Abstract
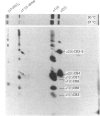
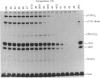
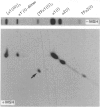
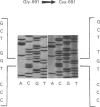
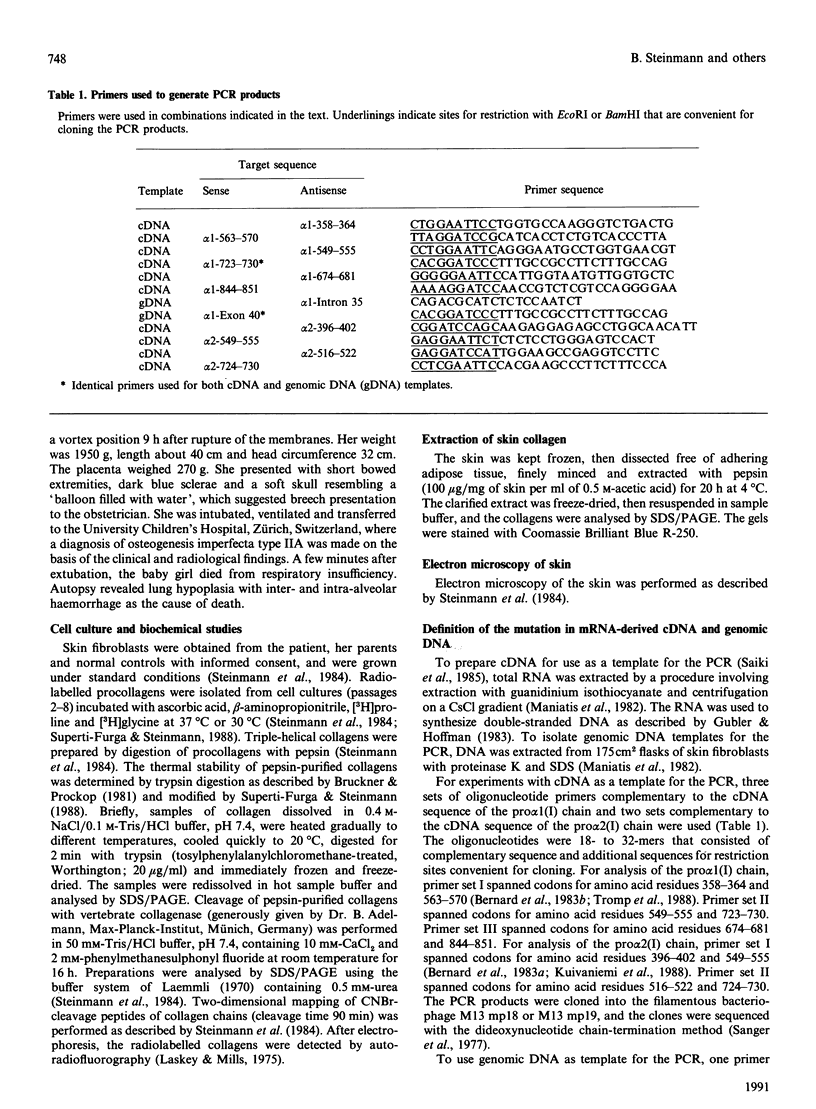
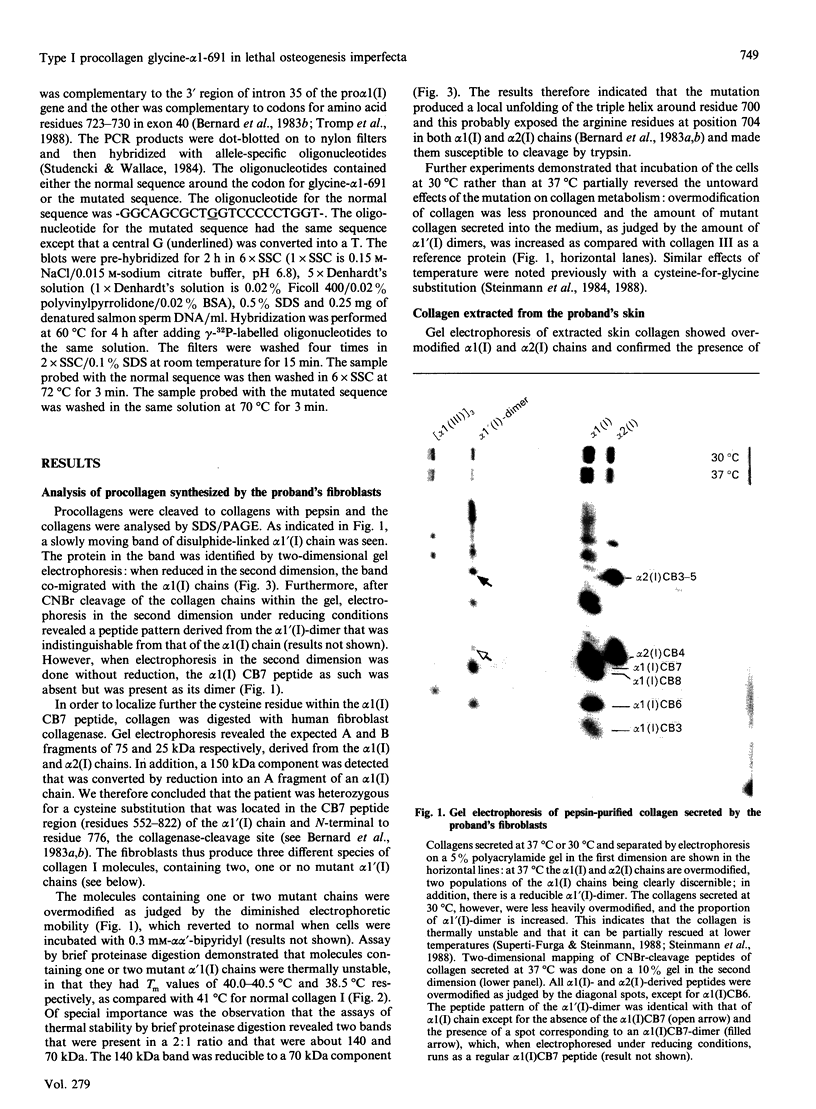
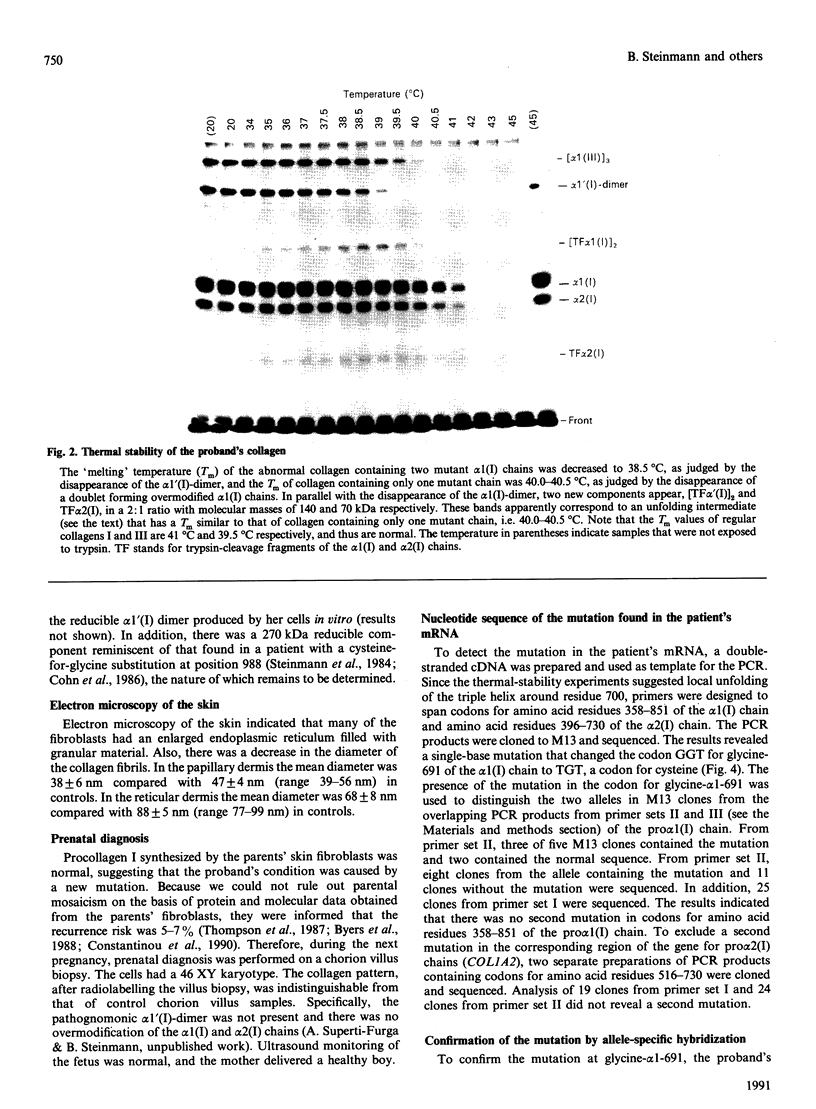
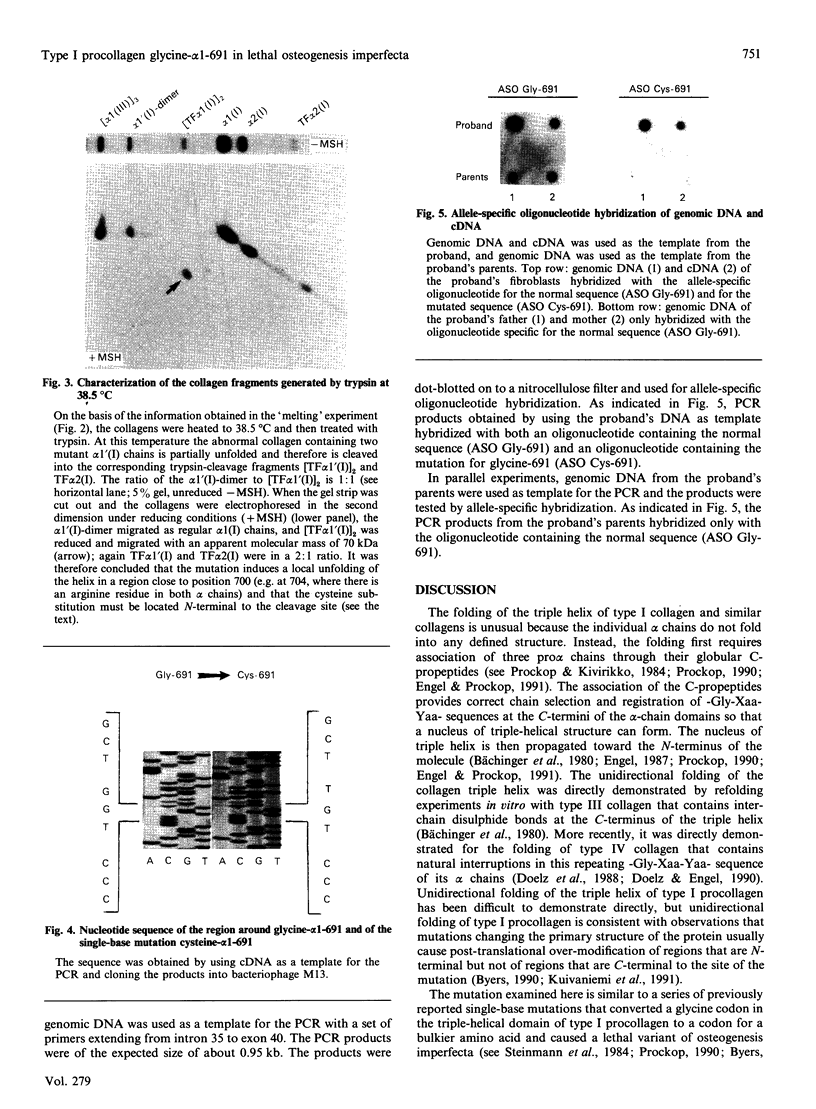
Skin fibroblasts from a proband with lethal osteogenesis imperfecta synthesized a type I procollagen containing a cysteine residue in the alpha 1(I) helical domain. Assay of thermal stability of the triple helix by proteinase digestion demonstrated a decreased temperature for thermal unfolding of the protein. Of special importance was the observation that assays of thermal stability by proteinase digestion revealed two bands present in a 2:1 ratio of about 140 and 70 kDa; the 140 kDa band was reducible to a 70 kDa band. Further analysis of the fragments demonstrated that the cysteine mutation produced a local unfolding of the triple helix around residue 700 and apparently exposed the arginine residue at position 704 in both the alpha 1(I) and alpha 2(I) chains. Analysis of cDNAs and genomic DNAs demonstrated a single-base mutation that changed the GGT codon for glycine-691 of the alpha 1(I) chain to a TGT codon for cysteine. The mutation was not found in DNA from either of the proband's parents. Since the proteinase assay of helical stability generated a fragment of 700 residues that retained disulphide-bonded cysteine residues at alpha 1-691, the results provide one of the first indications that glycine substitutions in type I procollagen can alter the conformation of the triple helix at a site that is C-terminal to the site of the substitution.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Bächinger H. P., Bruckner P., Timpl R., Prockop D. J., Engel J. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Role of disulfide bridges and peptide bond isomerization. Eur J Biochem. 1980 May;106(2):619–632. doi: 10.1111/j.1432-1033.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C. D., Pack M., Young S. B., Prockop D. J. Phenotypic heterogeneity in osteogenesis imperfecta: the mildly affected mother of a proband with a lethal variant has the same mutation substituting cysteine for alpha 1-glycine 904 in a type I procollagen gene (COL1A1). Am J Hum Genet. 1990 Oct;47(4):670–679. [PMC free article] [PubMed] [Google Scholar]
- Dölz R., Engel J., Kühn K. Folding of collagen IV. Eur J Biochem. 1988 Dec 15;178(2):357–366. doi: 10.1111/j.1432-1033.1988.tb14458.x. [DOI] [PubMed] [Google Scholar]
- Engel J., Prockop D. J. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu Rev Biophys Biophys Chem. 1991;20:137–152. doi: 10.1146/annurev.bb.20.060191.001033. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kadler K. E., Torre-Blanco A., Adachi E., Vogel B. E., Hojima Y., Prockop D. J. A type I collagen with substitution of a cysteine for glycine-748 in the alpha 1(I) chain copolymerizes with normal type I collagen and can generate fractallike structures. Biochemistry. 1991 May 21;30(20):5081–5088. doi: 10.1021/bi00234a035. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Sabol C., Tromp G., Sippola-Thiele M., Prockop D. J. A 19-base pair deletion in the pro-alpha 2(I) gene of type I procollagen that causes in-frame RNA splicing from exon 10 to exon 12 in a proband with atypical osteogenesis imperfecta and in his asymptomatic mother. J Biol Chem. 1988 Aug 15;263(23):11407–11413. [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Steinmann B., Superti-Furga A., Royce P. M. Imperfect collagenesis in osteogenesis imperfecta. The consequences of cysteine-glycine substitutions upon collagen structure and metabolism. Ann N Y Acad Sci. 1988;543:47–61. doi: 10.1111/j.1749-6632.1988.tb55315.x. [DOI] [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Steinmann B. Impaired secretion of type III procollagen in Ehlers-Danlos syndrome type IV fibroblasts: correction of the defect by incubation at reduced temperature and demonstration of subtle alterations in the triple-helical region of the molecule. Biochem Biophys Res Commun. 1988 Jan 15;150(1):140–147. doi: 10.1016/0006-291x(88)90497-4. [DOI] [PubMed] [Google Scholar]
- Thompson E. M., Young I. D., Hall C. M., Pembrey M. E. Recurrence risks and prognosis in severe sporadic osteogenesis imperfecta. J Med Genet. 1987 Jul;24(7):390–405. doi: 10.1136/jmg.24.7.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stacey A., Shikata H., Baldwin C. T., Jaenisch R., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 1(I) chain of human type I procollagen. Biochem J. 1988 Aug 1;253(3):919–922. doi: 10.1042/bj2530919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Westerhausen A., Kishi J., Prockop D. J. Mutations that substitute serine for glycine alpha 1-598 and glycine alpha 1-631 in type I procollagen. The effects on thermal unfolding of the triple helix are position-specific and demonstrate that the protein unfolds through a series of cooperative blocks. J Biol Chem. 1990 Aug 15;265(23):13995–14000. [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]