Abstract
Background
The use of prehospital tranexamic acid (TXA) in patients with trauma has attracted considerable attention. This systematic review and meta-analysis aimed to provide the best evidence for clinicians.
Methods
All related literature in PubMed, Embase, and the Cochrane Central Register of Controlled Trials (Central) databases were searched systematically from their establishment to July 1, 2023. The outcome measures included 24-hour and 28–30-day mortality and adverse events (multiple organ dysfunction syndrome, acute respiratory distress syndrome, thrombotic events, and infection events). The Revised Cochrane Risk of Bias Tool for Randomized Trials was used to evaluate the quality of the randomized controlled trials (RCTs). The Methodological Index for Nonrandomized Studies (MINORS) was used to evaluate the risk of bias in non-RCTs. The required information size was estimated using trial sequential analysis. The Grading of Recommendations, Assessment, Development, and Evaluation approach was used to evaluate the evidence quality.
Results
Eleven studies (comprising 11,259 patients) were included; two of these were RCTs. The overall risks of bias were low in the RCTs. ROBINS-I risk of bias was Moderate in 3 studies, serious in 5 studies, and critical in 1 study. A significant reduction in 24-hour mortality was observed (odds ratio [OR], 0.82; 95% confidence interval [CI], 0.71–0.94). A subgroup analysis that included only RCTs revealed that prehospital TXA was associated with reduced 28–30-day mortality (OR, 0.80; 95% CI, 0.66–0.97) and increased risks of thromboembolism (OR, 1.22; 95% CI, 1.03–1.44) and infection (OR, 1.13; 95% CI, 1.00–1.28) events. The blood products for transfusion decreased by 2.3 units on average (weighted mean difference [WMD], − 2.30; 95%CI, − 3.59 to − 1.01).
Conclusions
This updated systematic review showed that prehospital TXA reduced the 24-hour and 28–38-day mortality and blood transfusion but increased the risks of infection and thromboembolism in patients with trauma. Future RCTs with larger and more homogeneous samples will help verify our results.
Keywords: Tranexamic acid, Prehospital, Trauma, Meta-analysis, Trial sequential analysis
Introduction
Trauma is a leading cause of death and disability worldwide, accounting for 10% of all deaths [1]. Traumatic hemorrhage is the most common cause of early death in injured individuals [2, 3]. Approximately 25% of trauma victims have immediate coagulative malfunction and up to 40% die from hemorrhagic shock [4, 5]. Early treatment of coagulopathy consequently and hemorrhagic shock significantly decreases posttraumatic death [6]. Approximately 7% of patients with trauma have high fibrinolysis, which is a key component of trauma-induced coagulopathy and is associated with bleeding-related mortality, making it a potential therapeutic target [7–9]. Tranexamic acid (TXA) is an antifiber solvent that can improve clot stability by inhibiting plasminogen activation and fibrinolysis; thus, it may be an effective treatment [10].
Two major multicenter randomized controlled trials (RCTs) examined TXA in patients with trauma in the hospital [11, 12]. The results showed that TXA administered within 3 h of injury lowered the 28-day mortality in patients with suspected bleeding (CRASH-2 trial [12]) and mild and severe traumatic brain injury (CRASH-3 trial [11]). Despite ongoing concerns regarding the efficacy, dose, and indications of TXA, its low cost and risk make it commonly used in mature healthcare systems [6, 13]. However, developed countries still lack evidence regarding the beneficial effects of prehospital usage of TXA. The meta-analysis by Almuwallad et al. [14], including four trials with 2347 patients, suggested that prehospital TXA significantly reduced early (24-h) mortality, with no associated increase in the risk of venous thromboembolism. However, the authors reported no significant reduction in 28–30-day mortality.
Nevertheless, the enthusiasm of researchers for exploring the effects of prehospital TXA has not diminished. Many experiments on prehospital TXA have been published since the meta-analysis by Almuwallad et al., and the cumulative sample size has increased approximately five-fold. Therefore, this systematic review and meta-analysis were conducted to update the existing medical evidence.
Methods
We completed the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023418399) registration before the start of the study and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15] throughout the study. Two authors independently agreed on each assessment during the study, and a third author arbitrated any disagreements.
Eligibility criteria
Studies that met the inclusion criteria were included according to the Participant-Intervention-Comparator-Outcomes-Study (PICOS) principles (Participant [P]: patients suspected or diagnosed with traumatic bleeding [including internal and external bleeding] or traumatic brain injury; Intervention (I): prehospital TXA administration; Comparator (C): no prehospital TXA; Outcomes (O): at least one of the following should be reported: mortality, adverse events, consumption of blood products, or quantity of supplementary fluids; and Study (S): RCT or cohort study).
The exclusion criteria were: (1) secondary analysis of RCTs, 2) previously published cohort studies involving the same population, and 3) protocols and meeting abstracts.
Search strategy and selection
We conducted the search using a combination of subject words and free words and constructed search expressions using logical symbols, wildcards, and Boolean logic operators. “Tranexamic acid” and “prehospital” were the two subjects for which a thorough literature search was conducted to determine all keywords and search terms. The retrieval databases included PubMed, Embase, and the Cochrane Central Register of Controlled Trials (Central). The retrieval time was limited from database inception to July 1, 2023. We placed no restrictions on the publication year, language, or region.
References were managed using EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA). After removing duplicate records, the titles and abstracts were initially screened by two independent investigators, and full-text publications were evaluated for all potentially relevant articles.
Data collection
To synthesize the evidence, a standardized, prepiloted Microsoft Excel (Microsoft Corp., Redmond, WA, USA) form was used to tabulate and extract data from the included studies. Separately, two authors extracted the following data: study design, number of patients, first author, year of publication, baseline characteristics of patients, duration of follow-up, Injury Severity Score (ISS), systolic blood pressure (SBP) upon emergency room arrival, number of patients with SBP < 90 mmHg, duration of the prehospital phase, adverse events (multiple organ dysfunction syndrome [MODS], acute respiratory distress syndrome [ARDS], thrombotic events, infection events), mortality (at 24 h and 28–30 days), blood product consumption, and crystalloid fluid input.
Risk of bias assessment
We used the Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2.0) to evaluate bias from areas such as reporter (selective reporting), attrition (incomplete outcome data), detection (blinding of outcome assessment), performance (blinding of participants and personnel), selection (random sequence, allocation concealment), and others. Each domain was categorized as low, unclear, or high risk according to the risk classification system. For a cohort study, the ROBINS-I tool was used in accordance with Cochrane and GRADE guidelines. Each of the seven domains of the ROBINS-I tool were rated as being at low, moderate, serious or critical risk of bias (RoB), or no information. Additionally, if at least 10 trials were found, Egger’s test was used to examine publication bias.
Quantitative data synthesis
The data in these studies were presented in various ways. To calculate the standard deviation (SD) of the mean from the standard error, 95% confidence interval (CI), and P value, we followed the recommendations of the Cochrane Handbook [16]. When data were presented as median and interquartile range or median and range, the mean and SD were calculated using the method described by Wan et al. [17].
STATA Version 12 (STATA Corp., College Station, TX, USA) was used for all statistical analyses. The continuity of variables was expressed as the weighted mean difference (WMD) and 95% CI. Dichotomous data were reported as odds ratios (ORs) and 95% CI. The results are graphically represented using forest plots. P < 0.05 was regarded as statistically significant in each study. To assess the statistical heterogeneity of the combined studies, we also produced I2 statistics. In studies with significant heterogeneity (I2 > 50%), the sources of heterogeneity were further examined. The meta-analysis employed a random-effects model after removing overt clinical heterogeneity. If the methods utilized in several studies varied significantly, a random-effects model was also applied. Conversely, the fixed-effects model was used when I2 < 50%, indicating nonexistent or little heterogeneity. A sensitivity analysis was performed to estimate the stability of the results by individually removing each study from the analysis. Subgroup analysis was conducted according to the study design (RCT or cohort study).
Trial sequential analysis
As the results of a meta-analysis may be biased by the presence of systematic errors (bias) or random errors (play of chance) owing to sparse data and repeated significance testing [18], we performed trial sequential analysis (TSA) using TSA software (version 0.9.5.10; Copenhagen Trial Unit, Copenhagen, Denmark). The optimal information size was set to a two-sided alpha of 0.05, beta of 0.80, and relative risk reduction of 30% using a DerSimonian–Laird random-effects model. TSA allows for the evaluation of the reliability of the statistical results of meta-analyses. TSA can be used to determine whether the CI and P values in the meta-analysis are sufficient to show the expected effects [19]. The required information size (RIS) and trial sequence monitoring boundary (TSMB) were adjusted for the meta-analysis. TSMB determines whether the evidence in a meta-analysis is reliable and conclusive [20]. If the cumulative Z-curve enters the futility boundaries or crosses TSMB, the expected intervention effect shows conclusive evidence. Otherwise, the evidence is deemed absent. For dichotomous data, we calculated RIS according to the average incidence of all the included RCTs. For continuous data, we estimated RIS based on a D2 of 50% and the average difference and variance according to empirical assumptions, which were automatically generated by the software.
Quality of the evidence
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to evaluate evidence quality [21]. Five factors contributed to reduced grades: limitation of the study design (more than a quarter of the studies were considered to have a serious risk of bias), inconsistency (substantial heterogeneity, I2 > 50%), indirectness (dissimilar populations, interventions, outcomes, and time points), imprecision (pooled sample size < 300), and potential publication bias (funnel plot assessment and Egger’s test two-tailed P < 0.1, or study quantity < 10). The quality of evidence was categorized as high, moderate, low, or very low.
Results
Study selection and characteristics
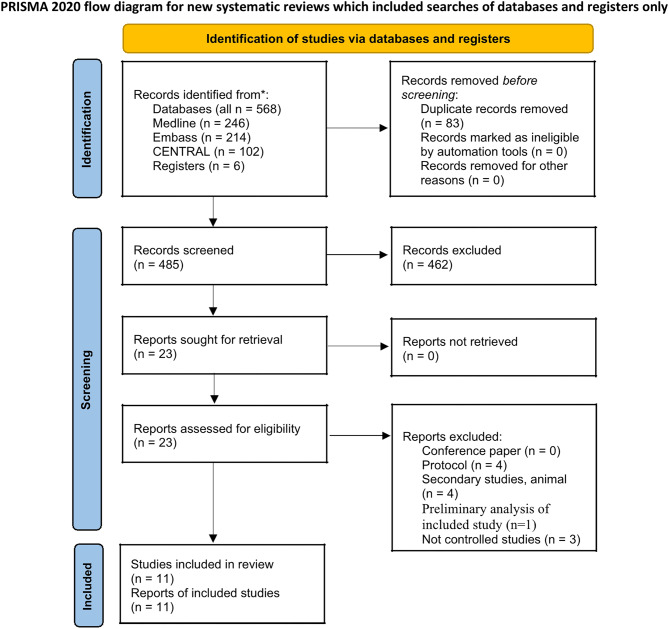
Overall, 568 articles were identified. After removing duplicates, 485 articles remained. The title- and abstract-eligibility checks resulted in the exclusion of 462 studies. After screening the entire texts of 23 potentially pertinent publications, 12 additional studies were excluded. Finally, 11 studies that met the inclusion criteria were included in the meta-analysis. The studies included two RCTs [22, 23] and nine cohort studies [24–32] (Fig. 1).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flowchart showing the search and selection process
The characteristics of the included studies are shown in Table 1. The 11 studies included in this meta-analysis involved 5304 cases and 5955 controls. All patients were diagnosed with or suspected of having a hemorrhagic injury or traumatic brain injury. Blunt injury was the dominant mechanism. Only one study [26] included more than half of the patients with penetrating injuries. The mean ISS ranged 16–41. Most studies only include patients who reach the emergency department or trauma center and intensive care unit. Two studies [30, 31] only excluded patients who died at the scene. The pre-hospital TXA dose of 9 included literatures was 1 g. In-hospital TXA was based on the decision of clinicians. The dosage of TXA was not clear in two studies [25, 32]. It is worth noting that Bossers et al.’ s research [32] focused on patients with severe traumatic brain injury.
Table 1.
Main characteristics of all included studies
| Source | Study Design | Intervention | Sample size | Age* | Gender (Male/female) | ISS* | SBP* (mmHg) | SBP≤90 mmHg (n) | Duration of prehospital phase* (min) | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Leenen 2021 | Prospective cohort study | Prehospital TXA | 207 | 40 | 152/55 | 29 | 120 | 45 | NA | 28 days |
| No prehospital TXA | 215 | 49 | 146/69 | 29 | 120 | 41 | NA | |||
| Gulickx 2023 | Prospective cohort study | Prehospital TXA | 124 | 36.1 | 97/27 | 28 | NA | 24 | 50.4 | 30 days |
| No prehospital TXA | 353 | 42.7 | 238/115 | 18 | NA | 31 | 42.0 | |||
| Neeki 2018 | Prospective cohort study | Prehospital TXA | 362 | 37.96 | 293/69 | 16.08 | 78.42 | NA | NA | 28 days |
| No prehospital TXA | 362 | 37.64 | 293/69 | 17.15 | 83.66 | NA | NA | |||
| Imach 2021 | Prospective cohort study | Prehospital TXA | 2275 | 47.6 | 1679/596 | 32.4 | 113 | 539 | 77 | 30 days |
| No prehospital TXA | 2275 | 47.5 | 1688/587 | 32 | 111 | 571 | 77 | |||
| Menyar 2019 | Retrospective cohort study | Prehospital TXA | 102 | 31.4 | 98/4 | 22 | 107.3 | 55 | 74 | 30 days |
| No prehospital TXA | 102 | 31.5 | 91/11 | 22 | 102.4 | 74 | 62 | |||
| Wafaisade 2016 | Prospective cohort study | Prehospital TXA | 258 | 43 | 187/71 | 24 | 114 | 51 | 77.2 | 30 days |
| No prehospital TXA | 258 | 41 | 187/71 | 24 | 117 | 50 | 74.2 | |||
| Boudreau 2018 | Retrospective cohort study | Prehospital TXA | 62 | 44.5 | 45/17 | 22 | 126 | NA | NA | NA |
| No prehospital TXA | 54 | 33 | 45/9 | 26 | 110 | NA | NA | |||
| Wessem 2021 | Prospective cohort study | Prehospital TXA | 120 | 42 | 80/40 | 34 | 120 | 23 | 61 | 28 days |
| No prehospital TXA | 114 | 53 | 77/37 | 29 | 127 | 15 | 61 | |||
| Guyette 2020 | Randomized controlled trial | Prehospital TXA | 447 | 41 | 327/120 | 12 | 123 | NA | 39 | 30 days |
| Placebo | 456 | 42 | 341/115 | 12 | 126 | NA | 39 | |||
| Russell 2023 | Randomized controlled trial | Prehospital TXA | 657 | 44.1 | 459/198 | 29 | NA | 464 | NA | 6 months |
| Placebo | 643 | 44.2 | 459/184 | 29 | NA | 445 | NA | |||
| Bossers 2020 | Prospective cohort study | Prehospital TXA | 693 | 47 | 486/207 | 27 | 142 | NA | NA | 12 months |
| No prehospital TXA | 1134 | 45 | 797/337 | 26 | 143 | NA | NA |
TXA, tranexamic acid; ISS, Injury Severity Score; SBP, systolic blood pressure
* Data are expressed as means
Risk of bias
The two RCTs showed low risks of bias [22, 23] in different domains (Fig. 2). The non-RCTs (n = 9) were evaluated using ROBINS-I. Table 2 shows the quality checklist for the risk of bias for each cohort study. A low RoB was not found in any of this articles; three (33.3%) had moderate RoB, five (55.6%) serious RoB, and one (11.1%) critical RoB. Confounding bias, participant selection bias, and bias due to intervention classification are the most important ROBINS-I domains that contribute to moderate, serious, or critical RoB.
Fig. 2.
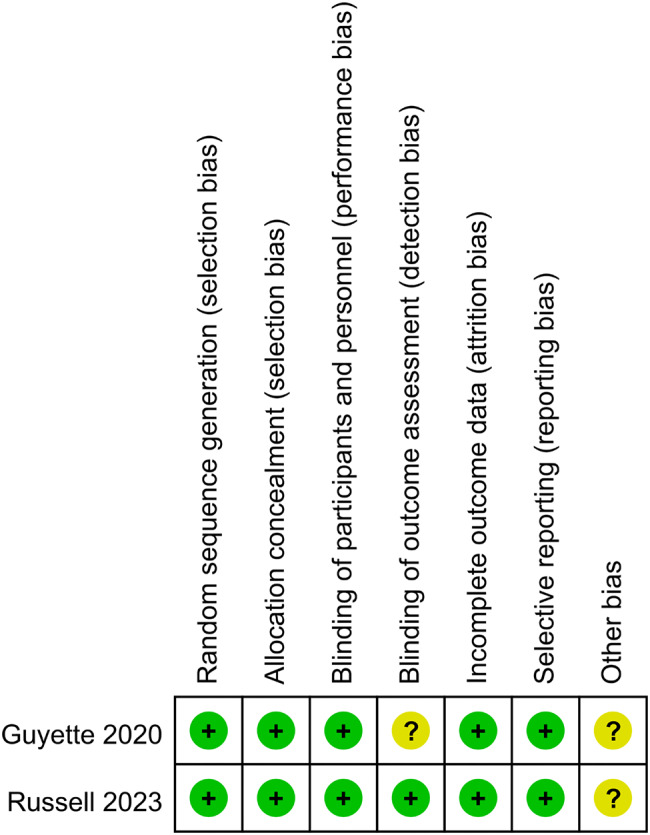
Summary of risk of bias assessment of the randomized controlled trials
Table 2.
ROBINS-I assessment of study bias for included studies
| Bias domain | Boudreau et al. | Wessem et al. | Leenen et al. | Gulickx et al. | Neeki et al. | Imach et al. | Menyar et al. | Wafaisade et al. | Bossers et al. |
|---|---|---|---|---|---|---|---|---|---|
| Due to confounding | Critical | Serious | Serious | Serious | Moderate | Serious | Moderate | Moderate | Moderate |
| Selection of participants | Serious | Low | Low | Low | Low | Moderate | Low | Low | Low |
| Classification of interventions | Moderate | Low | Moderate | Serious | Low | Low | Low | Low | Serious |
| Deviation from intended | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Missing data | Low | Low | Low | Low | Low | Moderate | Low | Low | Low |
| Measurement of outcomes | Moderate | Low | Low | Low | Low | Low | Low | Low | Low |
| Selection of the reported result | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Overall risk of bias | Critical | Serious | Serious | Serious | Moderate | Serious | Moderate | Moderate | Serious |
Meta-analysis
Mortality
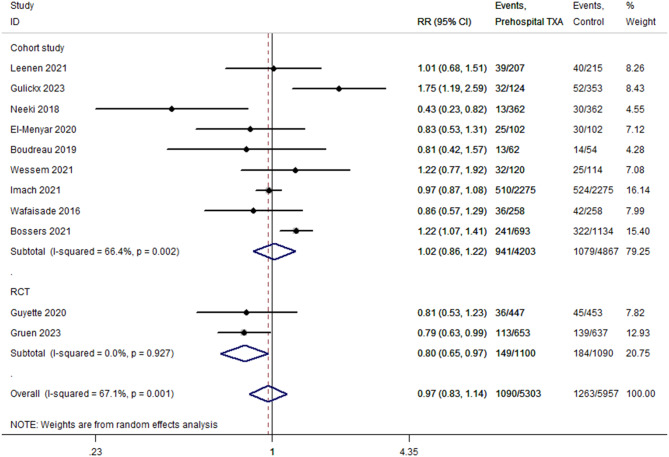
All 11 included studies reported 28–30-day mortality. The TXA and non-TXA groups included 5,303 and 5,957 patients, respectively. Comprehensive analysis showed that prehospital TXA had no advantage in reducing 28–30-day mortality in patients with trauma (P = 0.710; OR, 0.97; 95% CI, 0.83–1.14; I2 = 67.1%; Fig. 3). The sensitivity analysis showed that when one study was excluded at a time, the combined results did not change (OR, 1.01; 95% CI, 0.80–1.22). TSA showed that the cumulative Z-curve did not cross TSMB but crossed the futility boundary (Table 3). TSA of the pooled meta-analysis showed no evidence of the anticipated intervention effect. Publication bias was not evident in Egger’s test (P = 0.878). Subgroup analyses were conducted according to the study design. Meta-analysis of the RCTs showed that prehospital TXA reduced 28–30-day mortality (P = 0.024; OR, 0.80; 95% CI, 0.66–0.97; I2 = 0%; Fig. 3). TSA showed that the cumulative Z-curve did not cross TSMB and did not reach RIS (the cumulative information size was 2190). We observed no significant difference in the pooled analysis of cohort studies (P = 0.815; OR, 1.02; 95% CI, 0.86–1.22; I2 = 66.4%; Fig. 3).
Fig. 3.
Pooled and subgroup analyses of 28–30-day mortality
Table 3.
Meta- and trial sequential analyses of the outcomes
| Meta-analysis | TSA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | OR | 95%CI | P | I2% | RRR% | IIA% | ICA% | D2% | CIS | RIS | Cross TSMB | Cross FB | Evidence |
| Mortality (at 28–30 days) | 0.97 | 0.83, 1.14 | 0.71 | 67.4 | 19.25 | 13.63 | 16.88 | 78 | 11,260 | 17,841 | NO | YES | AE |
| Mortality (at 24 h) | 0.82 | 0.71, 0.94 | 0.004 | 46.3 | 25.94 | 7.25 | 9.79 | 71 | 8464 | 13,282 | YES | NO | FE |
| Thromboembolism events | 1.22 | 1.03, 1.44 | 0.019 | 30.9 | -27.34 | 16.3 | 12.8 | 0 | 8964 | 9823 | YES | NO | FE |
| Infection events | 1.13 | 1.00, 1.28 | 0.046 | 0 | -17.86 | 28.44 | 24.13 | 0 | 7706 | 3275 | NO | NO | AE |
AE, absent evidence; CI, confidence intervals; D2, diversity; FB, futility boundary; FE, firm evidence; ICA, incidence in control arm; CIS, cumulative information size, RIS, required information size; IIA, incidence in intervention arm; OR, odds ratio; RRR, relative risk reduction; TSMB, trial sequential monitoring boundary; TSA, trial sequential analysis
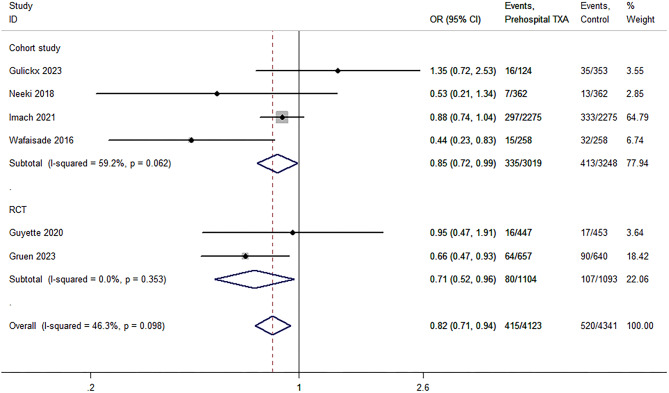
Six studies [22, 23, 25, 26, 30, 31] calculated the mortality in patients with trauma 24 h after admission. The TXA and non-TXA groups included 4,123 and 4,341 patients, respectively. The results of the meta-analysis showed that prehospital TXA was associated with reduced 24-hour mortality (P = 0.004; OR, 0.82; 95% CI, 0.71–0.94; I2 = 46.3%; Fig. 4). The sensitivity analysis showed that the results were unstable (OR, 0.84; 95% CI, 0.51–1.02). TSA revealed strong evidence (Table 3); however, subgroup analysis suggested similar results between RCTs (P = 0.027; OR, 0.71; 95% CI, 0.52–0.96; I2 = 0%; Fig. 4) and cohort studies (P = 0.035; OR, 0.85; 95% CI, 0.72–0.99; I2 = 59.2%; Fig. 4).
Fig. 4.
Pooled and subgroup analyses of 24-hour mortality
Thromboembolic events
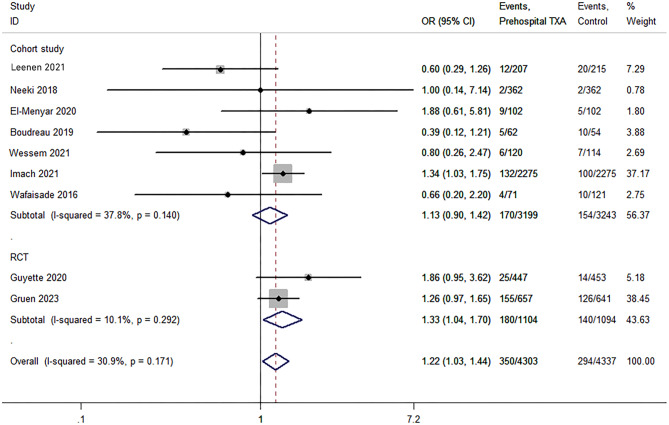
Thromboembolic events include deep vein thrombosis, pulmonary embolism, myocardial infarction, and ischemic stroke. Data on thromboembolism events were available from nine studies [22–24, 26–31]. The total numbers of patients in the TXA and non-TXA groups were 4,303 and 4,337, respectively. The TXA group showed a 19% higher thromboembolism rate compared to the non-TXA group (P = 0.019; OR, 1.22; 95% CI, 1.03–1.44; I2 = 30.9%; Fig. 5). The sensitivity analysis showed that the results were stable (odds ratio [OR], 1.27; 95% CI, 1.00–1.49). TSA revealed strong evidence (Table 3). Subgroup analysis showed that only the aggregate analysis of RCTs was statistically significant (P = 0.022; OR, 1.33; 95% CI, 1.04–1.70; I2 = 10.1%; Fig. 5) and a RIS of 3978 (cumulative information size, 2198).
Fig. 5.
Pooled and subgroup analyses of thromboembolic events
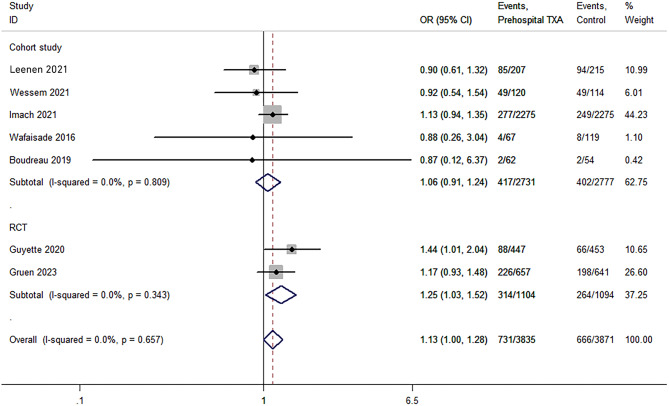
Infection events
Seven studies reported on infection events [22–24, 28–31]. The meta-analysis included 3,835 and 3,871 patients with and without TXA treatment, respectively. The infection rate was 9.8% higher in the TXA group (P = 0.046; OR, 1.13; 95% CI, 1.00–1.28; I2 = 0%; Fig. 6). Sensitivity analysis showed that the results were unstable (OR, 1.14; 95% CI, 0.97–1.31). TSA revealed an absence of evidence (Table 3). Subgroup analysis showed that the summary analysis of RCTs (P = 0.024; OR, 1.25; 95% CI, 1.03–1.52; I2 = 0%; Fig. 6) was statistically significant, while that in the cohort studies was not (P = 0.453; OR, 1.06; 95% CI, 0.91–1.24; I2 = 0%; Fig. 6).
Fig. 6.
Pooled and subgroup analyses of infection events
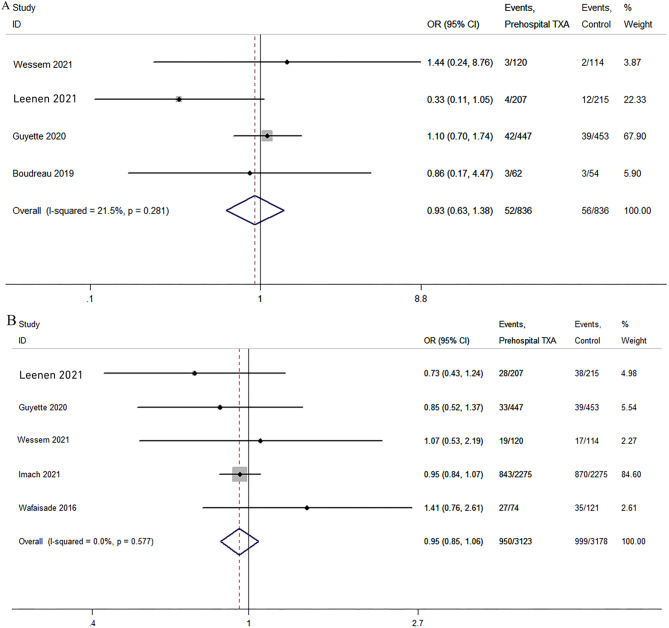
ARDS and MODS
Four studies reported data on ARDS [23, 24, 28, 29]. The results of the meta-analysis showed no significant difference between the TXA and non-TXA groups (P = 0.804; OR, 0.95; 95% CI, 0.64–1.42; I2 = 21.2%; Fig. 7). The pooled analysis of five studies [23, 24, 29–31] showed no significant difference in the incidence of MODS between the two groups (P = 0.351; OR, 0.95; 95% CI, 0.85–1.06; I2 = 0%; Fig. 7).
Fig. 7.
Pooled analyses of acute respiratory distress syndrome (ARDS) (A) and multiple organ dysfunction syndrome (MODS) (B)
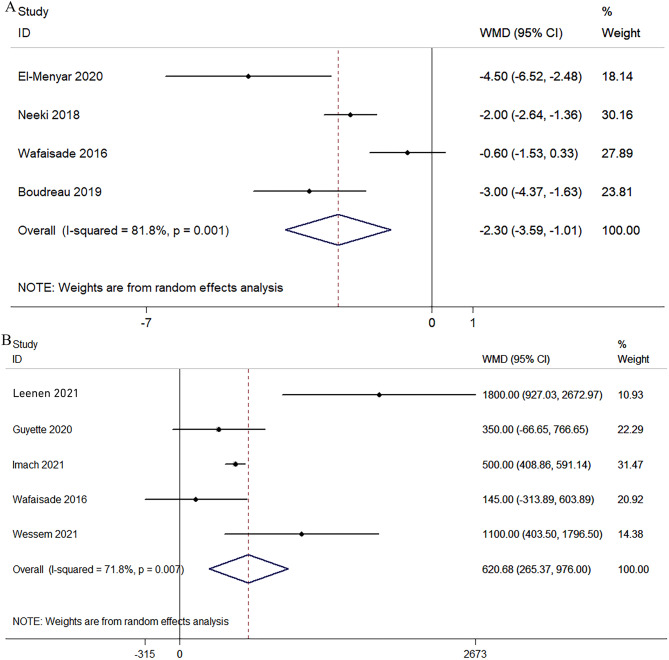
Total blood products transfused and crystalloid infusion volume
Four studies [26–28, 31] reported on the total blood products transfused. Compared with the group without TXA, the blood products for transfusion by TXA decreased by an average of 2.3 units (P = 0.000; WMD, − 2.30; 95% CI, − 3.59 to − 1.01; I2 = 81.8%; Fig. 8). Data from five studies [23, 24, 29–31] showed that the crystalloid infusion volume in the TXA group increased by an average of 620 mL (P = 0.001; WMD, 620.68; 95% CI, 265.37–976.00; I2 = 71.8%; Fig. 8).
Fig. 8.
Pooled and analyses of total blood products transfused (A) and crystalloid infusion volume (B)
Quality of the evidence
The grade evaluations of the quality of the evidence are shown in Table 4. Overall, the quality of the evidence was very low or low, mostly due to study limitations, inconsistencies, and potential publication bias.
Table 4.
Summary of the findings and assessment of the quality of the evidence
| Summary of findings | Quality of evidence assessment (GRADE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Trials | Participants | I2, % | WMD/OR (95% CI) | Study limitation | Inconsistency | Imprecision | Publication bias | Quality |
| Mortality (at 28–30 days) | 11 | 11,260 | 67.4 | 0.97 (0.83, 1.14) | -1 | -1 | None | None | Low |
| Mortality (at 24 h) | 6 | 8464 | 46.3 | 0.82 (0.71, 0.94) | -1 | None | None | -1 | Low |
| Thromboembolism events | 9 | 8964 | 30.9 | 1.22 (1.03, 1.44) | -1 | None | None | -1 | Low |
| Infection events | 7 | 7706 | 0 | 1.13 (1.01, 1.28) | -1 | None | None | -1 | Low |
| MODS | 5 | 6301 | 0 | 0.95 (0.85, 1.06) | -1 | None | None | -1 | Low |
| ARDS | 4 | 1672 | 21.2 | 0.93 (0.63, 1.38) | -1 | None | None | -1 | Low |
| Total blood products transfused (in units) | 4 | 1560 | 81.8 | -2.30 (-3.59, -1.01) | -1 | -1 | None | -1 | Very low |
| Crystalloid infusion volume (mL) | 5 | 6434 | 71.8 | 620.68 (265.37, 976.00) | -1 | -1 | None | -1 | Very low |
MODS, multiple organ dysfunction syndrome; ARDS, acute respiratory distress syndrome; WMD, weighted mean difference; OR, odds ratio
Discussion
This meta-analysis of the curative effect of prehospital TXA in patients with trauma showed that compared to no TXA, prehospital TXA reduced 24-hour mortality. However, this effect did not persist for 28–30-day mortality. These results were consistent with those reported by Almuwallad et al. [14], although our study had a larger sample size. Notably, prehospital TXA reduced the 28–30-day mortality in the RCT subgroup. Compared to those of cohort studies, the results of the RCTs were more accurate and reliable. Selective bias may have contributed to the insignificant results of the cohort studies. While TSA showed that more RCTs are needed to verify the effects of prehospital TXA on 28–30-day mortality, the present study is the first to report the effect of prehospital TXA in this regard.
Previous studies showed the benefits of TXA in treating polytrauma [33–35]. These benefits include bleeding control, hemostasis, and resuscitation, which reduce mortality in patients with trauma. TSA in the current meta-analysis identified the early (within 24 h) benefits of TXA. Prehospital TXA reduced blood product consumption. Conversely, the infusion of crystalloids increased. However, the largest clinical trial (CRASH-2) [36] so far has not found any substantial reduction in the amount of blood transfusion or transfusion received by trauma patients treated by TXA. There are several possible reasons for this difference. First, the main measure of traumatic bleeding was surgical hemostasis, and the difference of procedures was conceivable. Second, Prehospital TXA enables patients to receive antifibrinolytic treatment earlier, resulting in less bleeding. Finally, the meta-analysis data showed statistical heterogeneity after conversion. In their meta-analysis of 129 trials involving more than 10,000 patients, Ker et al. [37] showed that TXA use was associated with a 38% reduction in the number of allogeneic blood transfusions. In summary, a reduction in blood transfusion by prehospital TXA was initially observed, which warrants further exploration.
TXA should be used with caution as antifibrinolytic therapy may be associated with increased risks of seizures [38, 39], myocardial infarction [40] and other thrombotic complications [41, 42]. However, the relationship between TXA and vascular occlusion remains controversial. Robert et al. [43] conducted an RCT to explore the effect of high-dose TXA and the influence of thromboembolic events in patients with acute gastrointestinal bleeding. The authors found increased venous thromboembolism events (deep venous thrombosis or pulmonary embolism) in the TXA group than in the placebo group (risk ratio [RR], 1.85; 95% CI, 1.15–2.98). Xie et al. [44] suggested that TXA was associated with the total incidence of vascular occlusion after total knee arthroplasty (P < 0.001). However, a meta-analysis [45] of 216 studies involving 125,550 patients by Taeuber et al. reported no association between TXA levels and the overall risk of thromboembolic events. Previous meta-analyses [14] also reported similar conclusions. However, our study found that prehospital TXA increased the risk of vascular occlusion (OR 1.22; 95% CI, 1.03–1.44). Further investigation revealed that this discrepancy solely affected RCTs. TSA suggested that this result requires further verification. In view of the fact that the current research has not found the benefit of pre-hospital TXA on the overall mortality of trauma patients, and it will lead to an increase in the risk of thromboembolic events, TXA provided in hospital according to coagulation parameters may be a better choice. Interestingly, prehospital TXA administration increased the incidence of nosocomial infections. This may be because the suppression of plasminogen activation by TXA exacerbates staphylococcal infectious arthritis and sepsis [46].
Previous researches suggested that TXA should be administered as soon as possible after arriving at a trauma treatment location [11, 12, 36]. Most deaths due to bleeding in patients with trauma occur within hours of arrival at the trauma center, emphasizing the need for early prehospital assistance to provide helpful treatment [47–49]. Consequently, guidelines for early treatment have recently been developed, including the use of prehospital TXA after trauma [28, 34, 50]. The results of this study suggest that pre-hospital TXA may be beneficial to the early survival of trauma patients. However, stratified studies of the dosage and timing of drug administration are scarce. Further studies should not only focus on improving the survival of trauma patients but also on the relationship between thromboembolic events, infectious complications, and early mortality.
The present study had some limitations. First, only two of the 11 included studies were RCTs, and the lack of randomization created a risk of confusion and bias. We attempted to explain these hazards using a subgroup analysis. The two RCTs demonstrated differing significance and homogeneity from cohort studies. Second, the different TXA doses and administration times may have resulted in heterogeneity. Third, the ISS varied considerably between studies and within some studies, which may understate the efficacy of TXA in investigations with larger ISS values. Fourth, some studies lacked sufficient data to measure the dispersion for effect measurements (SD or SE). Data translated using this formula may have resulted in statistical heterogeneity. Finally, the differences in trauma investigation, including transport times, severity of injury, blunt/penetrating trauma, level 1/level 2 trauma center, civil/military study setting etc., are also factors are also factors that cause bias. Nevertheless, we evaluated the quality of evidence using a validated tool and considered the level of certainty of the evidence for each result.
Conclusions
The results of this meta-analysis of published studies showed that prehospital TXA significantly reduced the 24-hour mortality of patients with trauma. This effect was also observed for 28–30-day mortality in the RCT subgroup. Additionally, prehospital TXA was associated with increased risks of thromboembolism and infection. These findings suggest the need to reconsider the risk-benefit ratio of TXA in the prehospital setting.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- GRADE
Grading of recommendations, assessment, development, and evaluation
- ISS
Injury Severity Score
- MINORS
Methodological Index for nonrandomized studies
- MODS
Multiple organ dysfunction syndrome
- OR
Odds ratio
- PICOS
Participant-Intervention-Comparator-Outcomes-Study
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
randomized controlled trial
- RR
Risk ratio
- RIS
Required information size
- RoB 2.0
Revised Cochrane Risk of Bias tool for randomized trials
- SBP
Systolic blood pressure
- SD
Standard deviation
- TSA
Trial sequential analysis
- TSMB
Trial sequence monitoring boundary
- TXA
Tranexamic acid
- WMD
Weighted mean difference
Author contributions
Conceived and designed the study: H-Y C, W-T XPerformed the study: H-Y C, L-G W, C-C F, W Y, W-T XAnalyzed the data: H-Y C, L-G W, C-C FWrote the manuscript: H-Y C, W Y, W-T X.
Funding
No funding was disclosed by the author(s).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable. The study was waived by the Ethics Committee of the People’s Hospital of Nanchuan District.
Consent for publication
Not applicable.
Conflict of interest
The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gosselin RA, Spiegel DA, Coughlin R, Zirkle LG. Injuries: the neglected burden in developing countries. Bull World Health Organ. 2009;87(4):246–a246. 10.2471/blt.08.052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perel P, Prieto-Merino D, Shakur H, Clayton T, Lecky F, Bouamra O, Russell R, Faulkner M, Steyerberg EW, Roberts I. Predicting early death in patients with traumatic bleeding: development and validation of prognostic model. BMJ (Clinical Res ed). 2012;345:e5166. 10.1136/bmj.e5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. The Journal of trauma 2008; 64(6): 1459-63; discussion 1463-5. 10.1097/TA.0b013e318174e8bc [DOI] [PubMed]
- 4.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 5.Paudyal P, Smith J, Robinson M, South A, Higginson I, Reuben A, Shaffee J, Black S, Logan S. Tranexamic acid in major trauma: implementation and evaluation across South West England. Eur J Emerg Medicine: Official J Eur Soc Emerg Med. 2017;24(1):44–8. 10.1097/mej.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 6.Brown JB, Neal MD, Guyette FX, Peitzman AB, Billiar TR, Zuckerbraun BS, Sperry JL. Design of the study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) Trial: addressing the knowledge gaps. Prehospital Emerg care. 2015;19(1):79–86. 10.3109/10903127.2014.936635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. The Journal of trauma 2008; 64(5): 1211-7; discussion 1217. 10.1097/TA.0b013e318169cd3c [DOI] [PubMed]
- 8.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. The journal of trauma and acute care surgery 2012; 73(2): 365 – 70; discussion 370. 10.1097/TA.0b013e31825c1234 [DOI] [PubMed]
- 9.Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, Simmen HP, Spahn DR, Baulig W. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg. 2011;113(5):1003–12. 10.1213/ANE.0b013e31822e183f. [DOI] [PubMed] [Google Scholar]
- 10.Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015;5Cd004896. 10.1002/14651858.CD004896.pub4.
- 11.CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet (London England). 2019;394(10210):1713–23. 10.1016/s0140-6736(19)32233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet (London England). 2011;377(9771):1096–e10111011. 10.1016/s0140-6736(11)60278-x. [DOI] [PubMed] [Google Scholar]
- 13.Howard JT, Stockinger ZT, Cap AP, Bailey JA, Gross KR. Military use of tranexamic acid in combat trauma: does it matter? J Trauma Acute care Surg. 2017;83(4):579–88. 10.1097/ta.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 14.Almuwallad A, Cole E, Ross J, Perkins Z, Davenport R. The impact of prehospital TXA on mortality among bleeding trauma patients: a systematic review and meta-analysis. J Trauma Acute care Surg. 2021;90(5):901–7. 10.1097/ta.0000000000003120. [DOI] [PubMed] [Google Scholar]
- 15.Arya S, Kaji AH, Boermeester MA. PRISMA Reporting guidelines for Meta-analyses and systematic reviews. JAMA Surg. 2021;156(8):789–90. 10.1001/jamasurg.2021.0546. [DOI] [PubMed] [Google Scholar]
- 16.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):Ed000142. 10.1002/14651858.Ed000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive–trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38(1):287–98. 10.1093/ije/dyn188. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen JC, Gluud C, Winkel P, Lange T, Wetterslev J. The thresholds for statistical and clinical significance - a five-step procedure for evaluation of intervention effects in randomised clinical trials. BMC Med Res Methodol. 2014;14:34. 10.1186/1471-2288-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takwoingi Y, Hopewell S, Tovey D, Sutton AJ. A multicomponent decision tool for prioritising the updating of systematic reviews. BMJ (Clinical Res ed). 2013;347:f7191. 10.1136/bmj.f7191. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Res ed). 2008;336(7650):924–6. 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruen RL, Mitra B, McArthur BSA, Burns CJ, Gantner B, Maegele DC, Cameron M, Dicker PA, Forbes B, Hurford AB, Martin S, Mazur CA, Medcalf SM, Murray RL, Myles LJ, Ng PS, Pitt SJ, Rashford V, Reade S, Swain MC, Trapani AH, Young T. Prehospital Tranexamic acid for severe trauma. N Engl J Med. 2023. 10.1056/NEJMoa2215457. [DOI] [PubMed] [Google Scholar]
- 23.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, Nirula R, Vercruysse GA, O’Keeffe T, Joseph B, Alarcon LH, Callaway CW, Zuckerbraun BS, Neal MD, Forsythe RM, Rosengart MR, Billiar TR, Yealy DM, Peitzman AB, Sperry JL. Tranexamic acid during Prehospital Transport in patients at risk for Hemorrhage after Injury: a Double-blind, Placebo-Controlled, Randomized Clinical Trial. JAMA Surg. 2020;156(1):11–20. 10.1001/jamasurg.2020.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Wessem KJP, Leenen LPH. Does liberal Prehospital and In-Hospital tranexamic acid influence outcome in severely injured patients? A prospective cohort study. World J Surg. 2021;45(8):2398–407. 10.1007/s00268-021-06143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulickx M, Lokerman RD, Waalwijk JF, Dercksen B, van Wessem KJP, Tuinema RM, Leenen LPH, van Heijl M. Pre-hospital tranexamic acid administration in patients with a severe hemorrhage: an evaluation after the implementation of tranexamic acid administration in the Dutch pre-hospital protocol. Eur J Trauma Emerg Surgery: Official Publication Eur Trauma Soc. 2023. 10.1007/s00068-023-02262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neeki MM, Dong F, Toy J, Vaezazizi R, Powell J, Wong D, Mousselli M, Rabiei M, Jabourian A, Niknafs N, Burgett-Moreno M, Vara R, Kissel S, Luo-Owen X, O’Bosky KR, Ludi D, Sporer K, Pennington T, Lee T, Borger R, Kwong E. Tranexamic acid in Civilian Trauma Care in the California Prehospital Antifibrinolytic Therapy Study. Western J Emerg Med. 2018;19(6):977–86. 10.5811/westjem.2018.8.39336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Menyar A, Sathian B, Wahlen BM, Abdelrahman H, Peralta R, Al-Thani H, Rizoli S. Prehospital administration of tranexamic acid in trauma patients: a 1:1 matched comparative study from a level 1 trauma center. Am J Emerg Med. 2020;38(2):266–71. 10.1016/j.ajem.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 28.Boudreau RM, Deshpande KK, Day GM, Hinckley WR, Harger N, Pritts TA, Makley AT, Goodman MD. Prehospital Tranexamic Acid Administration during Aeromedical Transport after Injury. J Surg Res. 2019;233:132–8. 10.1016/j.jss.2018.07.074. [DOI] [PubMed] [Google Scholar]
- 29.van Wessem KJP, Jochems D, Leenen LP. H. The effect of prehospital tranexamic acid on outcome in polytrauma patients with associated severe brain injury. Eur J Trauma Emerg Surgery: Official Publication Eur Trauma Soc. 2022;48(3):1589–99. 10.1007/s00068-021-01827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imach S, Wafaisade A, Lefering R, Böhmer A, Schieren M, Suárez V, Fröhlich M. The impact of prehospital tranexamic acid on mortality and transfusion requirements: match-pair analysis from the nationwide German TraumaRegister DGU®. Crit Care (London England). 2021;25(1):277. 10.1186/s13054-021-03701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wafaisade A, Lefering R, Bouillon B, Böhmer AB, Gäßler M, Ruppert M. Prehospital administration of tranexamic acid in trauma patients. Crit Care (London England). 2016;20(1):143. 10.1186/s13054-016-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossers SM, Loer SA, Bloemers FW, Den Hartog D, Van Lieshout EMM, Hoogerwerf N, van der Naalt J, Absalom AR, Peerdeman SM, Schwarte LA, Boer C. Schober P. Association between Prehospital Tranexamic Acid Administration and outcomes of severe traumatic brain Injury. JAMA Neurol. 2021;78(3):338–45. 10.1001/jamaneurol.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishida T, Kinoshita T, Yamakawa K. Tranexamic acid and trauma-induced coagulopathy. J Intensive care. 2017;5:5. 10.1186/s40560-016-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napolitano LM. Prehospital tranexamic acid: what is the current evidence? Trauma Surg Acute care open. 2017;2(1):e000056. 10.1136/tsaco-2016-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Benov A, Darlington DN, Keesee JD, Liu B, Cap AP. Effect of tranexamic acid administration on acute traumatic coagulopathy in rats with polytrauma and hemorrhage. PLoS ONE. 2019;14(10):e0223406. 10.1371/journal.pone.0223406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2) a randomised, placebo-controlled trial. West Indian Med J. 2010;59(6):612–24. [PubMed] [Google Scholar]
- 37.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ (Clinical Res ed). 2012;344:e3054. 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70–3. 10.1016/j.seizure.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Lier H, Maegele M, Shander A. Tranexamic acid for Acute Hemorrhage: a narrative review of Landmark studies and a critical reappraisal of its Use over the last decade. Anesth Analg. 2019;129(6):1574–84. 10.1213/ane.0000000000004389. [DOI] [PubMed] [Google Scholar]
- 40.Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, Christensen H, Ciccone A, Collins R, Czlonkowska A, Dineen RA, Egea-Guerrero DL, England JJ, Krishnan TJ, Laska K, Law AC, Ozturk ZK, Pocock S, Roberts SJ, Robinson I, Roffe TG, Seiffge C, Scutt D, Thanabalan P, Werring J, Whynes D. Bath P. M. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet (London England). 2018;391(10135):2107–15. 10.1016/s0140-6736(18)31033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg. 2015;261(2):390–4. 10.1097/sla.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 42.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Archives of surgery (Chicago, Ill.: 1960) 2012; 147(2): 113-9. 10.1001/archsurg.2011.287 [DOI] [PubMed]
- 43.HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet (London England). 2020;395(10241):1927–36. 10.1016/s0140-6736(20)30848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie J, Ma J, Kang P, Zhou Z, Shen B, Yang J, Pei F. Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases. Thromb Res. 2015;136(2):234–8. 10.1016/j.thromres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Taeuber I, Weibel S, Herrmann E, Neef V, Schlesinger T, Kranke P, Messroghli L, Zacharowski K, Choorapoikayil S, Meybohm P. Association of Intravenous Tranexamic Acid with thromboembolic events and mortality: a systematic review, Meta-analysis, and Meta-regression. JAMA Surg. 2021;156(6):e210884. 10.1001/jamasurg.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kłak M, Anäkkälä N, Wang W, Lange S, Jonsson IM, Tarkowski A, Jin T. Tranexamic acid, an inhibitor of plasminogen activation, aggravates staphylococcal septic arthritis and sepsis. Scand J Infect Dis. 2010;42(5):351–8. 10.3109/00365540903510690. [DOI] [PubMed] [Google Scholar]
- 47.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O’Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC. McKinley B. A. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–9. 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 49.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC. Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock (Augusta Ga). 2017;47(5):567–73. 10.1097/shk.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huebner BR, Dorlac WC, Cribari C. Tranexamic Acid Use in Prehospital uncontrolled hemorrhage. Wilderness Environ Med. 2017;28(2s):S50–60. 10.1016/j.wem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.