physical activity, energy intake, duration of dietary pattern, alcohol intake, smoking status, level of education, BMI as a mediator and the addition of EPA/DHA supplement use in the analyses of plasma lipids [46]. The regular-meat diet was considered as the reference group and PBDs as the exposure variable. Additional diagnostic procedures for identification of potential confounders involved the inspection of residual plots for normality and homogeneity. A sensitivity analysis was performed to assess how robust the results were to the previously mentioned possible confounders by comparing E-values to beta-coefficients. Significance level was calculated via Bonferroni corrected alpha P < 0.001 (n = 10 tests) and P < 0.05 were also reported. For appropriate graphical comparison between PBDs and a regular meat-eating diet, adjusted β coefficients ± SD of the significant variables are presented as figures. Percentage difference was calculated by using the adjusted mean difference between the exposure and reference group, divided by the reference group, multiplied by 100. Statistical analyses was conducted using StataCorp, 2016 (Stata Statistical Software: Release 17, College Station, TX, USA: StataCorp LP).
Results
Participant characteristics
In total 230 participants were included in this cross-sectional study. The mean age of participants was 54 ± 10 years, two thirds were female, just under half (40%) were overweight or obese, 6% were smokers, 80% had a higher education, and physical activity levels were comparable across groups (Table 1). Vegans were significantly younger and had a shorter duration of following dietary patterns in comparison to RMEs. 11% of the sample supplemented with omega-3 poly-unsaturated fatty acids (n-3 PUFAs), majority were EPA/DHA based, and reported use was higher among RMEs compared to those adhering to a PBD. Prevalence of MetS, individual metabolic components and HOMAR-IR were not significantly different between plant-based and regular-meat diets, except for elevated FBG which was higher among regular-eating diets compared to PBDs.
Table 1.
Characteristics of participants across different dietary pattern groups
| Total sample (n = 230) | Vegan (n = 48) |
Lacto-ovo vegetarian (n = 47) | Pesco-vegetarian (n = 46) | Semi-vegetarian (n = 44) | Regular meat eater (n = 45) | P | |
|---|---|---|---|---|---|---|---|
| Women | 177 (78.0%) | 34 (77.0%) | 36 (%76.6) | 37 (80.4%) | 36 (81.8%) | 34 (75.6%) | 0.754 |
| Age (years) | 53.5 ± 10.3 | 47.8 ± 10.0a | 53.4 ± 9.9b | 55.9 ± 11.2b | 54.8 ± 8.8b | 55.9 ± 9.7b | < 0.001 |
| BMI (kg/m2) | 24.3 ± 4.1 | 24.5 ± 4.1 | 24.4 ± 3.3 | 25.2 ± 4.6 | 23.9 ± 4.8 | 23.9 ± 3.5 | 0.559 |
| Overweight or obese | 93 (40.4%) | 17 (35.4%) | 23 (48.0%) | 18 (39.1%) | 13 (29.5%) | 22 (48.9%) | 0.248 |
| Higher education | 203 (88.3%) | 42 (87.5%) | 40 (85.1%) | 42 (91.3%) | 37 (84.1%) | 42 (99.3%) | 0.599 |
| Physical Activity (METs) | 5326 ± 5326 | 5775 ± 4036 | 6972 ± 8888 | 4945 ± 4119 | 4395 ± 3619 | 5163 ± 3671 | 0.179 |
| Current Smoker | 13 (5.7%) | 5 (10.4%) | 3 (6.4%) | 1 (2.2%) | 2 (4.6%) | 2 (4.4%) | 0.557 |
| Dietary pattern duration (yrs) | 16.6 + 17.9 | 6.8 ± 7.7a | 16.3 ± 13.8b | 16.2 ± 14.8b | 11.9 ± 14.1b | 32.4 ± 24.4c | < 0.001 |
| Supplement use (count (%)) d | |||||||
| N-3 PUFAs | 25 (10.9) | 3 (6.3) | 7 (14.9) | 4 (8.7) | 2 (4.6) | 9 (20.0) | 0.117 |
| EPA/DHA | 22 (9.6%) | 2 (4.2) | 5 (10.6) | 4 (8.7) | 2 (4.6) | 9 (20.0) | 0.099 |
| ALA | 3 (1.3) | 1 (2.1) | 2 (4.3) | 0 | 0 | 0 | 0.488 |
| Metabolic syndrome and components (count (%)) e | |||||||
| Metabolic syndrome | 14 (6.1) | 2 (4.2) | 2 (4.2) | 3 (6.5) | 4 (9.1) | 2 (4.4) | 0.855 |
| Abdominal obesity | 74 (32.2) | 12 (25.0) | 19 (40.4) | 15 (32.6) | 9 (20.5) | 19 (42.2) | 0.113 |
| Hypertriglyceridemia | 31 (13.5) | 6 (12.5) | 9 (19.2) | 5 (10.9) | 7 (15.9) | 4 (8.9) | 0.638 |
| Low HDL-C | 29 (12.6) | 11 (22.9) | 5 (10.6) | 6 (13.0) | 5 (11.4) | 2 (4.4) | 0.122 |
| Hypertension | 22 (9.6) | 2 (4.2) | 4 (8.5) | 5 (10.9) | 3 (6.8) | 8 (17.8) | 0.266 |
| Elevated FBG | 13 (6.7) | 1 (2.1) | 0 | 3 (6.5) | 3 (6.8) | 6 (13.3) | 0.042 |
| HOMAR-IR | 1.4 ± 1.4 | 1.2 ± 1.0 | 1.4 ± 1.0 | 1.3 ± 1.0 | 1.4 ± 1.0 | 1.5 ± 0.7 | 0.605 |
| HOMAR-IR (cut off 1.7) | 56 (24.4) | 10 (20.8) | 12 (25.5) | 10 (21.7) | 11 (25.0) | 13 (28.9) | 0.906 |
Data reported as means ± SD and for continuous variables and counts and (percentages) for categorical variables. Continuous data was compared using ANOVA and Tukey post-hoc test. Categorical data was compared using Fisher’s Exact
a, b,cValues within the same row without a common superscript letters are significantly different (P < 0.05)
dParticipant is currently taking n-3 PUFA supplement as per medical history and were defined as; EPA/DHA (fish and krill based), ALA (algae and flaxseed based)
eThe definition of metabolic syndrome and metabolic components are as per the National Cholesterol Education Program Adult Treatment Panel III further detailed in the methods section
ALA, Alpha-linolenic acid; BMI, body-mass index; EPA, eicosapentaenoic acid; FBG, fasting blood glucose; HbA1c, haemoglobin A1C; HOMA-IR, Homeostasis model assessment-estimated insulin resistance; DHA, docosahexaenoic acid; HDL-C, High-density lipoprotein cholesterol; IFFC, International Federation of Clinical Chemistry; MET, Metabolic equivalent of task minutes; NGSP, National Glycohemoglobin
Nutrient analyses
Overall individuals adhering to a PBD, specifically vegans, had a more favourable nutrient composition compared to RMEs, characterised by significantly lower intake of saturated fats, trans fats, cholesterol, discretionary choices (including sugar sweetened beverages) and higher intake of PUFAs, dietary fibre, fruit and legumes/nuts compare to RMEs (Table 2). Individuals adhering to a PV dietary pattern had significantly higher seafood intake compared to all other groups and long chain n-3 PUFAs (LC n-3 PUFAs) compared to SVs, LOVs and vegans. Total energy intake and fat as a percentage of total energy intake (EN%) was comparable across dietary patterns and RMEs had a higher protein intake (EN%) compared to those following a PBD. In comparison to RMEs, carbohydrate (EN%) and dietary fibre intake were significantly higher in those following a vegan, LOV, and SV dietary pattern, additionally, dietary fibre intake of vegans was almost two times that of RMEs. Sugar intake was comparable, although intake of starch was significantly higher in vegans compared to RMEs.
Table 2.
Dietary intake across dietary pattern groups derived from an average of two dietitian-administered diet histories (quantitative data) and the AES® FFQ (qualitative data)
| Nutrient/food group serves (per/day) | Total sample (n = 230) | Vegan (n = 48) |
Lacto-ovo vegetarian (n = 47) | Pesco-vegetarian (n = 46) | Semi-vegetarian (n = 44) | Regular meat-eater (n = 45) | P |
|---|---|---|---|---|---|---|---|
| Quantitative data | |||||||
| Energy (kJ) | 9584 ± 2691 | 9957 ± 2712 | 9767 ± 3337 | 9041 ± 2325 | 9651 ± 2598 | 9489 ± 2361 | 0.545 |
| Protein (%)e | 16.5% ± 4.1 | 15.4% ± 3.6a, b | 14.5% ± 4.0a | 17.0% ± 3.1b | 15.4% ± 2.9a, b | 20.4% ± 4.3c | < 0.001 |
| Carbohydrate (%)e | 40.1% ± 9.3 | 43.9% ± 8.4a | 41.7% ± 8.6a, b | 37.8% ± 9.1b, c | 41.9% ± 8.3a, b | 35.0% ± 9.8c | < 0.001 |
| Sugar (g) | 86.0 ± 38.2 | 77.3 ± 26.4 | 92.4 ± 46.7 | 82.2 ± 32.4 | 92.0 ± 29.6 | 88.3 ± 49.8 | 0.241 |
| Starch (g) | 147.5 ± 76.9 | 184.7 ± 86.9a | 153.2 ± 79.1a, b | 125.6 ± 55.8b | 155.4 ± 76.9a, b | 116.5 ± 64.0b | < 0.001 |
| Total fat (%)e | 37.4% ± 8.2 | 35.2% ± 8.4 | 38.1% ± 8.4 | 38.7% ± 7.8 | 36.9% ± 7.0 | 38.1% ± 8.9 | 0.236 |
| Saturated fat (g) | 28.5 ± 13.3 | 23.3 ± 11.2a | 28.0 ± 14.3a, b | 27.8 ± 13.2a, b | 30.7 ± 11.5a, b | 33.1 ± 14.6b | 0.006 |
| Saturated fat (%)e | 11.0% ± 4.1 | 8.7% ± 4.1a | 10.6% ± 4.1a | 11.4% ± 4.1b | 11.8% ± 3.2b | 12.8% ± 3.6b | < 0.001 |
| Trans fats (g) | 0.9 ± 0.6 | 0.5 ± 0.7a | 0.8 ± 0.5a | 1.0 ± 0.6b | 1.0 ± 0.6b | 1.3 ± 0.6b | < 0.001 |
| MUFAs (g) | 39.0 ± 15.3 | 37.0 ± 15.6 | 40.0 ± 15.9 | 40.3 ± 16.3 | 38.1 ± 14.8 | 39.8 ± 14.2 | 0.806 |
| PUFAs (g) | 20.3 ± 10.4 | 25.8 ± 13.6a | 21.3 ± 10.1a, b | 18.5 ± 7.5b | 19.2 ± 10.0b | 16.2 ± 6.9b | < 0.001 |
| LCn-3 PUFAs (g) | 0.5 ± 0.7 | 0.0 ± 0.0a | 0.0 ± 0.0a, b | 1.0 ± 0.8c | 0.4 ± 0.7d | 0.8 ± 0.8c | < 0.001 |
| ALA (g) | 2.6 ± 2.4 | 3.7 ± 3.4a | 2.8 ± 2.1a, b | 2.1 ± 1.3b | 2.6 ± 3.0a, b | 1.6 ± 0.9b | < 0.001 |
| n-6 PUFAs (g) | 17.5 ± 8.8 | 22.3 ± 10.7a | 19.1 ± 8.8a | 15.5 ± 6.9b | 16.8 ± 8.2b | 13.6 ± 6.1b | < 0.001 |
| Cholesterol (mg) | 142.3 ± 138.4 | 33.6 ± 92.1a | 94.8 ± 122.5a, c | 160.7 ± 108.7b | 152.4 ± 112.6b, c | 279.1 ± 124.8d | < 0.001 |
| Dietary fibre (g) | 45.9 ± 20.9 | 58.0 ± 18.8a | 51.6 ± 29.7a | 41.5 ± 13.4b, c | 45.6 ± 16.7b | 31.8 ± 9.4c | < 0.001 |
| Alcohol (g) | 5.1 ± 10.0 | 1.8 ± 4.3a | 3.5 ± 7.2a | 6.7 ± 11.4a, b | 4.0 ± 8.1a, b | 9.4 ± 14.4b | 0.002 |
| Qualitative data | |||||||
| Vegetables | 5.9 ± 2.4 | 6.3 ± 2.5 | 6.2 ± 2.7 | 5.9 ± 2.1 | 6.0 ± 2.1 | 5.1 ± 2.7 | 0.126 |
| Non-Starchy | 4.9 ± 2.1 | 5.3 ± 2.0 | 5.0 ± 2.2 | 4.9 ± 1.9 | 5.0 ± 1.8 | 4.1 ± 2.5 | 0.103 |
| Grains | 2.7 ± 1.3 | 2.7 ± 1.1 | 2.6 ± 1.3 | 2.9 ± 1.6 | 2.7 ± 1.2 | 2.6 ± 1.5 | 0.911 |
| Non-refined | 2.1 ± 1.1 | 1.1 ± 1.0 | 2.0 ± 1.1 | 2.3 ± 1.5 | 1.9 ± 0.9 | 2.0 ± 1.2 | 0.599 |
| Fruit | 3.1 ± 1.7 | 3.8 ± 2.1a | 3.0 ± 1.3a.b | 3.0 ± 1.6a, b | 3.2 ± 1.5a, b | 2.7 ± 1.6b | 0.023 |
| Protein-rich foodsf | 1.9 ± 0.9 | 1.5 ± 0.7a | 1.3 ± 0.5a | 2.0 ± 0.6b | 1.9 ± 1.0a, b | 2.7 ± 0.7c | < 0.001 |
| Legumes/nuts | 1.1 ± 0.6 | 1.5 ± 0.7a | 1.1 ± 0.5b | 1.1 ± 0.5b | 1.0 ± 0.5b | 0.6 ± 0.5c | < 0.001 |
| Seafood | 0.2 ± 0.3 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.6 ± 0.4b | 0.2 ± 0.4c | 0.3 ± 0.2c | < 0.001 |
| Red meat | 0.2 ± 0.4 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.2 ± 0.2b | 1.0 ± 0.5c | < 0.001 |
| Dairy | 1.4 ± 1.4 | 0.0 ± 0.0a | 1.6 ± 1.5b | 1.9 ± 1.0b | 1.7 ± 1.2b | 2.0 ± 1.4b | < 0.001 |
| Discretionary choices | 1.7 ± 1.2 | 1.3 ± 1.0a | 1.6 ± 1.1a | 1.5 ± 1.1a | 1.8 ± 1.2a, b | 2.2 ± 1.5b | 0.008 |
| Sugar sweetened beverages | 0.4 ± 0.6 | 0.3 ± 0.4a | 0.3 ± 0.4a | 0.3 ± 0.4a | 0.3 ± 0.7a, b | 0.6 ± 0.8b | 0.024 |
Data are reported as absolute means ± SD and p-values reported from ANOVA and Tukey post-hoc test for pairwise comparisons
a, b,c, dValues within a row without a common superscript letters are significantly different (P < 0.05)
ePresented as % contribution of total energy intake
fProtein-rich foods include meats, poultry, seafood, eggs, legumes, and nuts. Assessment of meat exclusion among vegans and LOVs and dairy exclusion among vegans were derived from diet histories
ALA, α-linolenic acid; eq, equivalent; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids; LC n-3 PUFAs, long chain omega-3 polyunsaturated fatty acids; n-6 PUFAs, omega-6 polyunsaturated fatty acids
Plasma lipids
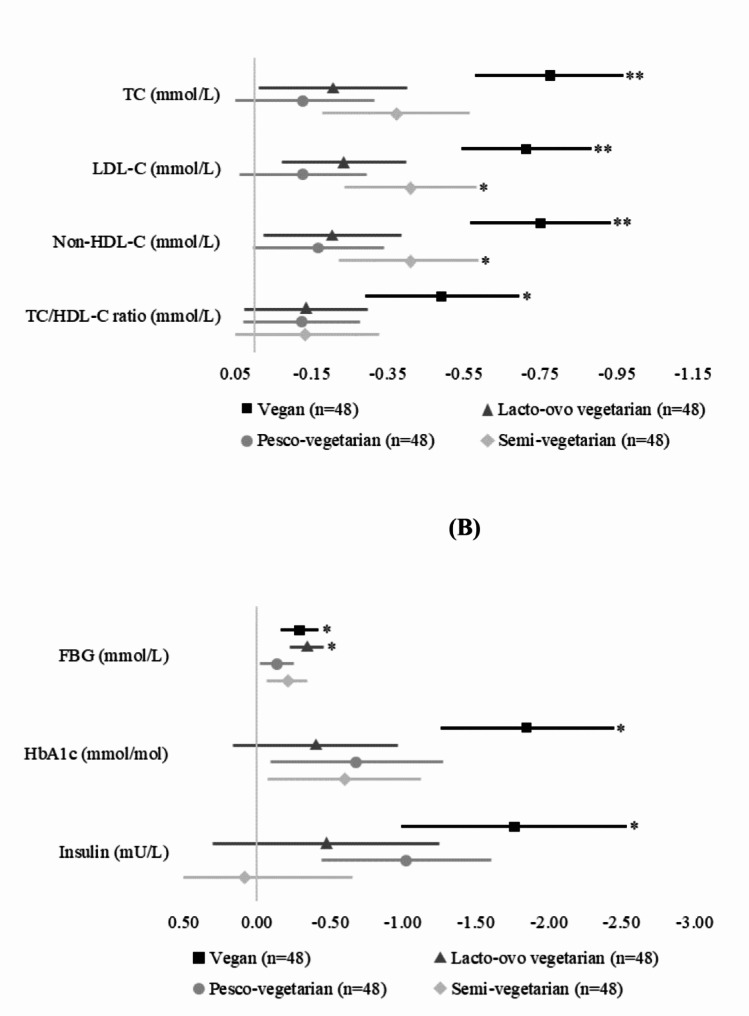
After adjustments, and compared to RMEs, the vegan dietary pattern was associated with significantly lower plasma lipids (Table 3; Fig. 1). Compared to RMEs, vegans had significantly lower TC by 14.9% (-0.77mmol/L,95% CI -1.15, -0.39, P < 0.001), LDL-C by 22.6% (-0.71mmol/L, 95% CI -1.05, -0.38, P < 0.001), non-HDL-C by 20.7% (-0.75mmol/L, 95% CI -1.11, -0.39, P < 0.001) and TC/HDL-ratio by 14.3% (-0.49mmol/L, 95% CI -0.87, -0.11, P = 0.012). In addition, SVs had significantly lower LDL-C by 12.4% (-0.41mmol/L, 95% CI -0.74, -0.08, P = 0.041) and non-HDL-C by 10.8% (-0.40mmol/L, 95% CI -0.76, -0.05, P = 0.026). Those following a LOV and PV dietary pattern did not have significantly different plasma lipids compared to RMEs. HDL-C and TG concentrations were comparable across plant-based and regular-meat dietary patterns. Supplementary Table 1 shows adjusted means, and 95% CIs used to calculate percentage differences in plasma lipids between PBDs and the regular meat-eating diet.
Table 3.
Adjusted mean differences in lipid and glycaemic indices across plant-based diets compared to a regular meat-eating diet
| Variable | Vegan (n = 48) | Lacto-ovo vegetarian (n = 47) | Pesco-vegetarian (n = 46) | Semi-vegetarian (n = 44) | |||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | ||
| Lipid lipids | |||||||||
| TC (mmol/L) | -0.77 (-1.15, -0.39) | < 0.001* | -0.20 (-0.58, 0.17) | 0.282 | -0.12 (-0.50, 0.26) | 0.526 | -0.37 (-0.74, 0.00) | 0.052 | |
| TG (mmol/L) | -0.08 (-0.31, 0.14) | 0.462 | 0.07 (-0.12, 0.26) | 0.487 | -0.09 (-0.30, 0.13) | 0.435 | -0.01 (-0.20, 0.19) | 0.948 | |
| LDL-C (mmol/L) | -0.71 (-1.05, -0.38) | < 0.001* | -0.23 (-0.56, 0.09) | 0.158 | -0.13 (-0.45, 0.19) | 0.439 | -0.41 (-0.74, -0.08) | 0.014 | |
| HDL-C (mmol/L) | -0.03 (-0.18, 0.12) | 0.693 | 0.02 (-0.12, 0.16) | 0.806 | 0.05 (-0.10, 0.20) | 0.517 | 0.04 (-0.10, 0.19) | 0.578 | |
| Non-HDL-C (mmol/L) | -0.75 (-1.11, -0.39) | < 0.001* | -0.20 (-0.54, 0.13) | 0.236 | -0.17 (-0.52, 0.19) | 0.356 | -0.40 (-0.76, -0.05) | 0.026 | |
| TC/HDL-C ratio (mmol/L) | -0.49 (-0.87, -0.11) | 0.012 | -0.13 (-0.43, 0.17) | 0.381 | -0.12 (-0.44, 0.19) | 0.448 | -0.13 (-0.52, 0.26) | 0.510 | |
| Glycaemic indices | |||||||||
| FBG (mmol/L) | -0.29 (-0.53, -0.06) | 0.014 | -0.34 (-0.56, -0.11) | 0.003 | -0.14 (-0.36, 0.09) | 0.232 | -0.21 (-0.48, 0.06) | 0.134 | |
| HbA1c (IFFC, mmol/mol) | -1.85 (-3.00, -0.71) | 0.002 | -0.40 (-1.51, 0.71) | 0.479 | -0.68 (-1.84, 0.48) | 0.249 | -0.60 (-1.63, 0.43) | 0.254 | |
| Insulin (mU/L) | -1.76 (-3.26, -0.26) | 0.021 | -0.47 (-2.00, 1.05) | 0.542 | -1.03 (-2.17, 0.11) | 0.078 | 0.09 (-1.36, 1.55) | 0.902 | |
Data is presented as β coefficients (95% CIs) and p-values. Multivariate regression analyses was used to adjust the model for age (years), sex (female, male), physical activity level (MET/week), total energy intake (kJ/day), duration of dietary pattern (years), alcohol intake (g), smoking status (yes, no), level of education (higher education yes, no), BMI (kg/m2) as a mediator and the addition of EPA/DHA supplement use (yes, no) for lipid lipids. Bold values indicate statistical significance (P < 0.05). P-values marked with an asterisk meet the Bonferroni corrected alpha (P = 0.001)
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; FBG, fasting blood glucose; HbA1c, haemoglobin A1C; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides
Fig. 1.
Adjusted mean differences ± SE in (a) lipid levels and (b) glycaemic indices of plant-based compared to regular meat-eating diets
FBG, fasting blood glucose; HbA1c, haemoglobin A1C; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides. *P < 0.05, **P = 0.001 (Bonferroni corrected alpha).
Glycaemic indices
After adjustments, and compared to RMEs, the vegan dietary pattern was associated with significantly lower glycaemic indices for all parameters (Table 3; Fig. 1). Compared to RMEs, vegans had significantly lower FBG by 6.2% (-0.29mmol/L, 95% CI -0.53, -0.06, P = 0.014), HbA1c by 5.8% (-1.85mmol/mol, 95% CI -3.00, -0.71, P = 0.002) and insulin by 28.7% (-1.76mU/L, 95% CI -3.26, -0.26, P = 0.021). In addition, LOVs had significantly lower FBG by 7.3% (-0.34mmol/L, 95% CI -0.56, -0.11, P = 0.003). Those following a PV and SV dietary pattern did not have significantly different glycaemic indices compared to RMEs. Supplementary Table 1 shows adjusted means, and 95% CIs used to calculate percentage differences in glycaemic indices between PBDs and the regular meat-eating diet.
Sensitivity analyses and multiple comparisons
After completing a sensitivity analyses, E-values were small and significant outcomes remained unchanged, indicating it was unlikely there was a missing confounder with an observed association and exposure. In addition, outcomes among vegans for TC, LDL-C, non-HDL-C and HbA1c met the Bonferroni corrected alpha of P = 0.001. This provides further evidence that the vegan dietary pattern is robustly associated with favourable plasma lipids and glycaemic indices when compared to RMEs.
Discussion
This cross-sectional study of adults found adherence to a vegan dietary pattern was associated with favourable plasma lipids and glycaemic indices when compared to a regular-meat dietary pattern characterised by lower concentrations of TC, LDL-C, non-HDL-C, TC/HDL ratio, FBG, HbA1c and insulin. Those following other PBDs demonstrated a less pronounced association, although SVs had significantly lower LDL-C and non-HDL-C and LOVs had significantly lower FBG when compared to RMEs. There were no differences observed between any plant-based and regular meat-eating dietary patterns on HDL-C and TG concentrations. These outcomes withstood adjustments for potential confounders, sensitivity analyses and were somewhat robust to adjustment for multiple testing.
This study indicates that vegans have a favourable lipid profile compared to RMEs, except there were no differences in TG and HDL-C. Our results align with multiple pooled meta-analyses of both RCTs and observational studies which reported that when compared RMEs, LOVs (including vegans) reduced or had lower concentrations of TC, LDL-C, non-HDL-C, although reported no difference in TG [25, 29–31]. These studies also reported lower HDL-C among vegetarians (including vegans), and one study found vegans to have higher TG, dissimilar to the current results. Crude analyses revealed a slightly lower HDL-C among vegans, the difference was not significant when compared to RMEs. Large overseas cross-sectional studies with similar methodologies found vegan and vegetarian dietary patterns to have significantly lower HDL-C, LDL-C, TC, TC/HDL-C ratio, and higher TG [47, 48]. Reasons why current HDL-C concentrations do not align with previous literature may be attributed to the ‘healthy user effect’ as participants in the current sample had comparable levels of MetS and metabolic components including obesity and hypertension, known to influence plasma lipids [49]. To the best of our knowledge, no other study has investigated plasma lipids across four categories of plant-based eating. The AHS-2 is the only other study to explore dietary patterns and health parameters among vegans, LOVs and PVs and reported that the prevalence of elevated TC and LDL-C were lower in all three groups compared to non-vegetarians [34]. Noteworthy, among this study, individuals were Seventh Day Adventists, whom are known to also practice other health-promoting behaviours such as abstaining from alcohol [34]. Another explanation for the absence of an effect of PBDs on HDL-C in the current study is that the design distinguishes between various PBDs, whilst other studies may have had increased power to detect differences in biomarkers by combining vegan and vegetarians into the same dietary category for analyses.
Nutrient composition of PBDs may explain differences in plasma lipids observed across diet groups. Although overall fat intake was comparable, when compared to RMEs, vegans and LOVs had significantly lower intake of saturated fat, trans-fat, and cholesterol and higher polyunsaturated fats (vegans only). LOVs had significantly lower trans-fat intake and PVs and SVs had comparable intakes to RMEs. These outcomes are aligned with previous larger observational studies with alike characterisations of vegan and LOV dietary patterns. Such studies observed vegans to have the lowest dietary intake of saturated fat, trans fats and cholesterol and highest intakes of PUFAs compared to a regular meat-eating diet [50–53]. Furthermore, analyses of daily food group intake provided deeper insight into how dietary habits contribute to dietary fat profiles. PBDs in the present study had significantly higher intakes of legumes/nuts as their source of protein rich foods, which are predominantly richer in dietary soluble fibre, unsaturated fats, whilst consuming minimal/nil animal-based protein, inherently rich in saturated and trans fats. It is well documented that dietary saturated and trans fats have been shown to increase LDL-C and TC concentrations, therefore increasing the risk of atherosclerotic CVD [54]. PUFAs, on the other hand, are known to benefit heart health by lowering TG and LDL-C concentrations through mechanisms such as reducing systemic inflammation, improving endothelial function, and acting as an antiatherogenic agent [55, 56]. RMEs had the highest saturated fat intake, lowest PUFAs intakes and highest plasma lipids levels. In contrast, vegans had the lowest saturated fat intake, highest PUFAs intakes and lowest plasma lipids.
Intake of n-3 PUFA are known to specifically reduce TG concentrations. As expected, intake of fish and seafood, and LC n-3 PUFAs was significantly higher among those adhering to a PV dietary pattern compared to all other groups (except for RMEs for LC n-3 PUFAs). However, levels of consumption were moderate (half a serve a day) which explain why a dietary pattern, although higher in n-3 PUFAs rich foods did not significantly lower TG concentrations. In addition, RMEs had the highest number of participants supplementing with EPH/DHA compared to PBDs. Although appropriately adjusted for, this may have an influence on crude TG concentrations. Lastly, intake of dietary fibre can also play a key role in reducing absorption of saturated and trans fats, which therefore lowers LDL-C and TC and has been proved to reduce risk of CVD [57]. Soluble fibre lowers lipid levels by binding to cholesterol particles therefore preventing absorption [57]. Moreover, soluble fiber can also bind to bile acids which results in a reduction of cholesterol content within the liver cells, upregulation of LDL receptors and clearance of circulating LDL-C [58]. In the present sample, compared to RMEs, individuals adhering to a PBD consumed more dietary fibre, vegetables, fruit, legumes/nuts and fruits which are rich in soluble fibre, which is supported by previous large cross-sectional and prospective studies [50, 51, 53]. Detailed collection of micro and macro nutrient compositions related to intake of dietary fats and fibre helps to cement our understanding of the independent effect of dietary pattern on cardiometabolic risk factors.
In the current study, compared to RMEs, those adhering to a vegan dietary pattern were associated with favourable glycaemic indices characterised by significantly lower FBG, HbA1c, and insulin. In addition, LOVs had significantly lower FBG compared to RMEs. PBDs have been offered as a strategy for managing glycaemic control in previous literature [24]. A meta-analysis of six RCTs following vegetarian dietary patterns for ≥ 4 weeks found they were associated with significant reduction in HbA1c and a non-significant reduction in FBG [59]. Another meta-analysis investigating the effect of replacing animal protein with plant protein in RCTs with a median follow up of 8 weeks reported a significant reduction in HbA1c, FBG and insulin [60]. In addition, a meta-analysis of fourteen observational studies found a vegetarian (including vegans) dietary pattern was inversely associated with risk of T2D compared to non-vegetarians [61]. Very few studies have investigated the effects of PBDs, inclusive of PVs and SVs on glycaemic indices and risk of T2D. The AHS-2 study found vegans, LOV, SV but not PV were associated with a substantial and independent reduced risk in T2D after a two year follow up period [28]. The same results were observed in the EPIC-Oxford study, except for PVs, which had a follow up period of eighteen years [62]. Moreover, an Australian-based study using the same definition of PBDs as the current study, found prevalence of impaired glucose tolerance was lower in vegans, LOVs, SV, and PVs, compared to RMEs [15]. Both meta-analyses and similar observational studies corroborate results seen in the present study which demonstrate those following a vegan and or LOV dietary pattern had lower FBG and HbA1c compared to RMEs.
Our study demonstrated that the vegan dietary pattern had lower insulin levels when compared to RMEs. Findings are corroborated by a previous, age and sex-matched cross-sectional study which reported that a vegan dietary pattern had lower insulin and HOMA-IR, however, significance for LOVs was lost after adjustment for confounders such as BMI, physical activity, and alcohol consumption [63]. Furthermore, two separate RCTs reported both vegans and LOVs interventions reduce insulin resistance (HOMA-IR) and insulin levels when compared to an omnivorous intervention after 16 weeks and 24 weeks, respectively [64, 65]. Few studies have explored the relationship between PVs and SVs and insulin levels. Results from a small cross-sectional study found individuals who maintained a long-term SV had lower insulin concentrations and insulin resistance (HOMA-IR) when compared to non-vegetarians [66]. This study strengthens the developing recognition of vegan and LOV dietary patterns as effective tools in the prevention and management of glycaemic indices and potential improvement in insulin sensitivity which are key risk factors for CVD [16, 67]. There is not enough research to conclusively define influence of SV and PV dietary patterns on glycaemic indices and insulin sensitivity, although emerging findings are favourable. Future longitudinal studies evaluating prevalence of T2D, glycaemic indices, and insulin resistance across various PBDs, inclusive of SV and PVs are warranted to substantiate outcomes observed in the current study and small pool of previous literature.
Nutrient composition across dietary patterns may be linked to differences in glycaemic indices. Vegans, LOVs and SV had significantly higher carbohydrates (EN%) than RMEs, and vegans also had significantly higher intake of starch. However, as vegans had significantly lower glycaemic indices than RMEs, it is apparent that consumption of food groups as opposed to individual nutrients may better explain observed differences. PBDs, specifically vegans had higher intake of legumes/nuts, fruit and lower intakes of discretionary choices characterised by nutrient poor foods high in added sugars, energy, salt, saturated fats, compared to RMEs [68]. Evidence suggest that a dietary pattern rich in fibre and wholefood groups and lower in processed meat, red meat and discretionary choices including sugar sweetened beverages can reduce risk of T2D [69]. Added sugar foods and high glycaemic rich carbohydrate are rapidly absorbed after consumption leading to an increase in blood glucose and insulin responses, which if sustained over time can lead to glucose intolerance and insulin resistance, corner stones for T2D [70]. Previous literature not only confirms the ability of PBDs to favourably modulate glycaemic indices and risk of T2D, but also suggest that a vegetarian dietary pattern may have greater capacity to improve insulin sensitivity compared with a conventional diabetic diet, characterised by lower carbohydrate and higher protein intakes [65].
The novelty of this study is such that it is the first ever Australian-based study to purposefully recruit individuals habitually following several types of PBDs from the community to evaluate health status and risk of cardiometabolic biomarkers. Majority of the previous literature on PBDs are from secondary analyses, which have not specifically recruited individuals habitually following PBDs from the community, therefore, can only evaluate indexes, for example healthy and unhealthy plant-based indexes. This study design did not result in retro-fitting participants into indices which allows for better translation to clinical practice and nutrition policy interpretation. Results contribute to existing literature by providing distinguished differences between various PBDs and regular meat-eating diets and their association with key risk factors for CVD and T2D, not previously explored. Outcomes are underpinned by comprehensive investigation of dietary patterns, a primary lifestyle risk factor for these chronic conditions.
Strengths include use of validated and dietitian administered detailed dietary collection tools which provide comprehensive qualitative and quantitative nutrient intake data fundamental to reflect the complex nature of human dietary patterns [38]. Second, the duration in which participants were following dietary patterns was long enough to exert an effect on primary outcomes. Third, several potential covariates were controlled for to avoid confounding and therefore appropriately examine the association between PBDs and lipaemic and glycaemic indices whilst considering other lifestyle factors. Lastly, the level of difference between the vegan dietary pattern and RMEs for LDL-C and HbA1c was deemed clinically significant as defined by a ≥ 10% and ≥ 0.5% difference, respectively, and therefore translational and practical for use in health care and nutrition policy [71]. However, several limitations need to be considered. Being a cross-sectional study, findings should be interpreted with caution and no inferences on causation can be employed. While some data were self-reported, all questionnaires were reviewed by study investigators with participants, and use of validated data collection methods such as the AES® FFQ [36] and IPAQ were used [44]. Although adequately powered, the sample size is modest and future larger studies using similar validated dietary collection methodologies are warranted.
Conclusion
In conclusion, this cross-sectional study of healthy middle-aged adults found individuals adhering to a vegan dietary pattern had favourable plasma lipids and glycaemic indices compared to regular-meat dietary patterns characterised by significantly lower concentrations of TC, LDL-C, non-HDL-C, TC/HDL ratio, FBG, HbA1c and insulin. Other PBDs had a moderate association; SVs had lower LDL-C and non-HDL-C; and LOVs had lower FBG. HDL-C and TG were comparable across plant-based and regular meat-eating dietary patterns. This study highlights the importance of detailed dietary collection methodologies to effectively evaluate links between dietary patterns, metabolic biomarkers, and overall cardiovascular health. Findings from this study have direct application to clinical practice, suggestive of at a minimum, the consideration of plant-forward dietary patterns for the assistance of managing elevated blood lipid and/or glycaemic parameters among those at higher risk of CVD. In addition, outcomes warrant further consideration of PBDs to be incorporated alongside dietary advice relating to cardiometabolic disease risk reduction, as well, as among national population-based dietary guidelines. Larger forthcoming prospective studies utilising similar methodologies to define and evaluate PBDs, inclusive of PVs and SVs, are warranted to substantiate findings and further examine effects of PBDs on the development of chronic diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank all study participants for their efforts.
Abbreviations
- AHS-2
Adventist Health-2 study
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FBG
fasting blood glucose
- HbA1c
haemoglobin A1C
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- IPAQ
International Physical Activity Questionnaire
- LDL-C
low-density lipoprotein cholesterol
- LOV
lacto-ovo vegetarian
- METs
metabolic equivalent of tasks
- MetS
metabolic syndrome
- LC N-3 PUFAs
long chain omega-3 poly-unsaturated fatty acids
- PBD
plant-based diet
- PV
pesco-vegetarian
- RCT
randomised controlled trial
- RME
regular meat-eater
- SV
semi-vegetarian
- TC
total cholesterol
- TG
triglycerides
- T2D
type 2 diabetes mellitus
- EN%
percentage total energy intake
Author contributions
GA: conceptualization, data curation, formal analysis, investigation, methodology, Software, validation, Visualization, writing of original draft. JF: conceptualization, funding acquisition, investigation, methodology, supervision, validation, visualization, review & editing. SE: data curation, review & editing. CO: review & editing. LW: writing, review & editing. MG: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was partially supported by the pilot grant from the College of Health, Medicine, and Well-being at the University of Newcastle (grant no. 10-32804), Bridging Scholarship and an Early Career small grant for statistical support from the Hunter Medical Research Institute (grant no. 2101041), and Hunter Medical Research Institute Philanthropy funds (grant no. 2200517).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethics committee approval was obtained by the University of Newcastle’s Human Research Ethics Committee (HREC 2020 − 0195). Informed and signed consent was required by all participants prior to commencing the study.
Consent for publication
Not applicable.
Competing interests
JF is a part-time postdoctoral research fellow at the University of Newcastle and is also currently employed part-time by Sanitarium Health Food Company, which had no input in the study and is not financially supporting or sponsoring part of this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
The original online version of this article was revised: the authors noticed that the competing interest statement was not included. In addition, the first letter of the word “australians” in the article title must be capitalize.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/7/2024
The original online version of this article was revised: the authors noticed that the competing interest statement was not included. In addition, the first letter of the word “australians” in the article title must be capitalize.
Change history
11/8/2024
A Correction to this paper has been published: 10.1186/s12944-024-02362-z
References
- 1.Statistics ABo. Diabetes: Canberra: ABS; 2022 [cited 2024 April 17]. https://www.abs.gov.au/statistics/health/health-conditions-and-risks/diabetes/latest-release
- 2.Statistics ABo. High cholesterol: Canberra; ABS. 2022 [cited 2024 April 4]. https://www.abs.gov.au/statistics/health/health-conditions-and-risks/high-cholesterol/latest-release
- 3.Mendis S, Graham I, Narula J. Addressing the Global Burden of Cardiovascular diseases; need for scalable and sustainable frameworks. Glob Heart. 2022;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaggini M, Gorini F, Vassalle C. Lipids in atherosclerosis: pathophysiology and the role of calculated lipid indices in assessing Cardiovascular Risk in patients with Hyperlipidemia. Int J Mol Sci. 2022;24(1). [DOI] [PMC free article] [PubMed]
- 5.Lusis AJ. Atherosclerosis Nat. 2000;407(6801):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes Mellitus and Cardiovascular Disease: genetic and epigenetic links. Front Endocrinol (Lausanne). 2018;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res. 2016;167(1):257–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parhofer KG. Interaction between glucose and lipid metabolism: more than Diabetic Dyslipidemia. Diabetes Metab J. 2015;39(5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Research RM. The slow but steady rise of vegetarianism in Australia: Roy Morgan; 2016 [cited 2023 30 June]. https://www.roymorgan.com/findings/the-slow-but-steady-rise-of-vegetarianism-in-australia
- 11.Raven P. How many Britons will attempt a vegan diet and lifestyle in January 2023? YouGov UK; 2023 [cited 2023 Jun 30]. https://yougov.co.uk/topics/society/articles-reports/2022/12/29/how-many-britons-will-attempt-vegan-diet-and-lifes
- 12.Dressler J, Storz MA, Müller C, Kandil FI, Kessler CS, Michalsen A et al. Does a plant-based Diet stand out for its favorable composition for Heart Health? Dietary Intake Data from a Randomized Controlled Trial. Nutrients. 2022;14(21). [DOI] [PMC free article] [PubMed]
- 13.Ahmad SR. Plant-based diet for obesity treatment. Front Nutr. 2022;9:952553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bye ZL, Keshavarz P, Lane GL, Vatanparast H. What role do plant-based diets play in supporting the Optimal Health and Well-being of canadians? A scoping review. Adv Nutr. 2021;12(6):2132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baleato CL, Ferguson JJA, Oldmeadow C, Mishra GD, Garg ML. Plant-based dietary patterns versus meat consumption and prevalence of impaired glucose intolerance and diabetes Mellitus: a cross-sectional study in Australian women. Nutrients. 2022;14(19):4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin G, Ferguson JJA, Garg ML. Effects of Plant-based diets on Weight Status in Type 2 diabetes: a systematic review and Meta-analysis of Randomised controlled trials. Nutrients. 2021;13(11). [DOI] [PMC free article] [PubMed]
- 17.Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a Meta-Analysis of Randomized controlled trials. J Gen Intern Med. 2016;31(1):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson JJA, Oldmeadow C, Mishra GD, Garg ML. Plant-based dietary patterns are associated with lower body weight, BMI and waist circumference in older Australian women. Public Health Nutr. 2022;25(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baleato CL, Ferguson JJA, Oldmeadow C, Mishra GD, Garg ML. Plant-based dietary patterns versus meat consumption and prevalence of impaired glucose intolerance and diabetes Mellitus: a cross-sectional study in Australian women. Nutrients. 2022;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson JJA, Austin G, Oldmeadow C, Garg ML. Plant-based dietary patterns and Cardiovascular Disease Risk in australians: protocol for a cross-sectional study. Nutrients. 2023;15(13):2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin G, Ferguson JJA, Eslick S, Oldmeadow C, Wood LG, Garg ML. Cardiovascular Disease Risk in individuals following plant-based dietary patterns compared to regular meat-eaters. Nutrients. 2024;16(7). [DOI] [PMC free article] [PubMed]
- 22.Mihrshahi S, Ding D, Gale J, Allman-Farinelli M, Banks E, Bauman AE. Vegetarian diet and all-cause mortality: evidence from a large population-based Australian cohort - the 45 and up study. Prev Med. 2017;97:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Diseases WEOftPaCoN. Plant-based diets and their impact on health, sustainability and the environment: A review of the evidence. 2021.
- 24.Adokwe JB, Waeyeng D, Suwan K, Camsanit K, Kaiduong C, Nuanrat P, et al. Plant-based Diet and Glycemic Control in Type 2 diabetes: evidence from a Thai health-promoting hospital. Nutrients. 2024;16(5):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch CA, Kjeldsen EW, Frikke-Schmidt R. Vegetarian or vegan diets and blood lipids: a meta-analysis of randomized trials. Eur Heart J. 2023;44(28):2609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannesen CO, Dale HF, Jensen C, Lied GA. Effects of Plant-based diets on outcomes related to glucose metabolism: a systematic review. Diabetes Metab Syndr Obes. 2020;13:2811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picasso MC, Lo-Tayraco JA, Ramos-Villanueva JM, Pasupuleti V, Hernandez AV. Effect of vegetarian diets on the presentation of metabolic syndrome or its components: a systematic review and meta-analysis. Clin Nutr. 2019;38(3):1117–32. [DOI] [PubMed] [Google Scholar]
- 28.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metabolism Cardiovasc Dis. 2013;23(4):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott PS, Kharaty SS, Phillips CM. Plant-based diets and lipid, lipoprotein, and inflammatory biomarkers of Cardiovascular Disease: a review of Observational and Interventional studies. Nutrients. 2022;14(24):5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and Meta-analysis of Randomized controlled trials. J Am Heart Assoc. 2015;4(10):e002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama Y, Levin SM, Barnard ND. Association between plant-based diets and plasma lipids: a systematic review and meta-analysis. Nutr Rev. 2017;75(9):683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riaz H, Khan SU, Rahman H, Shah NP, Kaluski E, Lincoff AM, et al. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(5):533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299(21):2524–32. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto S, Beeson WL, Shavlik DJ, Siapco G, Jaceldo-Siegl K, Fraser G, et al. Association between vegetarian diets and cardiovascular risk factors in non-hispanic white participants of the Adventist Health Study-2. J Nutr Sci. 2019;8:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Body mass index (BMI) and waist measurement: Australian Goverment, Department of Health and Aged Care., 2021 [cited 2023 9 Sep]. https://www.health.gov.au/topics/overweight-and-obesity/bmi-and-waist
- 36.Collins CE, Boggess MM, Watson JF, Guest M, Duncanson K, Pezdirc K, et al. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin Nutr. 2014;33(5):906–14. [DOI] [PubMed] [Google Scholar]
- 37.National Health and Medical Research Council. Australian Dietary guidelines Summary. Canberra: National Health and Medical Research Council; 2013. [Google Scholar]
- 38.Bailey RL. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr Opin Biotechnol. 2021;70:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezaianzadeh A, Namayandeh SM, Sadr SM. National Cholesterol Education Program Adult Treatment Panel III Versus International Diabetic Federation Definition of metabolic syndrome, which one is Associated with Diabetes Mellitus and Coronary Artery Disease? Int J Prev Med. 2012;3(8):552–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for americans of Mexican descent using machine learning. PLoS ONE. 2011;6(6):e21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin SY, Li WC, Yang TA, Chen YC, Yu W, Huang HY, et al. Optimal threshold of Homeostasis Model Assessment of Insulin Resistance to identify metabolic syndrome in a Chinese Population aged 45 years or younger. Front Endocrinol (Lausanne). 2021;12:746747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metabolism. 2010;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada C, Moriyama K, Takahashi E. Optimal cut-off point for homeostasis model assessment of insulin resistance to discriminate metabolic syndrome in non-diabetic Japanese subjects. J Diabetes Investig. 2012;3(4):384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 45.Li D, Sinclair A, Mann N, Turner A, Ball M, Kelly F, et al. The association of diet and thrombotic risk factors in healthy male vegetarians and meat-eaters. Eur J Clin Nutr. 1999;53(8):612–9. [DOI] [PubMed] [Google Scholar]
- 46.Kim H, Lee K, Rebholz CM, Kim J. Plant-based diets and incident metabolic syndrome: results from a South Korean prospective cohort study. PLoS Med. 2020;17(11):e1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu YF, Hsu CC, Chiu TH, Lee CY, Liu TT, Tsao CK, et al. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: a matched cohort study. Br J Nutr. 2015;114(8):1313–20. [DOI] [PubMed] [Google Scholar]
- 48.Dawczynski C, Weidauer T, Richert C, Schlattmann P, Dawczynski K, Kiehntopf M. Nutrient intake and Nutrition Status in vegetarians and vegans in comparison to omnivores - the nutritional evaluation (NuEva) study. Front Nutr. 2022;9:819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawczynski C, Weidauer T, Richert C, Schlattmann P, Dawczynski K, Kiehntopf M. Nutrient intake and Nutrition Status in vegetarians and vegans in comparison to omnivores - the nutritional evaluation (NuEva) study. Front Nutr. 2022;9. [DOI] [PMC free article] [PubMed]
- 51.Nebl J, Schuchardt JP, Wasserfurth P, Haufe S, Eigendorf J, Tegtbur U, et al. Characterization, dietary habits and nutritional intake of omnivorous, lacto-ovo vegetarian and vegan runners - a pilot study. BMC Nutr. 2019;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6(3):259–69. [DOI] [PubMed] [Google Scholar]
- 53.Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113(12):1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep. 2010;12(6):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ander BP, Dupasquier CM, Prociuk MA, Pierce GN. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp Clin Cardiol. 2003;8(4):164–72. [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson JJA, Dias CB, Garg ML. Omega-3 polyunsaturated fatty acids and Hyperlipidaemias. In: Hegde MV, Zanwar AA, Adekar SP, editors. Omega-3 fatty acids: Keys to Nutritional Health. Cham: Springer International Publishing; 2016. pp. 67–78. [Google Scholar]
- 57.Soliman GA. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients. 2019;11(5). [DOI] [PMC free article] [PubMed]
- 58.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis*. Am J Clin Nutr. 1999;69(1):30–42. [DOI] [PubMed] [Google Scholar]
- 59.Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4(5):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viguiliouk E, Stewart SE, Jayalath VH, Ng AP, Mirrahimi A, de Souza RJ, et al. Effect of replacing animal protein with plant protein on Glycemic Control in Diabetes: a systematic review and Meta-analysis of Randomized controlled trials. Nutrients. 2015;7(12):9804–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee Y, Park K. Adherence to a vegetarian Diet and Diabetes Risk: a systematic review and Meta-analysis of Observational studies. Nutrients. 2017;9(6):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papier K, Appleby PN, Fensom GK, Knuppel A, Perez-Cornago A, Schmidt JA, et al. Vegetarian diets and risk of hospitalisation or death with diabetes in British adults: results from the EPIC-Oxford study. Nutr Diabetes. 2019;9(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui X, Wang B, Wu Y, Xie L, Xun P, Tang Q, et al. Vegetarians have a lower fasting insulin level and higher insulin sensitivity than matched omnivores: a cross-sectional study. Nutr Metab Cardiovasc Dis. 2019;29(5):467–73. [DOI] [PubMed] [Google Scholar]
- 64.Kahleova H, Petersen KF, Shulman GI, Alwarith J, Rembert E, Tura A, et al. Effect of a low-Fat Vegan Diet on Body Weight, insulin sensitivity, Postprandial Metabolism, and Intramyocellular and hepatocellular lipid levels in overweight adults: a Randomized Clinical Trial. JAMA Netw Open. 2020;3(11):e2025454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28(5):549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim MH, Bae YJ. Comparative study of serum leptin and insulin resistance levels between Korean Postmenopausal Vegetarian and non-vegetarian women. Clin Nutr Res. 2015;4(3):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14(5):342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson BJ, Bell LK, Zarnowiecki D, Rangan AM, Golley RK. Contribution of Discretionary Foods and Drinks to Australian Children’s Intake of Energy, Saturated Fat, Added Sugars and Salt. Child (Basel). 2017;4(12). [DOI] [PMC free article] [PubMed]
- 69.Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm C, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vlachos D, Malisova S, Lindberg FA, Karaniki G. Glycemic Index (GI) or glycemic load (GL) and dietary interventions for optimizing Postprandial Hyperglycemia in patients with T2 diabetes: a review. Nutrients. 2020;12(6). [DOI] [PMC free article] [PubMed]
- 71.Bradley R, Kozura E, Buckle H, Kaltunas J, Tais S, Standish LJ. Description of clinical risk factor changes during naturopathic care for type 2 diabetes. J Altern Complement Med. 2009;15(6):633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.