Abstract
Background
Dementia preventive interventions targeting multiple modifiable risk factors are a promising approach. However, the impact of modifiable risk factors in the presence of beta-amyloid or phosphorylated-tau (p-tau) pathology is unclear.
Methods
The objective of the study was to examine the role of modifiable risk factors (vascular factors, depression, and smoking) in the progression to mild cognitive impairment (MCI) or dementia among 434 cognitively unimpaired (CU) and 611 individuals with MCI from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database. Vascular risk factors were summarized with the Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) score, dichotomized into higher versus lower risk. Depression and smoking (yes/no) were categorised according to medical history or current symptoms. Analyses were stratified by beta-amyloid negative (A-) and positive (A +), p-tau negative (T-) and positive (T +), or beta-amyloid and p-tau negative (A-T-) and positive (A + T +) biomarker status. Cox proportional hazard models were adjusted for age, sex, education, baseline MMSE score, baseline hippocampal volume and ApoE4 carrier status.
Results
Higher CAIDE score was associated with increased risk of progression to all-cause dementia in most MCI subgroups: adjusted hazard ratios (aHR) [95% CI] were 3.1 [1.43; 6.53] in the A- subgroup, 1.7 [1.20–2.27] in T + , 2.6 [1.06–6.59] in A-T-, and 1.6 [1.15–2.22] in the A + T + subgroup. Smoking (yes/no) was associated with increased dementia aHR in the A + MCI subgroup: 1.6 [1.07–2.34]. Depression increased dementia aHR in the T + MCI subgroup: 1.5 [1.06–2.02]. No significant associations were found in the CU biomarker subgroups.
Conclusion
Addressing modifiable risk factors carries an important potential for reducing the risk of dementia even after the onset of Alzheimer's pathology. Knowledge of biomarker status can further optimize prevention strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01602-9.
Keywords: Modifiable risk factors, CAIDE, Depression, Smoking, Amyloid, Tau, MCI
Background
Alzheimer’s disease (AD) and other forms of dementia are major causes of years lived with disability and represent a substantial long-term economic challenge for society. As the population ages, the consequences of dementia are anticipated to become even more severe [1]. Although there have been recent advances in anti-amyloid agents [2], current pharmacological therapeutic options have limited benefits. Addressing modifiable risk factors e.g. via lifestyle-based intervention programs in early risk and/or disease stages has been recommended for dementia risk reduction [3]. Major risk factors including e.g. smoking, depression, high blood pressure, and obesity, were estimated to account for about 40% of dementia cases [4, 5]. These risk factors have been linked to both AD and cerebrovascular damage [6–16].
To estimate an individual’s risk of developing dementia based on vascular factors, risk scores such as CAIDE (Cardiovascular Risk Factors, Aging, and Incidence of Dementia) have been developed [17]. The CAIDE score is based on age, education, sex, blood pressure, body mass index, total cholesterol, and physical activity. It provides a comprehensive and integrated assessment of an individual’s risk profile, allowing a more accurate estimate of the overall dementia risk, simplifying complex information into a single score, and making it more accessible to individuals and health professionals. From the above risk factors, obesity, high blood pressure, and hyperlipidemia are well known to increase the risk of vascular disease and, thus, the likelihood of cerebrovascular damage. They may also play a role in the development of AD [18, 19]. In the context of obesity and hyperlipidemia, adipokines and cholesterol have been described to modulate amyloid precursor protein degradation and thus beta-amyloid (Aβ) accumulation. Hypertension may also impair Aβ clearance, and may thus directly contribute to AD [20, 21].
The CAIDE dementia risk score has been previously tested in observational studies in relation to various cerebrospinal fluid (CSF) and neuroimaging markers, and post-mortem brain pathology [22], and higher scores correlate with signs of neurodegeneration such as reduced cortical thickness, increased medial temporal atrophy, white matter lesions, reduced brain perfusion, increased neuroinflammation, and changes in CSF Aβ and total tau [23–27]. It was also used to identify older at-risk individuals from the general population in the Finnish Geriatric Intervention study to prevent cognitive impairment and disability (FINGER). The FINGER trial showed cognitive and other related health benefits for a 2-year multidomain lifestyle intervention versus regular health advice [28]. In the Multidomain Alzheimer's Preventive Trial (MAPT), cognitive benefits from the multidomain intervention were shown in participants with a higher CAIDE score [29]. While a higher CAIDE score may reflect the potential for lifestyle-based dementia risk reduction in individuals without substantial impairment, its associations with dementia risk are less clear in populations with specific cognitive and neuropathological profiles.
The harmful effects of smoking on blood vessels, including in the brain, are well known [30, 31]. Smokers have an increased risk of dementia compared to those who have never smoked [14]. Moreover, there is evidence suggesting direct impact on AD development. Older smokers have reduced grey matter density in brain regions associated with the early stages of AD [32]. In vitro and animal studies have shown that cigarette smoke exposure consistently promotes amyloidogenic and tau abnormalities [15, 16]. Smoking is associated with cerebral oxidative stress, which promotes hyperphosphorylation of tau proteins and increases β-secretase cleavage of amyloid precursor protein involved in the production of Aβ oligomers and extracellular fibrillar Aβ aggregation [11].
Depression has been indicated as a risk factor for cognitive impairment in the context of vascular conditions as it is associated with adverse cerebrovascular effects, including increased risk of stroke and vascular pathological changes, which contribute to cognitive decline, and are also strongly associated with AD [6, 9, 33, 34]. Some studies have also reported that individuals with mild cognitive impairment (MCI) and pathological Aβ levels who have depressive symptoms progress more quickly to dementia than those without depressive symptoms [35–37].
The typical pathological changes in Aβ and tau proteins associated with Alzheimer’s disease appear decades before cognitive symptoms [38]. Detection of these protein changes in cognitively unimpaired (CU) or MCI individuals indicates a significant increase in the risk of cognitive decline [39–41]. While modifiable risk factors may provide room for dementia risk reduction, associations of the CAIDE risk score and additional risk factors such as depression and smoking with clinical progression in populations with more specific cognitive-neuropathological profiles is not fully clear. In the present study, we aimed to examine the role of defined modifiable risk factors, namely the CAIDE score, depression, and smoking, in the progression to MCI or all-cause dementia among biomarker-homogeneous (in terms of Aβ and p-tau) CU and MCI subgroups. This was accomplished by performing a comparative analysis of progression data between participants who were either positive or negative for these modifiable risk factors within each subgroup, classified according to Aβ, p-tau, and both Aβ and p-tau pathology.
Methods
Study population
Data from 1045 (611 with MCI and 434 cognitively unimpaired) participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) were used. ADNI is a publicly available (https://adni.loni.usc.edu/) follow-up study cohort at more than 60 clinical sites in the US and Canada that uses a variety of biomarkers, neuroimaging, and clinical assessments to study Alzheimer's disease and dementia. Enrolled participants were categorised into CU, MCI and all-cause dementia groups using the Clinical Dementia Rating (CDR) score (CDR = 0 for CU, CDR = 0.5 for MCI and > 0.5 for dementia) and education level adjusted MMSE and Wechsler Logical Memory II subscale tests to aid in the diagnostic process. Participants were aged between 55 and 90 years and underwent a comprehensive medical examination. Individuals with severe neurological or psychiatric disorders and systemic diseases affecting cognition were excluded from the study. Full details of the enrolment process are available at https://adni.loni.usc.edu/help-faqs/adni-documentation/. The date of the ADNI database download was May 05, 2022, with data captured from 2005 onwards. CU and MCI were assessed using participant-level follow-up data (see Supplementary Appendix 1 for detailed ADNI data management) [42].
CU and MCI subgroups were classified according to Aβ, p-tau, or both Aβ and p-tau pathology. Analyses of the various dementia risk factors for the CU group were performed on data from 434 participants when considering Aβ pathology alone, 331 participants when considering p-tau pathology alone, and 219 participants when considering both Aβ and p-tau pathology. Analyses of the MCI group for Aβ pathology alone were based on data from 611 participants, for p-tau pathology alone on 551 participants, and on 417 participants when both pathologies were considered together. The median follow-up for both CU and MCI participants was four years. Detailed baseline data and progression to MCI or all-cause dementia during follow-up are shown in Tables 1 and 2.
Table 1.
Baseline information - Cognitively Unimpaired (CU)
| Amyloid | n | All (n = 434) | Amyloid positive (n = 152) | Amyloid negative (n = 282) | statistics |
| Age: mean (SD) years | 434 | 73.3 ( 6.2) | 74.7 ( 5.9) | 72.5 ( 6.2) | t = -4.3,df = 346.0,p = < .0001 * |
| Baseline MMSE | 434 | 29.1 ( 1.2) | 29.0 ( 1.1) | 29.1 ( 1.2) | t = 0.1, df = 363.2, p = 0.9501 |
| ApoE4 carrier status | 434 | 124 ( 28.6%) | 70 ( 46.1%) | 54 ( 19.1%) | ChiSq = 35.0,df = 1.0,p = < .0001 * |
| Baseline Hippocampus Volume (mm3) | 434 | 7483 ( 864) | 7336 ( 860) | 7562 ( 857) | t = 2.6, df = 307.7, p = 0.0089 |
| Female gender: n (%) | 434 | 240 ( 55.3%) | 94 ( 61.8%) | 146 ( 51.8%) | ChiSq = 4.1, df = 1.0, p = 0.0441 |
| Higher CAIDE score: n (%) | 428 | 131 ( 30.6%) | 48 ( 31.8%) | 83 ( 30.0%) | ChiSq = 0.2, df = 1.0, p = 0.6956 |
| CAIDE Total Score | 428 | 5.8 ( 1.5) | 5.8 ( 1.5) | 5.8 ( 1.4) | t = -0.3, df = 318.7, p = 0.7691 |
| Depression as risk: n (%) | 434 | 74 ( 17.1%) | 28 ( 18.4%) | 46 ( 16.3%) | ChiSq = 0.3, df = 1.0, p = 0.5773 |
| Smokers: n (%) | 356 | 40 ( 11.2%) | 17 ( 13.6%) | 23 ( 10.0%) | ChiSq = 1.1, df = 1.0, p = 0.2988 |
| Follow-up time: median(IQR) | 434 | 48 ( 24- 96) | 48 ( 24- 90) | 60 ( 24- 96) | ChiSq = 3.2, df = 1.0, p = 0.0724 |
| Progression to MCI or dementia: n (%) | 434 | 83 ( 19.1%) | 38 ( 25.0%) | 45 ( 16.0%) | ChiSq = 5.2, df = 1.0, p = 0.0223 |
| p-tau181 | n | All (n = 331) | p-tau181 positive (n = 114) | p-tau181 negative (n = 217) | statistics |
| Age: mean (SD) years | 331 | 74.0 ( 5.8) | 75.7 ( 6.1) | 73.1 ( 5.4) | t = -3.9,df = 220.4,p = 0.0001 * |
| Baseline MMSE | 331 | 29.0 ( 1.2) | 29.0 ( 1.2) | 29.0 ( 1.1) | t = 0.1, df = 227.2, p = 0.8962 |
| ApoE4 carrier status | 331 | 88 ( 26.6%) | 39 ( 34.2%) | 49 ( 22.6%) | ChiSq = 5.2, df = 1.0, p = 0.0229 |
| Baseline Hippocampus Volume (mm3) | 331 | 7452 ( 856) | 7313 ( 881) | 7525 ( 836) | t = 2.1, df = 219.4, p = 0.0354 |
| Female gender: n (%) | 331 | 171 ( 51.7%) | 59 ( 51.8%) | 112 ( 51.6%) | ChiSq = 0.0, df = 1.0, p = 0.9805 |
| Higher CAIDE score: n (%) | 327 | 105 ( 32.1%) | 41 ( 36.6%) | 64 ( 29.8%) | ChiSq = 1.6, df = 1.0, p = 0.2087 |
| CAIDE Total Score | 327 | 5.8 ( 1.5) | 5.9 ( 1.5) | 5.8 ( 1.5) | t = -0.7, df = 241.3, p = 0.5003 |
| Depression as risk: n (%) | 331 | 68 ( 20.5%) | 23 ( 20.2%) | 45 ( 20.7%) | ChiSq = 0.0, df = 1.0, p = 0.9043 |
| Smokers: n (%) | 331 | 37 ( 11.2%) | 10 ( 8.8%) | 27 ( 12.4%) | ChiSq = 1.0, df = 1.0, p = 0.3139 |
| Follow-up time: median(IQR) | 331 | 72 ( 36–102) | 66 ( 36- 96) | 72 ( 36–102) | ChiSq = 0.1, df = 1.0, p = 0.7322 |
| Progression to MCI or dementia: n (%) | 331 | 72 ( 21.8%) | 39 ( 34.2%) | 33 ( 15.2%) | ChiSq = 15.9,df = 1.0, p = < .0001 * |
| Amyloid and p-tau181 | n | All (n = 219) | Amyloid and p-tau181 positive (n = 60) | Amyloid and p-tau181 negative (n = 159) | statistics |
| Age: mean (SD) years | 219 | 73.7 ( 5.6) | 76.4 ( 5.2) | 72.6 ( 5.5) | t = -5.1,df = 119.5,p = < .0001 * |
| Baseline MMSE | 219 | 29.1 ( 1.2) | 29.1 ( 1.1) | 29.1 ( 1.2) | t = -0.4, df = 122.3, p = 0.6813 |
| ApoE4 carrier status | 219 | 56 ( 25.6%) | 28 ( 46.7%) | 28 ( 17.6%) | ChiSq = 19.3,df = 1.0,p = < .0001 * |
| Baseline Hippocampus Volume (mm3) | 219 | 7492 ( 800) | 7245 ( 817) | 7585 ( 776) | t = 2.8, df = 101.7, p = 0.0064 |
| Female gender: n (%) | 219 | 109 ( 49.8%) | 33 ( 55.0%) | 76 ( 47.8%) | ChiSq = 0.9, df = 1.0, p = 0.3418 |
| Higher CAIDE score: n (%) | 216 | 70 ( 32.4%) | 23 ( 39.0%) | 47 ( 29.9%) | ChiSq = 1.6, df = 1.0, p = 0.2056 |
| CAIDE Total Score | 216 | 5.8 ( 1.5) | 5.9 ( 1.5) | 5.8 ( 1.5) | t = -0.7, df = 108.2, p = 0.5080 |
| Depression as risk: n (%) | 219 | 42 ( 19.2%) | 12 ( 20.0%) | 30 ( 18.9%) | ChiSq = 0.0, df = 1.0, p = 0.8495 |
| Smokers: n (%) | 219 | 22 ( 10.0%) | 6 ( 10.0%) | 16 ( 10.1%) | ChiSq = 0.0, df = 1.0, p = 0.9890 |
| Follow-up time: median(IQR) | 219 | 72 ( 36- 96) | 54 ( 36- 90) | 72 ( 36–102) | ChiSq = 4.7, df = 1.0, p = 0.0305 |
| Progression to MCI or dementia: n(%) | 219 | 46 ( 21.0%) | 23 ( 38.3%) | 23 ( 14.5%) | ChiSq = 15.0,df = 1.0,p = 0.0001 * |
CU Cognitively Unimpaired, MCI Mild Cognitive Impairment, MMSE Mini-Mental State Examination, ApoE4 Apolipoprotein E epsilon 4 carriers, CAIDE Cardiovascular Risk Factors, Aging, and Dementia, p-tau181 Phosphorylated tau 181, CSF Cerebrospinal Fluid, n Number of participants, SD Standard Deviation, IQR Interquartile Range, ChiSq Chi-Square Test, df Degrees of Freedom, p p-value
*p-values indicate significant differences between biomarker positives and negatives (after correction for multiple comparisons p < 0.05/11, where 11 is the number of parameters compared), and are based on T-tests or Wilcoxon tests (follow-up time) in case of continuous variables and Chi-Square tests in case of categorical variables
Table 2.
Baseline information - Mild Cognitive Impairment (MCI)
| Amyloid | n | All (n = 611) | Amyloid positive (n = 377) | Amyloid negative (n = 234) | statistics |
| Age: mean (SD) years | 610 | 72.5 ( 7.4) | 73.5 ( 6.8) | 70.9 ( 8.1) | t = -4.0,df = 481.4,p = < .0001 * |
| Baseline MMSE | 611 | 27.8 ( 1.8) | 27.4 ( 1.8) | 28.4 ( 1.5) | t = 8.2,df = 630.7,p = < .0001 * |
| ApoE4 carrier status | 611 | 300 ( 49.1%) | 246 ( 65.3%) | 54 ( 23.1%) | ChiSq = 102.8,df = 1.0,p = < .0001 * |
| Baseline Hippocampus Volume (mm3) | 611 | 6865 (1133) | 6631 (1064) | 7242 (1142) | t = 6.6,df = 474.7,p = < .0001 * |
| Female gender: n (%) | 611 | 255 ( 41.7%) | 156 ( 41.4%) | 99 ( 42.3%) | ChiSq = 0.1, df = 1.0, p = 0.8210 |
| Higher CAIDE score: n (%) | 606 | 223 ( 36.8%) | 141 ( 37.8%) | 82 ( 35.2%) | ChiSq = 0.4, df = 1.0, p = 0.5172 |
| CAIDE Total Score | 606 | 5.9 ( 1.4) | 5.8 ( 1.4) | 5.9 ( 1.5) | t = 0.8, df = 543.7, p = 0.4211 |
| Depression as risk: n (%) | 611 | 192 ( 31.4%) | 110 ( 29.2%) | 82 ( 35.0%) | ChiSq = 2.3, df = 1.0, p = 0.1290 |
| Smokers: n (%) | 576 | 70 ( 12.2%) | 48 ( 13.5%) | 22 ( 10.0%) | ChiSq = 1.5, df = 1.0, p = 0.2138 |
| Follow-up time: median(IQR) | 611 | 48 ( 30- 78) | 48 ( 24- 60) | 48 ( 36- 96) | ChiSq = 17.8,df = 1.0,p = < .0001 * |
| Progression to dementia: n(%) | 611 | 221 ( 36.2%) | 195 ( 51.7%) | 26 ( 11.1%) | ChiSq = 103.2,df = 1.0,p = < .0001 * |
| p-tau181 | n | All (n = 551) | p-tau181 positive (n = 305) | p-tau181 negative (n = 246) | statistics |
| Age: mean (SD) years | 551 | 72.4 ( 7.5) | 73.4 ( 7.4) | 71.1 ( 7.4) | t = -3.4,df = 563.7,p = 0.0007 * |
| Baseline MMSE | 551 | 27.7 ( 1.8) | 27.4 ( 1.8) | 28.1 ( 1.7) | t = 5.2,df = 578.7,p = < .0001 * |
| ApoE4 carrier status | 551 | 273 ( 49.5%) | 192 ( 63.0%) | 81 ( 32.9%) | ChiSq = 49.1,df = 1.0,p = < .0001 * |
| Baseline Hippocampus Volume (mm3) | 551 | 6820 (1150) | 6582 (1077) | 7116 (1171) | t = 5.5,df = 504.2,p = < .0001 * |
| Female gender: n (%) | 551 | 230 ( 41.7%) | 132 ( 43.3%) | 98 ( 39.8%) | ChiSq = 0.7, df = 1.0, p = 0.4155 |
| Higher CAIDE score: n (%) | 548 | 196 ( 35.8%) | 102 ( 33.7%) | 94 ( 38.4%) | ChiSq = 1.3, df = 1.0, p = 0.2534 |
| CAIDE Total Score | 548 | 5.8 ( 1.4) | 5.7 ( 1.3) | 6.0 ( 1.5) | t = 2.2, df = 522.8, p = 0.0309 |
| Depression as risk: n (%) | 551 | 183 ( 33.2%) | 90 ( 29.5%) | 93 ( 37.8%) | ChiSq = 4.2, df = 1.0, p = 0.0398 |
| Smokers: n (%) | 551 | 68 ( 12.3%) | 41 ( 13.4%) | 27 ( 11.0%) | ChiSq = 0.8, df = 1.0, p = 0.3814 |
| Follow-up time: median(IQR) | 551 | 48 ( 36- 84) | 48 ( 36- 66) | 48 ( 36- 90) | ChiSq = 12.6,df = 1.0, p = 0.0004 * |
| Progression to dementia: n (%) | 551 | 213 ( 38.7%) | 163 ( 53.4%) | 50 ( 20.3%) | ChiSq = 63.0,df = 1.0,p = < .0001 * |
| Amyloid and p-tau181 | n | All (n = 418) | Amyloid and p-tau181 positive (n = 257) | Amyloid and p-tau181 negative (n = 160) | statistics |
| Age: mean (SD) years | 417 | 72.1 ( 7.6) | 73.4 ( 7.1) | 69.9 ( 7.8) | t = -4.4,df = 343.3, p = < .0001 * |
| Baseline MMSE | 417 | 27.7 ( 1.8) | 27.3 ( 1.8) | 28.4 ( 1.5) | t = 7.3,df = 419.3, p = < .0001 * |
| ApoE4 carrier status | 417 | 213 ( 51.1%) | 177 ( 68.9%) | 36 ( 22.5%) | ChiSq = 84.9,df = 1.0, p = < .0001 * |
| Baseline Hippocampus Volume (mm3) | 417 | 6763 (1142) | 6477 (1026) | 7221 (1174) | t = 6.6,df = 303.4, p = < .0001 * |
| Female gender: n (%) | 417 | 184 ( 44.1%) | 113 ( 44.0%) | 71 ( 44.4%) | ChiSq = 0.0, df = 1.0, p = 0.9353 |
| Higher CAIDE score: n (%) | 414 | 144 ( 34.8%) | 88 ( 34.5%) | 56 ( 35.2%) | ChiSq = 0.0, df = 1.0, p = 0.8827 |
| CAIDE Total Score | 414 | 5.8 ( 1.4) | 5.7 ( 1.3) | 5.9 ( 1.6) | t = 1.3, df = 332.2, p = 0.2014 |
| Depression as risk: n (%) | 417 | 144 ( 34.5%) | 78 ( 30.4%) | 66 ( 41.3%) | ChiSq = 5.2, df = 1.0, p = 0.0228 |
| Smokers: n (%) | 417 | 45 ( 10.8%) | 33 ( 12.8%) | 12 ( 7.5%) | ChiSq = 2.9, df = 1.0, p = 0.0874 |
| Follow-up time: median(IQR): | 417 | 48 ( 36- 78) | 48 ( 36- 60) | 60 ( 36- 96) | ChiSq = 20.7,df = 1.0, p = < .0001 * |
| Progression to dementia: n (%) | 417 | 178 ( 42.7%) | 158 ( 61.5%) | 20 ( 12.5%) | ChiSq = 96.7,df = 1.0, p = < .0001 * |
CU Cognitively Unimpaired, MCI Mild Cognitive Impairment, MMSE Mini-Mental State Examination, ApoE4 Apolipoprotein E epsilon 4 carriers, CAIDE Cardiovascular Risk Factors, Aging, and Dementia, p-tau181 Phosphorylated tau 181, CSF Cerebrospinal Fluid, n Number of participants, SD Standard Deviation, IQR Interquartile Range, ChiSq Chi-Square Test, df Degrees of Freedom, p p-value
*p-values indicate significant differences between biomarker positives and negatives (after correction for multiple comparisons p < 0.05/11, where 11 is the number of parameters compared), and are based on T-tests or Wilcoxon tests (follow-up time) in case of continuous variables and Chi-Square tests in case of categorical variables
Risk factors
The examined risk factors, such as depression, smoking, high blood pressure, obesity, and hyperlipidemia, were treated as dichotomous variables, and participants were categorised as having vs not having a risk factor according to their medical history. The CAIDE score was calculated based on age, sex, education, hypertension (Systolic Blood Pressure > 140 mm Hg), obesity (body mass index (BMI) > 30 kg/m2) and hyperlipidemia (total cholesterol > = 6.5 mmol/L) as previously described in detail (Supplementary eTable 1) [17]. All risk factors were measured at baseline, which was the starting (zero) point of the survival analyses. Physical activity could not be included in the CAIDE calculation because data were unavailable in the ADNI database. Based on the median CAIDE score and the cut-off previously used in the FINGER study [28], we used six points as a cut-off for high dementia risk.
Assignment to the smoking group was based on the participants' medical records. Similary, based on a history of depression documented in medical records, or baseline depressive symptoms, participants were divided into depression and no depression groups. Depressive symptoms were assessed using the Neuropsychiatric Inventory-Questionnaire (NPI-Q) in ADNI 1 or the Neuropsychiatric Inventory (NPI) in ADNI GO, ADNI 2, and ADNI 3 [43–45]. Following criteria established in previous studies for the CU and MCI populations, the cut-off point for categorizing depression was a severity score of ≥ 2 on the NPI-Q [46, 47] or a severity × frequency score of ≥ 4 on the NPI [48, 49].
Aβ and p-tau status
We used the 18F-Florbetapir (AV45) PET data as the default Aβ measurement where available. Florbetapir standardized uptake value ratio (SUVR) was created by averaging the four cortical regions and dividing them by the cerebellum as a reference. According to the ADNI recommendation, we applied the SUVR cut-off of 1.11 and used the whole cerebellum region as a reference [50]. In a previous study [51] Florbetapir positivity defined using the same cut-off was shown to be strongly correlated with post-mortem autopsy results. If PET data were unavailable, we used Aβ1-42 CSF measurements (Roche Elecsys) to maximize the analysis sample size. As previously indicated [52], we applied a cut-off of 977 pg/ml for Aβ1-42 measurements since this cut-off value showed the highest agreement with amyloid PET results (overall percent agreement 87%, 95% CI 84.2–89.5). Participants were defined as p-tau positive by CSF p-tau181 levels (INNO-BIA AlzBio3) above 23 pg/ml, a cut-off shown in a previous study on autopsy-based AD cases to have the best classification power [53].
The rationale for analyzing data on Aβ status separately was twofold. First, p-tau status was available for a smaller number of participants, thus focusing only on Aβ increased the statistical power. Second Aβ captures a broader risk group who may not (yet) have tau pathology. However, abnormal p-tau alongside Aβ indicates a more severe condition. Therefore, we studied A + T + /A-T- subgroups as well. We also analyzed groups subdivided by CSF p-tau181 pathology alone (T + /T-), reflecting the 2024 classification [54], which indicates that p-tau181 becomes abnormal alongside amyloid PET, but before tau PET.
Statistical analysis
The CU and MCI groups were divided into Aβ positive and negative (A + , A-), p-tau positive and negative (T + , T-) and Aβ and p-tau positive and negative (A + T + , A-T-) subgroups. Baseline characteristics of CU and MCI participants were compared between each biomarker positive and negative subgroup using t-test, Wilcoxon or Chi-square tests as appropriate. The associations of CAIDE score, depression, and smoking with progression to MCI and/or dementia were investigated in analyses stratified by cognitive and pathology status: CU A + /A-, CU T + /T-, CU A + T + /A-T-, MCI A + /A-, MCI T + /T-, MCI A + T + /A-T-.
We calculated the (adjusted) Hazard Ratios (HR) with their confidence interval (CI) from a Cox Proportional Hazard Model (PROC PHREG in SAS 9.4). Progression to dementia in the MCI group or progression to dementia and MCI combined (in the CU group) were the dependent (predicted) variables in separate models, while Aβ and p-tau positivity served as predictor variables together with modifiable risk factors such as CAIDE score, smoking, and depression. Cox regression (Cox) analyses of smoking and depression included age, sex, education, baseline MMSE score, baseline hippocampal volume and ApoE4 carrier status as covariates. Cox regression analyses of CAIDE score included age, baseline MMSE score, and baseline hippocampal volume and ApoE4 carrier status as covariates (sex and education were already included in the CAIDE score). In order to test the proportional hazard assumption we repeated all Cox regressions by including the interaction of time and risk factors as covariates. Since the interaction of time and risk factors were non-significant in all Cox regressions (all p values > 0.1) we can conclude that there is no evidence of the time dependency of the hazard ratios, i.e. the proportional hazard assumption were met in all cases. Death was included as a competing risk in the Cox regressions. All reported Hazard Ratios from Cox regressions are adjusted ones (aHR). Where a subgroup included < 20 participants, the survival analysis was not performed due to a high risk of bias.
The methodology described above was not applied to the CU and MCI A + T- and A-T + subgroups because of the high risk of bias due to the small sample size (< 20).
Sensitivity analyses
The Kaplan–Meier survival analyses were also performed for all biomarker groups and risk factors. The survival plots are included in the figures; therefore, the adjusted curves from the Cox regressions and the survival plots are easily comparable. In the results section, we also present the statistics (log-rank test and corresponding p values) from the Kaplan–Meier (KM) analyses.
Furthermore, we performed the Cox regression analysis with the CAIDE total score as a continuous variable and with seven as alternate cut-off value for the CAIDE score. Finally, we analyzed the effect of CAIDE as risk factor in the MCI sample regardless of biomarker status.
Results
Based on the analysis of 434 CU and 611 MCI participants, baseline characteristics did not differ significantly between the A-/A + , T-/T + , and A-T-/A + T + subgroups for either CU or MCI participants according to the percentage of participants with a higher CAIDE score, depression, and smoking. There were significant differences in age, ApoE4 carrier status, MMSE score, hippocampal volume and progression rate between the biomarker-negative and positive subgroups (Table 1 and 2).
A total of 103 CU and 60 MCI participants lacked p-tau data, with only data on their Aβ status available for analysis. The number of CU participants in each biomarker subgroup was 277 (A-), 151 (A +), 217 (T-), 114 (T +), 58 (A + T-), 53 (A-T +), 157 (A-T-), and 59 (A + T +). MCI participants included 234 (A-), 377 (A +), 246 (T-), 305 (T +), 86 (A + T-), 48 (A-T +), 160 (A-T-), and 257 (A + T +).
Higher CAIDE score and progression to MCI and/or dementia
Among CU participants with higher CAIDE scores, compared to those with lower scores, the risk of progression to MCI or dementia was not significantly increased in either the biomarker-negative or biomarker-positive subgroups (Table 3, Supplementary eFigure 1). The KM analyses did not show a statistically significant difference between any CU/CAIDE risk groups (all p values > 0.1).
Table 3.
The effect of modifiable risk factors on progression to MCI and/or dementia
| Effect | CU | MCI | ||||||
|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |||||
| Higher CAIDE score | A- | 1.6 (0.89; 2.93) | A + | 1.0 (0.49; 1.92) | A- | 3.1 (1.43; 6.53) | A + | 1.3 (0.98; 1.75) |
| T- | 1.1 (0.54; 2.40) | T + | 1.0 (0.55; 2.01) | T- | 1.6 (0.94; 2.83) | T + | 1.7 (1.20; 2.27) | |
| A-T- | 1.9 (0.80; 4.25) | A + T + | 0.9 (0.39; 2.15) | A-T- | 2.6 (1.06; 6.59) | A + T + | 1.6 (1.15; 2.22) | |
| Smoking | A- | n.e. | A + | n.e. | A- | 1.3 (0.39; 4.23) | A + | 1.6 (1.07; 2.34) |
| T- | n.e. | T + | n.e. | T- | 1.8 (0.89; 3.78) | T + | 1.5 (0.99; 2.31) | |
| A-T- | n.e. | A + T + | n.e. | A-T- | n.e. | A + T + | n.e. | |
| Depression | A- | 1.6 (0.81; 3.36) | A + | 1.0 (0.42; 2.60) | A- | 1.0 (0.48; 2.19) | A + | 1.2 (0.86; 1.57) |
| T- | 1.2 (0.44; 3.19) | T + | 1.1 (0.48; 2.45) | T- | 0.6 (0.30; 1.05) | T + | 1.5 (1.06; 2.02) | |
| A-T- | n.e. | A + T + | n.e. | A-T- | 0.6 (0.22; 1.49) | A + T + | 1.3 (0.94; 1.84) | |
Bold numbers indicate a significant increase
CU Cognitively Unimpaired, MCI Mild Cognitive Impairment, aHR adjusted Hazard Ratio, 95% CI 95% Confidence Interval, A- beta-amyloid negative, A + beta-amyloid positive, T- p-tau negative, T + p-tau positive, n.e. not estimated (due to small number of cases)
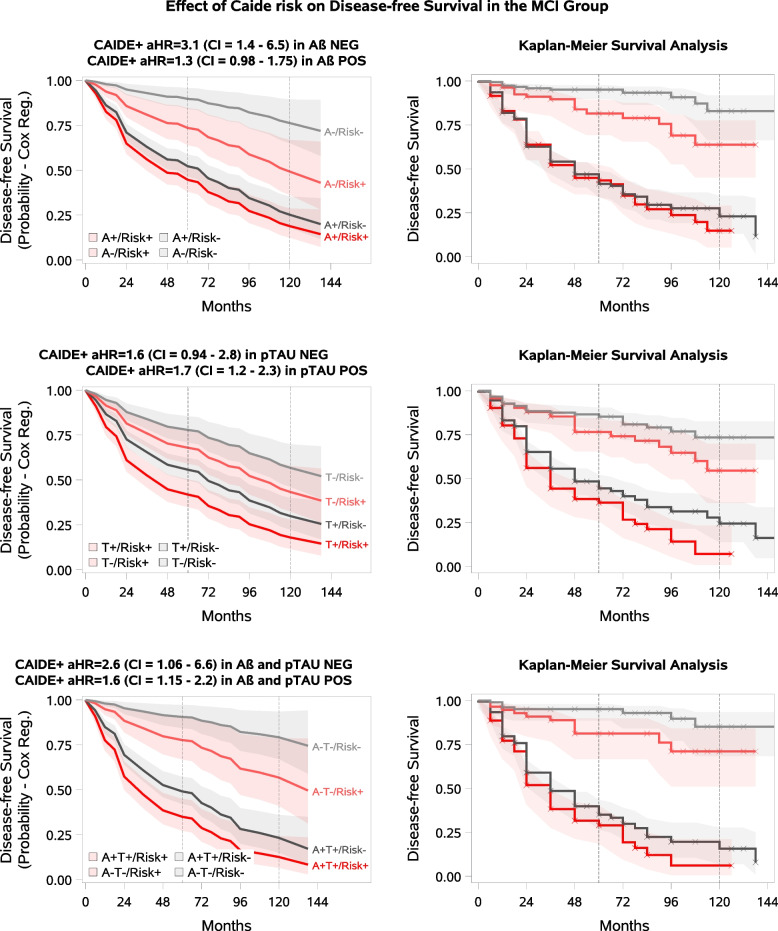
In the MCI population (Table 3, Fig. 1), the risk of progression to dementia was significantly increased among A- MCI participants with higher compared to lower CAIDE scores (Cox aHR = 3.1, 95% CI 1.43–6.53, KM log-rank chi-square (ChiSq) = 8.1, p = 0.004), while in the A + MCI subgroup a statistical trend-level association was observed (Cox aHR = 1.3, 95% CI 0.98–1.7, KM log-rank ChiSq = 0.16, p = 0.7). In the T + subgroup, higher CAIDE score was related to higher dementia risk compared with lower CAIDE score (Cox aHR = 1.7 95%CI 1.20–2.27, KM log-rank ChiSq = 5.0, p = 0.03), with a similar trend in the T- subgroup (Cox aHR = 1.6, 95%CI 0.94–2.83, KM log-rank ChiSq = 2.8, p = 0.096). Higher CAIDE score was significantly associated with an increased progression risk among both the A-T- (Cox aHR = 2.6, 95%CI 1.06–6.59, KM log-rank ChiSq = 4.7, p = 0.03) and A + T + (Cox aHR = 1.6, 95%CI 1.15–2.22, KM log-rank ChiSq = 2.6, p = 0.1) MCI subgroups.
Fig. 1.
CAIDE Score and Dementia Progression in MCI by beta-amyloid/p-tau Status. The pale lines in the figure represent the biomarker-negative group, the solid lines represent the biomarker-positive group, the red lines represent the modifiable risk factor-positive group, and the grey lines represent the modifiable risk factor-negative group. The shaded areas represent the confidence intervals. Disease-free survival means no progression to dementia. A CAIDE as a modifiable risk factor in MCI A-/A + participants. B CAIDE as a modifiable risk factor in MCI T-/T + participants. C CAIDE as a modifiable risk factor in MCI A-T-/A + T + participants
Sensitivity analysis for CAIDE score and risk for progression in the MCI group
According to the literature, cut-offs higher than six are acceptable [55]. We conducted the sensitivity analysis with the cut-off score of seven and also the CAIDE total score as a continuous variable in the MCI group. With seven as cutoff, none of the aHRs remained significant, while for CAIDE as a continuous variable, one point increase in the total score was associated with an increased risk in the A- (Cox aHR = 1.4, 95%CI 1.1–1.8), A-T- (Cox aHR = 1.4, 95%CI 1.01–1.9), A + T + (Cox aHR = 1.1, 95%CI 1.01–1.3), and T + groups (Cox aHR = 1.1, 95%CI 1.01–1.3). In the whole MCI sample, regardless of the biomarker status, higher CAIDE scores were associated with an increased risk of preogression (Cox aHR = 1.5, 95CI 1.1–1.9).
Smoking and progression to dementia
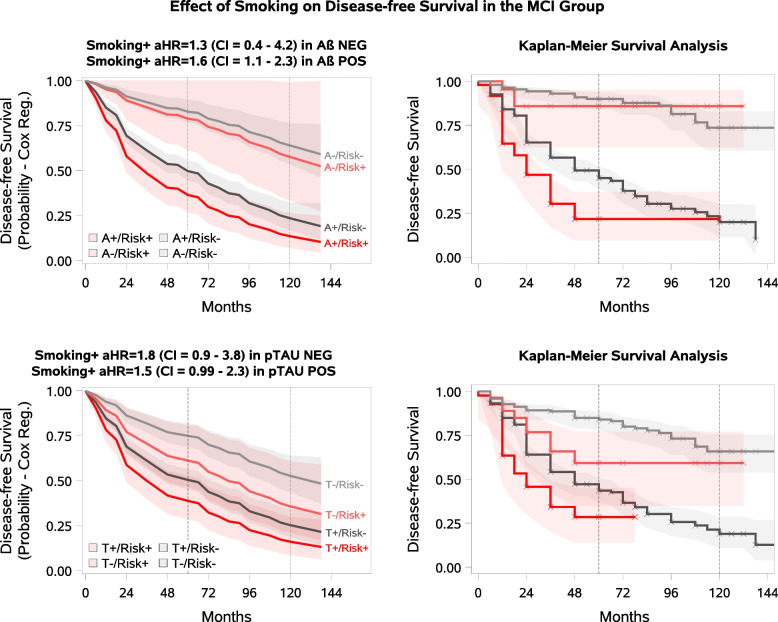
In the MCI population, the risk of progression to dementia was significantly increased in smokers compared to non-smokers in the A + (Cox aHR = 1.6, 95%CI 1.07–2.34, KM log-rank ChiSq = 11.5, p = 0.0007) subgroup, while a statistical trend-level association was observed in the T + subgroup (Cox aHR = 1.5, 95%CI 0.99–2.31, KM log-rank ChiSq = 8.0, p = 0.005). No association was observed in the A- and T- MCI subgroups (Table 3, Fig. 2). The analysis was not performed for MCI A-T- and A + T + subgroups, or any of the CU pathology subgroups due to the small number of smokers in each subgroup (ranging between 6 to 16, Table 2).
Fig. 2.
Smoking and Dementia Progression in MCI by beta-amyloid /p-tau Status. The pale lines in the figure represent the biomarker-negative group, the solid lines represent the biomarker-positive group, the red lines represent the modifiable risk factor-positive group, and the grey lines represent the modifiable risk factor-negative group. The shaded areas represent the confidence intervals. Disease-free survival means no progression to dementia. A Smoking as a modifiable risk factor in MCI A-/A + participants. B Smoking as a modifiable risk factor in MCI T-/T + participants
Depression and progression to MCI and/or dementia
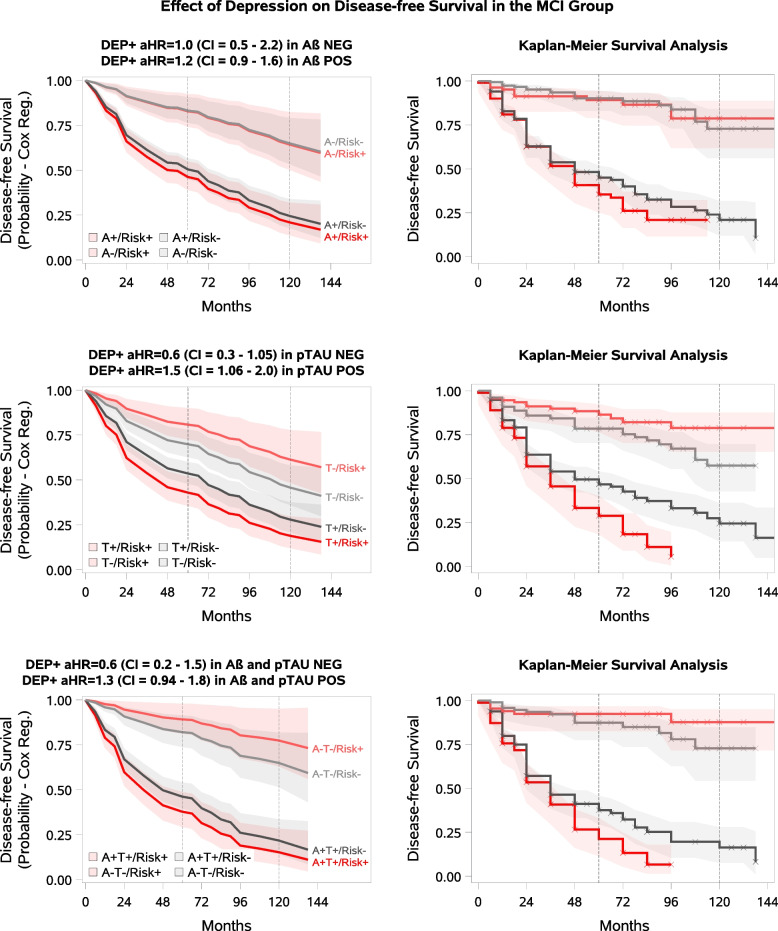
A comparison between participants with and without depression in the CU group showed no significant association with progression to MCI or dementia across the A-/A + and T-/T + biomarker subgroups (Table 3, Supplementary eFigure2). Analysis stratified by A-T-/A + T + status was not performed due to the small number of individuals with A + T + pathology and depression (n = 12, Table 2). The KM analyses showed no statistically significant difference between CU/Depression risk groups (all p values > 0.1).
In the MCI group, a significant difference in the risk of progression to dementia was observed between participants with and without depression in the T + subgroup (Cox aHR = 1.5, 95%CI 1.06–2.02, KM log-rank ChiSq = 8.2, p = 0.004), and a statistical trend-level associacion was observed in the A + T + subgroup (Cox aHR = 1.3, 95%CI 0.94–1.84, KM log-rank ChiSq = 3.9, p = 0.049) (Table 3, Fig. 3). No significant relation was identified between depression and progression to dementia in the biomarker-negative subgroups.
Fig. 3.
Depression and Dementia Progression in MCI by beta-amyloid /p-tau Status. The pale lines in the figure represent the biomarker-negative group, the solid lines represent the biomarker-positive group, the red lines represent the modifiable risk factor-positive group, and the grey lines represent the modifiable risk factor-negative group. The shaded areas represent the confidence intervals. Disease-free survival means no progression to dementia. A Depression at baseline as a modifiable risk factor in MCI A-/A + participants. B Depression at baseline as a modifiable risk factor in MCI T-/ T + participants. C Depression at baseline as a modifiable risk factor in MCI A-T-/A + T + participants
Discussion
We investigated to what extent the CAIDE dementia risk score, smoking, and depression (history of depression, or current symptoms) as modifiable risk factors were related to clinical progression of cognitive impairment in the presence or absence of Aβ and p-tau pathology. Analyzing the CU and MCI individuals separately, we found that the association of these risk factors with progression varied depending on the presence or absence of AD pathological changes.
The adverse association of the currently studied modifiable risk factors with the occurrence of Aβ and p-tau pathology is well documented in the literature. However, in this study no significant baseline differences were found in the occurrence of AD pathology between the subgroups with and without risk factors such as higher CAIDE score, smoking, or depression. While the influence of these modifiable factors on dementia risk is well established [3, 5, 6, 14, 17, 33, 34], the novelty of our research concerns their role specifically in the presence or absence of AD pathology.
A higher CAIDE score was associated with an increased risk of progression to dementia in MCI participants who were A-, T + , A-T-, and A + T + . Furthermore a statistical trend-level increase of risk was observed in the A + and T- subgroups. Associations were no longer significant when the CAIDE score cut-off was increased to seven, which may be due to smaller size of the higher risk group, since total CAIDE score as a continuous variable was related to an increased progression risk. Since higher CAIDE score was associated with higher progression risk in all almost MCI biomarker subgroups, and results were confirmed by a different unadjusted analytical approach (Kaplan–Meier survival analysis), these findings suggest that addressing modifiable vascular/lifestyle risk factors is critical to reducing the risk of progression due to non-AD pathology. Furthermore, even in the presence of AD pathology, managing these risk factors could significantly reduce the risk of dementia. Recent multimodal prevention models are combining e.g. FINGER lifestyle intervention with putative disease-modifying drugs [56]. The potential added benefit of lifestyle-based interventions would be particularly interesting to investigate in the context of new promising anti-Aβ therapies. Given the higher hazard ratios associated with higher CAIDE score in the non-AD MCI groups, our results further emphasize the importance of managing hypertension, obesity and hyperlipidaemia in dementia prevention, and highlight the potential for dementia risk reduction with vascular/lifestyle-based interventions in a significant group of cognitively impaired people who would most likely not be eligible for e.g. anti-Aβ therapies [57].
The detrimental relationship between depression and dementia is widely supported [6, 9, 33, 34]. Examining history of depression and depressive symptoms together, in the present study an increased risk of cognitive decline related to depression was found in the T + MCI subgroup, with a statistical trend-level association in the A + T + MCI subgroup. No statistically significant association with progression was observed in the A + and biomarker-negative MCI subgroups or in any CU subgroups studied (A + /A-,T + /T-). Notably, there was a significant difference in the prevalence of depression between the CU and MCI groups (17.1% vs 31.4%). One explanation for the link between depression and cognitive decline could be the serotonin and cholinergic deficits described as a consequence of depression [53, 58–62]. Depression is also associated with other risk factors for dementia, such as reduced physical activity, sleep disturbances, altered diet, and increased smoking [5, 63, 64]. Therefore, both direct and indirect effects of depression may increase the risk of dementia. An ongoing debate exists regarding whether mid- and late-life depression should be interpreted as a prodrome of dementia or as an independent risk factor [65, 66]. Our results highlight the importance of paying special attention to depressive symptoms, even in the presence of AD pathology, irrespective of whether depression is a risk factor or a consequence of the disease.
There is a well-established link between social activity and lower levels of depression [67, 68]. Social connections—including those facilitated by social media—have become increasingly important. Particularly for older adults who are at risk of isolation, social media platforms offer opportunities to maintain and enhance social interactions [69, 70]. Research suggests that certain types of social media use can have a positive impact on mental health, which may help to reduce certain dementia risk factors [71, 72]. Including social media use in lifestyle interventions may improve mental health and reduce the risk of dementia. Future research should explore the benefits of social media in vulnerable populations.
There was a significant association between smoking and progression to dementia in the MCI A + subgroup, and a trend-level association in the MCI T + subgroup, while the MCI A- and T- subgroups showed no correlation. Several mechanisms may explain the association between smoking and dementia [14, 30–32]. Some studies suggested that smoking may directly affect Aβ-associated degeneration [11, 14, 32, 73], accelerating its onset. In addition, smoking is known to have adverse effects on the vasculature [14, 30–32]. Other studies have shown that any factor that reduces oxygen supply leads to local Aβ deposition [74–76]. Preclinical research using AD-induced hypoxic models confirms that reduced brain vascularisation caused by smoking may contribute to an increased risk of dementia [74]. It should also be considered that smokers' lifestyles are often associated with other risk factors, such as a sedentary lifestyle or poor diet [77].
When interpreting our results for p-tau, it is important to note that the tau classification was based on CSF p-tau181, which is included in the Alzheimer's Association Workgroup Recommendation 2024 as a Core1 T1 biomarker and is recommended to be used primarily in conjunction with CSF Aβ42, as it has greater diagnostic value in this context. In addition, CSF p-tau181 becomes abnormal at the same time as amyloid PET and before tau PET. It is thought that the secretion of these tau fragments may represent a physiological response to Aβ plaques and may link Aβ proteinopathy to early tau proteinopathy [54]. At the same time, it is worth highlighting the role of p-tau181 as a prognostic factor. In our previous meta-analysis based on several studies measuring CSF p-tau181, we found that individuals identified as A + T + (using CSF p-tau181) had significantly higher odds ratios for cognitive decline compared to the A + or A + T- groups [41].
Finally, it is important to note that no significant association was identified between progression and the risk factors tested (CAIDE score, depression) in any of the CU biomarker subgroups. Given the well-established deleterious role of these risk factors in cognitive decline, we have two possible explanations. Firstly, the relatively low progression rate in the CU group (19.1% compared with 36.2% in MCI) may have reduced the statistical power to detect significant associations. Secondly, the median follow-up of the healthy group was four years, which may be insufficient for the adverse effects of these risk factors to become apparent in individuals who are cognitively intact.
Strenghts and limitations
This study used a large, well-characterised sample from the ADNI, including 434 CU and 611 MCI individuals, with a median follow-up of four years. However, the present study has several limitations. Aβ status was determined based on PET scans in most participants, and on CSF in the rest. Although PET is known to be more sensitive, both methods are widely used in practice, the concordance between the two methods is high, and CSF measurement is more widely available for financial reasons [38, 78].
The CAIDE scoring system provides a comprehensive and easy-to-use overview of cardiovascular and lifestyle risk factors. However, CAIDE was initially developed for a middle-aged population, and in the original study, it was used to predict the risk of dementia over 20 years. Since then, there have been examples of its use with shorter follow-ups and in older patients [3]. There is no uniform recommendation for the point value to separate the high- and low-risk groups so that this cut-off may differ in other populations. Nevertheless, utilizing the median for separating groups is appropriate for identifying the risk due to CAIDE factors. It should be noted that the lack of data on physical activity may lead to an underestimation of the association. However, the effect of physical activity is less weighted, changing the CAIDE score by only one point, compared with other modifiable risk factors, each of which contributes two points. Importantly, the accuracy and validity of cognitive tests and the CAIDE score may be influenced by cultural differences [79, 80]. To ensure that these assessments are globally applicable, future research should focus on validating and modifying them for a range of populations.
In the case of depression, it should be noted that the participants were classified based on their medical history. The severity of the depression or whether it was a late or early onset could not be considered. A more accurate classification method could further refine the results. Symptoms of depression at baseline were assessed by a detailed and comprehensive neuropsychiatric inventory developed for the detection of behavioral disturbances in dementia [43]. However, it has been utilised in preceding clinical trials with participants with MCI and CU and has been demonstrated to be a valid and reliable measure [46–48, 81]. Nevertheless, a clinically structured interview was not performed to diagnose depressive disorders according to the Diagnostic Diagnostic and Statistical Manual of Mental Disorders (DMS) [82]. In terms of smoking habits, only self-reported information was utilized, and a limitation of the study is the lack of consideration of the severity of smoking. Another limitation is that the potential confounding effect of these risk factors on each other is not included in our calculations. It also should be noted that the ADNI cohort is skewed towards white individuals and those with higher levels of education. This latter fact may restrict the generalisability of the findings to a more diverse population.
Another limitation is that the analyses could not take into account the effects of medications used for depression, hyperlipidaemia and hypertension. Therefore, these conditions were only included as categorical variables, as we could not take into account their treated or untreated status.
A limitation of the observations for CU participants is that analyses for smoking could not be performed due to the small number of cases, and analyses for depression were only partially performed. Additionally, the results for CAIDE scores and depression in the CU group are based on a moderately small sample size, resulting in lower statistical power compared to the MCI group.
We emphasise that our study aimed to investigate the role of modifiable risk factors in different biomarker subgroups, not to compare their effect between these different biomarker states. Due to statistical power limitations for interaction analyses, it remains unclear if the associations of CAIDE score, smoking and depression with clinical progression differ between the different biomarker subgroups.
Conclusion
Even after the onset of AD pathology, addressing modifiable risk factors remains critical to reducing the risk of dementia. As the effects of vascular/lifestyle-based interventions on dementia risk reduction are currently being investigated in randomized controlled trials, a key focus for future studies should be how the presence or absence of AD pathology may impact intervention effects, and potential added benefit of combining lifestyle-based and pharmacological therapies in populations who already have cognitive impairment and AD pathology.
Supplementary Information
Abbreviations
- A-
Non-pathologic levels of beta-amyloid
- A +
Pathologic levels of beta-amyloid
- Aβ
Beta-amyloid
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- aHR
Adjusted Hazard Ratio
- ChiSq
Chi-square
- CI
Confidance interval
- Cox
Cox regression analyses
- CU
Cognitively unimpaired
- CSF
Cerebrospinal fluid
- HR
Hazard ratio
- KM
Kaplan–Meier analyses
- MCI
Mild cognitive impairment
- PET
Positron emission tomography
- p-tau
Phosphorylated tau
- T-
Non-pathologic levels of phosphorylated tau
- T +
Pathologic levels of phosphorylated tau
Authors’ contributions
ZH: conceptualisation, methodology, formal analysis, writing—original draft; AS: conceptualisation, methodology, supervision, writing – review and editing; MAE: conceptualisation, writing – review and editing; VK: conceptualisation, writing – original draft; TT: supervision, writing – review and editing; ZM: supervision, writing – review and editing; PH: supervision, writing – review and editing; AH: conceptualisation, supervision, writing – review and editing; FM: conceptualisation, supervision, writing – review and editing; MK: conceptualisation, supervision, writing – review and editing; GC: conceptualisation, methodology, formal analysis, supervision, writing – review and editing, visualization.
Funding
The following grants supported our work:
1. OTKA FK 138385 (National Research, Development, and Innovation Fund)
2. This is an EU Joint Programme- Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organization under the aegis of JPND—www.jpnd.eu (National Research, Development and Innovation, Hungary, 2019–2.1.7-ERA-NET-2020–00006).
3. National Brain Research Program III (NAP2022-I-9/2022)
4. Momentum Research Grant of the Hungarian Academy of Sciences (Lendulet-2023_94)
5. AD-RIDDLE, supported by the Innovative Health Initiative Joint Undertaking (IHI JU) under grant agreement No. 101132933.
6. European Research Council [grant 804371]; EU Innovative Health Initiative (IHI) AD-RIDDLE grant; Alzheimerfonden (Sweden).
7. EU Innovative Health Initiative (IHI) AD-RIDDLE grant; EU Joint Programme—Neurodegenerative Disease Research (JPND) EURO-FINGERS grant; Region Stockholm (ALF, Sweden); Center for Innovative Medicine (CIMED) at Karolinska Institute (Sweden); Stiftelsen Stockholms sjukhem (Sweden); Swedish research council for health, working life and welfare (FORTE).
8. ERA PerMed Pattern-Cog grant.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
ADNI investigators obtained ethics approval from the local ethical committees of all involved sites. Access to all ADNI data was granted to us after registration to ADNI (https://adni.loni.usc.edu) and compliance with the data usage agreement. All work complied with ethical regulations for work with human participants. In accordance with the Declaration of Helsinki (consent for research), written informed consent was obtained from each participant or their designated representative. Ethics approval was obtained from the institutional review boards of each institution involved: Oregon Health and Science University; University of Southern California; University of California—San Diego; University of Michigan; Mayo Clinic, Rochester; Baylor College of Medicine; Columbia University Medical Center; Washington University, St. Louis; University of Alabama at Birmingham; Mount Sinai School of Medicine; Rush University Medical Center; Wien Center; Johns Hopkins University; New York University; Duke University Medical Center; University of Pennsylvania; University of Kentucky; University of Pittsburgh; University of Rochester Medical Center; University of California, Irvine; University of Texas Southwestern Medical School; Emory University; University of Kansas, Medical Center; University of California, Los Angeles; Mayo Clinic, Jacksonville; Indiana University; Yale University School of Medicine; McGill University, Montreal-Jewish General Hospital; Sunnybrook Health Sciences, Ontario; U.B.C. Clinic for AD & Related Disorders; Cognitive Neurology—St. Joseph’s, Ontario; Cleveland Clinic Lou Ruvo Center for Brain Health; Northwestern University; Premiere Research Inst (Palm Beach Neurology); Georgetown University Medical Center; Brigham and Women’s Hospital; Stanford University; Banner Sun Health Research Institute; Boston University; Howard University; Case Western Reserve University; University of California, Davis—Sacramento; Neurological Care of CNY; Parkwood Hospital; University of Wisconsin; University of California, Irvine—BIC; Banner Alzheimer’s Institute; Dent Neurologic Institute; Ohio State University; Albany Medical College; Hartford Hospital, Olin Neuropsychiatry Research Center; Dartmouth-Hitchcock Medical Center; Wake Forest University Health Sciences; Rhode Island Hospital; Butler Hospital; UC San Francisco; Medical University South Carolina; St. Joseph’s Health Care Nathan Kline Institute; University of Iowa College of Medicine; Cornell University and University of South Florida: USF Health Byrd Alzheimer’s Institute (https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization; 2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542796/. [PubMed]
- 2.Ramanan VK, Day GS. Anti-amyloid therapies for Alzheimer disease: finally, good news for patients. Mol Neurodegener. 2023;18(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–66. [DOI] [PubMed] [Google Scholar]
- 4.Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet. 2024;404(10452):572–628. [DOI] [PubMed] [Google Scholar]
- 5.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Depression as a modifiable factor to decrease the risk of dementia. Transl Psychiatry. 2017;7(5):e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjelik A, Bereczki E, Gonda S, Juhász A, Rimanóczy A, Zana M, et al. Human apoB overexpression and a high-cholesterol diet differently modify the brain APP metabolism in the transgenic mouse model of atherosclerosis. Neurochem Int. 2006;49(4):393–400. [DOI] [PubMed] [Google Scholar]
- 8.Chung JK, Plitman E, Nakajima S, Chow TW, Chakravarty MM, Caravaggio F, et al. Lifetime history of depression predicts increased Amyloid-β accumulation in patients with mild cognitive impairment. J Alzheimers Dis. 2015;45(3):907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43(1):32–7. [DOI] [PubMed] [Google Scholar]
- 10.Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, et al. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry. 2018;175(6):530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durazzo TC, Mattsson N, Weiner MW. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10(3 Suppl):S122–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein-Piekarski AN, Williams LM, Humphreys K. A trans-diagnostic review of anxiety disorder comorbidity and the impact of multiple exclusion criteria on studying clinical outcomes in anxiety disorders. Transl Psychiatry. 2016;6(6):e847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington KD, Gould E, Lim YY, Ames D, Pietrzak RH, Rembach A, et al. Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psychiatry. 2017;32(4):455–63. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AL, Nystrom NC, Piper ME, Cook J, Norton DL, Zuelsdorff M, et al. Cigarette smoking status, cigarette exposure, and duration of abstinence predicting incident dementia and death: a multistate model approach. J Alzheimers Dis. 2021;80(3):1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry. 2012;69(6):627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–41. [DOI] [PubMed] [Google Scholar]
- 18.Gabin JM, Tambs K, Saltvedt I, Sund E, Holmen J. Association between blood pressure and Alzheimer disease measured up to 27 years prior to diagnosis: the HUNT Study. Alzheimers Res Ther. 2017;9(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JQ, Tan L, Wang HF, Tan MS, Tan L, Xu W, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry. 2016;87(5):476–84. [DOI] [PubMed] [Google Scholar]
- 20.Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 2014;24(12):1982–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker KA, Power MC, Gottesman RF. Defining the Relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon A, Stephen R, Altomare D, Carrera E, Frisoni GB, Kulmala J, et al. Multidomain interventions: state-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services-part 4 of 6. Alzheimers Res Ther. 2021;13(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enache D, Solomon A, Cavallin L, Kåreholt I, Kramberger MG, Aarsland D, et al. CAIDE Dementia risk score and biomarkers of neurodegeneration in memory clinic patients without dementia. Neurobiol Aging. 2016;42:124–31. [DOI] [PubMed] [Google Scholar]
- 24.Hooshmand B, Polvikoski T, Kivipelto M, Tanskanen M, Myllykangas L, Mäkelä M, et al. CAIDE Dementia risk score, Alzheimer and cerebrovascular pathology: a population-based autopsy study. J Intern Med. 2018;283(6):597–603. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien JT, Firbank MJ, Ritchie K, Wells K, Williams GB, Ritchie CW, et al. Association between midlife dementia risk factors and longitudinal brain atrophy: the PREVENT-Dementia study. J Neurol Neurosurg Psychiatry. 2020;91(2):158–61. [DOI] [PubMed] [Google Scholar]
- 26.Stephen R, Liu Y, Ngandu T, Rinne JO, Kemppainen N, Parkkola R, et al. Associations of CAIDE Dementia risk score with MRI, PIB-PET measures, and cognition. J Alzheimers Dis. 2017;59(2):695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuorinen M, Spulber G, Damangir S, Niskanen E, Ngandu T, Soininen H, et al. Midlife CAIDE dementia risk score and dementia-related brain changes up to 30 years later on magnetic resonance imaging. J Alzheimers Dis. 2015;44(1):93–101. [DOI] [PubMed] [Google Scholar]
- 28.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. [DOI] [PubMed] [Google Scholar]
- 29.Chhetri JK, de Souto BP, Cantet C, Pothier K, Cesari M, Andrieu S, et al. Effects of a 3-Year multi-domain intervention with or without omega-3 supplementation on cognitive functions in older subjects with increased CAIDE Dementia Scores. J Alzheimers Dis. 2018;64(1):71–8. [DOI] [PubMed] [Google Scholar]
- 30.Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American heart association. . American heart association task force on risk reduction. Circulation. 1997;96(9):3243–7. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention (US); 2014. [PubMed]
- 32.Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(1):92–8. [DOI] [PubMed] [Google Scholar]
- 33.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brendel M, Pogarell O, Xiong G, Delker A, Bartenstein P, Rominger A. Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur J Nucl Med Mol Imaging. 2015;42(5):716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon B, Kim S, Park YH, Lim JS, Youn YC, Kim S, et al. Depressive symptoms are associated with progression to dementia in patients with amyloid-positive mild cognitive impairment. J Alzheimers Dis. 2017;58(4):1255–64. [DOI] [PubMed] [Google Scholar]
- 37.Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimäki M, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiat. 2017;74(7):712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ossenkoppele R, Pichet Binette A, Groot C, Smith R, Strandberg O, Palmqvist S, et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. 2022;28(11):2381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strikwerda-Brown C, Hobbs DA, Gonneaud J, St-Onge F, Binette AP, Ozlen H, et al. Association of elevated amyloid and tau positron emission tomography signal with near-term development of alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurol. 2022;79(10):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huszár Z, Engh MA, Pavlekovics M, Sato T, Steenkamp Y, Hanseeuw B, et al. Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis. Alzheimer’s Research & Therapy. 2024;16(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–9. 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. [DOI] [PubMed] [Google Scholar]
- 44.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9. [DOI] [PubMed] [Google Scholar]
- 45.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–5. [DOI] [PubMed] [Google Scholar]
- 46.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJH, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65(10):1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoro-Zohoun I, Nubukpo P, Houinato D, Mbelesso P, Ndamba-Bandzouzi B, Clément JP, et al. Neuropsychiatric symptoms among older adults living in two countries in Central Africa (EPIDEMCA study). Int J Geriatr Psychiatry. 2019;34(1):169–78. [DOI] [PubMed] [Google Scholar]
- 48.Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saari T, Koivisto A, Hintsa T, Hänninen T, Hallikainen I. Psychometric properties of the neuropsychiatric inventory: a review. J Alzheimers Dis. 2022;86(4):1485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landau SM, Fero A, Baker SL, Koeppe R, Mintun M, Chen K, Reiman EM, Jagust WJ. Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med. 2015;56(4):567-74. 10.2967/jnumed.114.148981. [DOI] [PMC free article] [PubMed]
- 51.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305(3):275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jack CR Jr, Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024;20(8):5143–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kivimäki M, Livingston G, Singh-Manoux A, Mars N, Lindbohm JV, Pentti J, et al. Estimating Dementia risk using multifactorial prediction models. JAMA Netw Open. 2023;6(6): e2318132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbera M, Lehtisalo J, Perera D, Aspö M, Cross M, De Jager Loots CA, et al. A multimodal precision-prevention approach combining lifestyle intervention with metformin repurposing to prevent cognitive impairment and disability: the MET-FINGER randomised controlled trial protocol. Alzheimer’s Res Ther. 2024;16(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg A, Öhlund-Wistbacka U, Hall A, Bonnard A, Hagman G, Rydén M, et al. β-Amyloid, Tau, Neurodegeneration Classification and Eligibility for Anti-amyloid Treatment in a Memory Clinic Population. Neurology. 2022;99(19):e2102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cirrito JR, Disabato BM, Restivo JL, Verges DK, Goebel WD, Sathyan A, et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci U S A. 2011;108(36):14968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14(2):158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the Cholinergic System. Curr Neuropharmacol. 2016;14(1):101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31(12):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J Affect Disord. 2013;148(1):12–27. [DOI] [PubMed] [Google Scholar]
- 64.Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45(5):649–57. [DOI] [PubMed] [Google Scholar]
- 65.Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184–90. [DOI] [PubMed] [Google Scholar]
- 66.Sinclair LI, Mohr A, Morisaki M, Edmondson M, Chan S, Bone-Connaughton A, et al. Is later-life depression a risk factor for Alzheimer’s disease or a prodromal symptom: a study using post-mortem human brain tissue? Alzheimers Res Ther. 2023;15(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans IEM, Martyr A, Collins R, Brayne C, Clare L. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J Alzheimers Dis. 2019;70(s1):S119–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gariépy G, Honkaniemi H, Quesnel-Vallée A. Social support and protection from depression: systematic review of current findings in Western countries. Br J Psychiatry. 2016;209(4):284–93. [DOI] [PubMed] [Google Scholar]
- 69.Kuo CY, Stachiv I, Nikolai T. Association of late life depression,(non-) modifiable risk and protective factors with dementia and Alzheimer’s disease: literature review on current evidences, preventive interventions and possible future trends in prevention and treatment of dementia. Int J Environ Res Public Health. 2020;17(20):7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wickramaratne PJ, Yangchen T, Lepow L, Patra BG, Glicksburg B, Talati A, et al. Social connectedness as a determinant of mental health: A scoping review. PLoS ONE. 2022;17(10):e0275004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly ME, Duff H, Kelly S, McHugh Power JE, Brennan S, Lawlor BA, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev. 2017;6(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shu S, Woo BK. Use of technology and social media in dementia care: Current and future directions. World J Psychiatry. 2021;11(4):109–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW, et al. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE. 2012;7(5): e36752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bragina OA, Sillerud LO, Kameneva MV, Nemoto EM, Bragin DE. Haemorheologic enhancement of cerebral perfusion improves oxygen supply and reduces Aβ plaques deposition in a mouse model of Alzheimer’s disease. Adv Exp Med Biol. 2022;1395:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang D, Chen F, Han Z, Yin Z, Ge X, Lei P. Relationship between Amyloid-β deposition and blood-brain barrier dysfunction in Alzheimer’s disease. Front Cell Neurosci. 2021;15:695479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Firth J, Solmi M, Wootton RE, Vancampfort D, Schuch FB, Hoare E, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vos SJB, Gordon BA, Su Y, Visser PJ, Holtzman DM, Morris JC, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Culture Ardila A, Testing Cognitive. In: Ardila A, editor. Historical development of human cognition: a cultural-historical neuropsychological perspective. Singapore: Springer Singapore; 2018. p. 135–59. [Google Scholar]
- 80.Kūkea Shultz P, Englert K. Cultural Validity as Foundational to Assessment Development: An Indigenous Example. Front Educ. 2021;6:701973. 10.3389/feduc.2021.701973.
- 81.Peters ME, Rosenberg PB, Steinberg M, Norton MC, Welsh-Bohmer KA, Hayden KM, et al. Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the Cache County Study. Am J Geriatr Psychiatry. 2013;21(11):1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Association AP. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc; 2013. p. 947. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.