Abstract
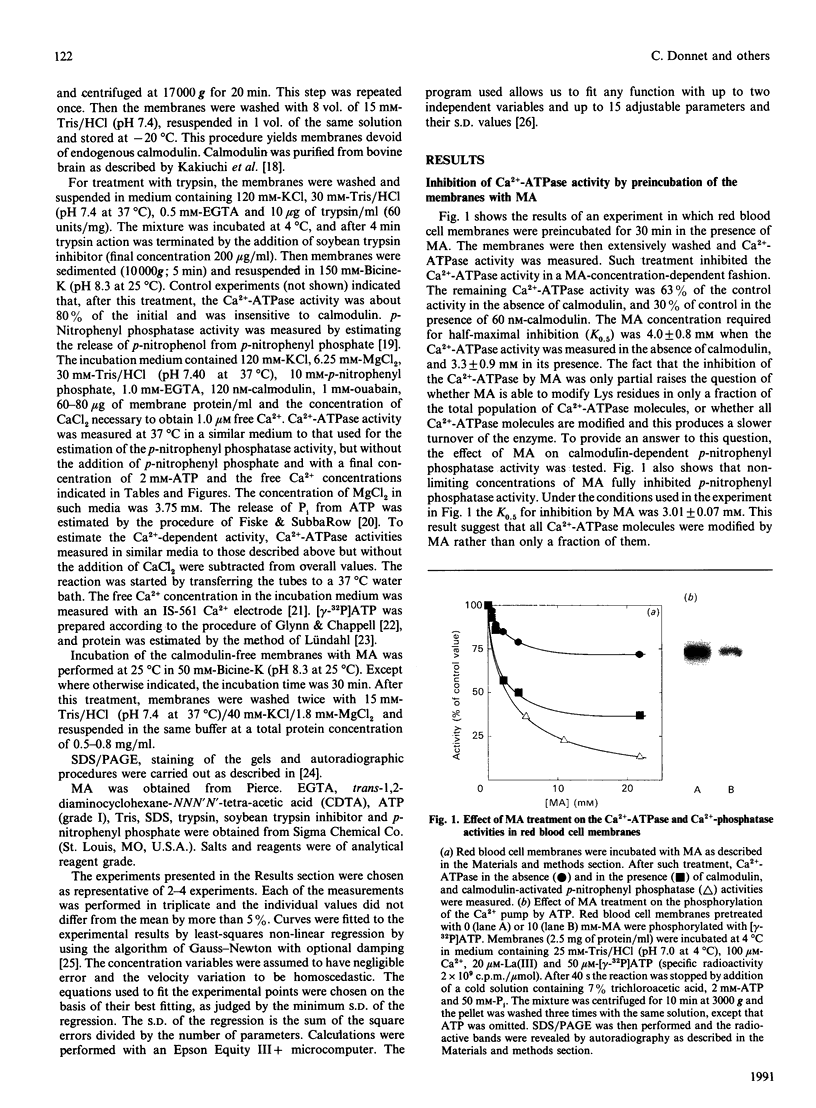
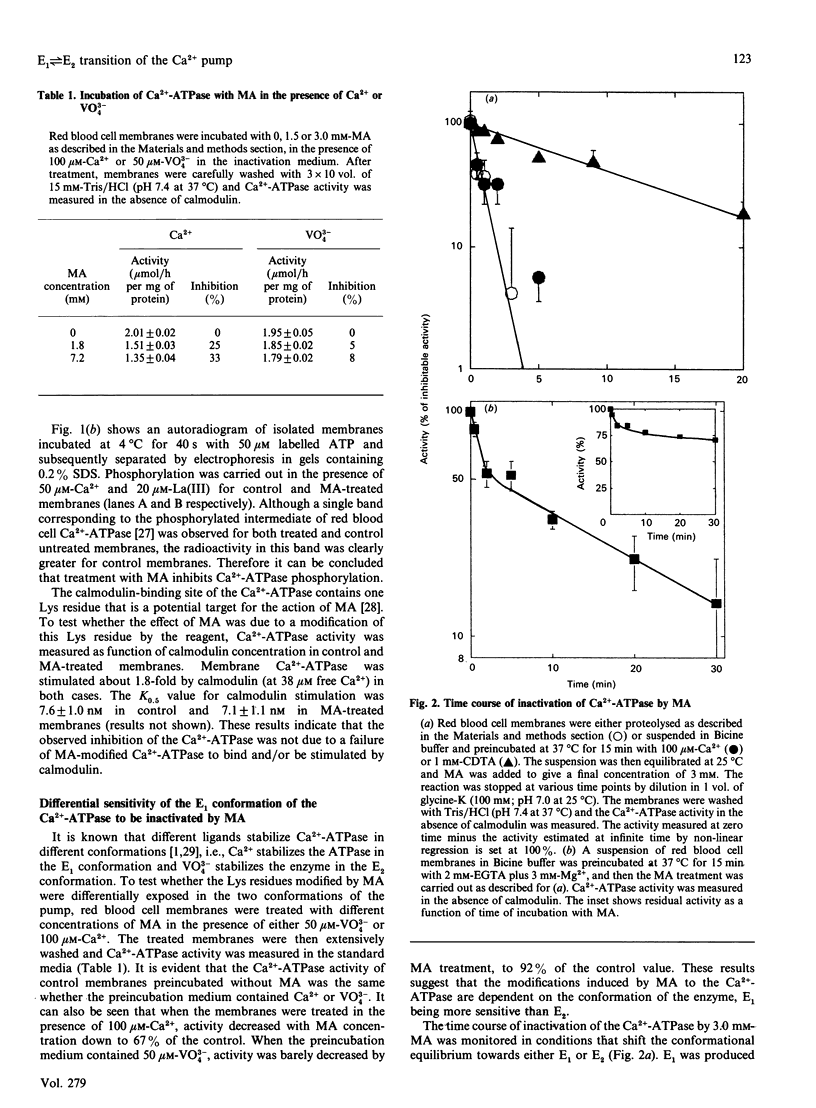
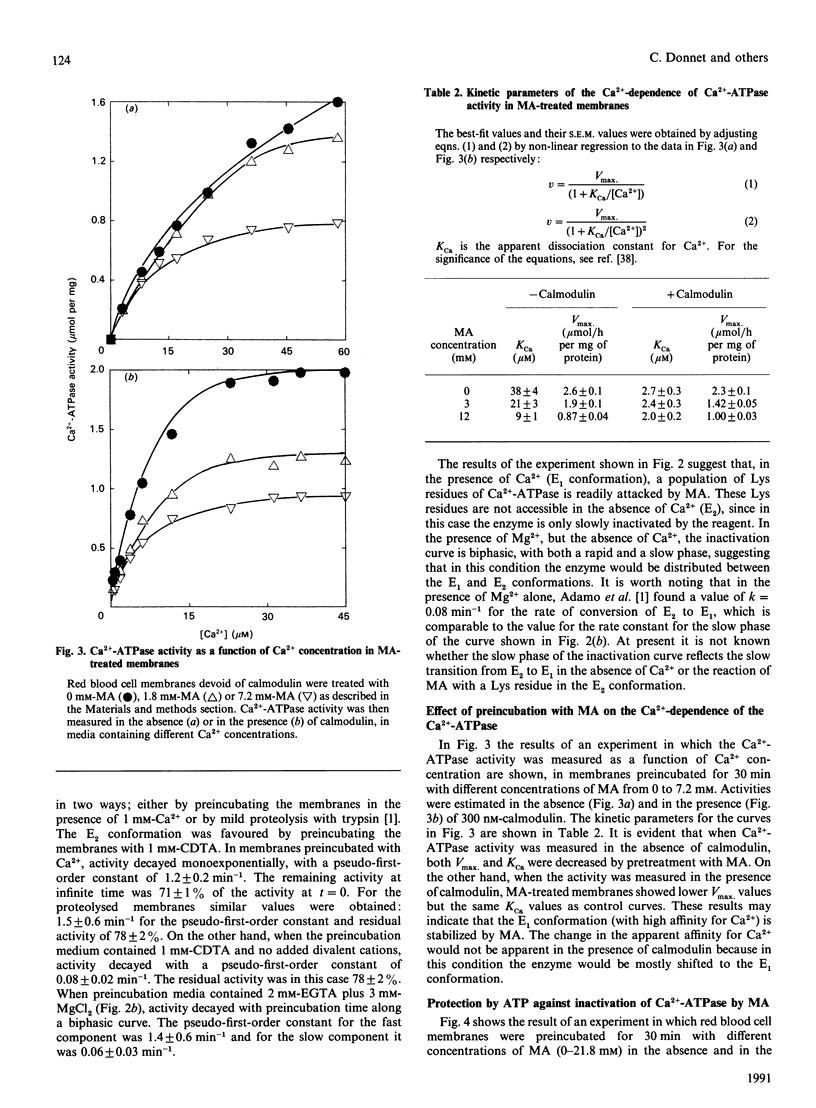
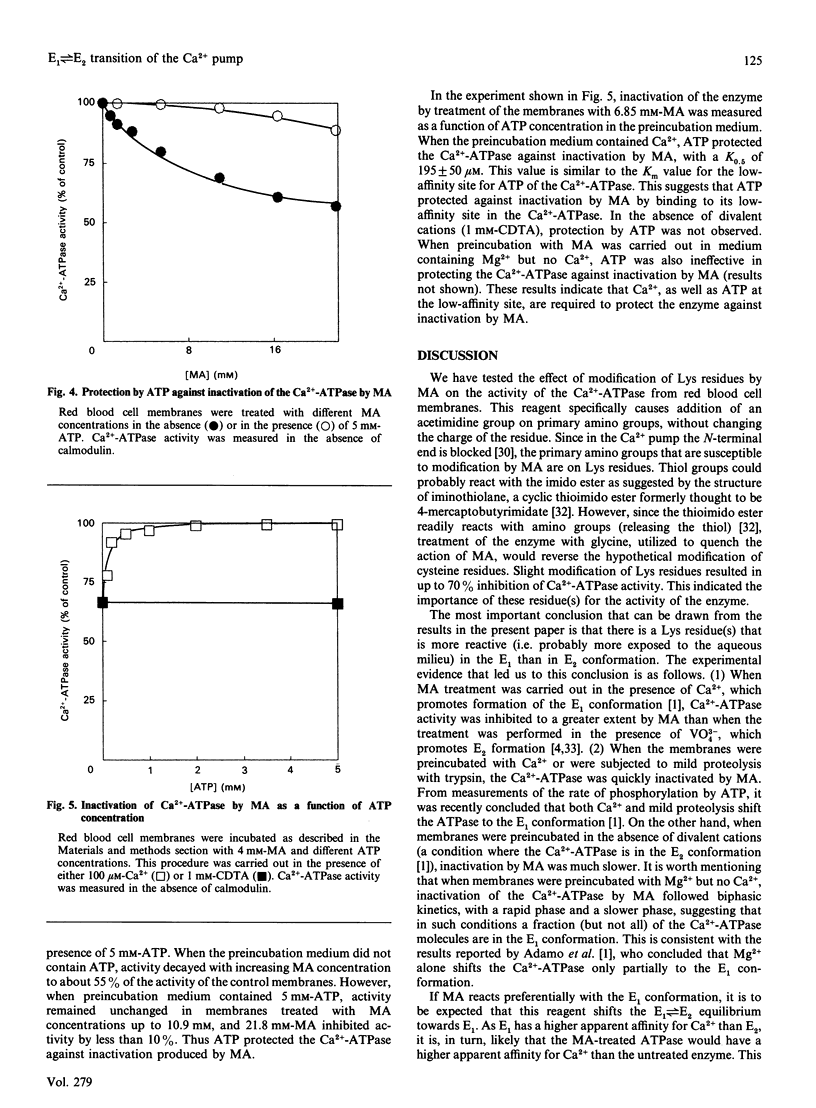
1. Modification of Lys residues of the Ca(2+)-ATPase from human red blood cells with methyl acetimidate (MA) inhibited up to 70% of the Ca(2+)-ATPase activity. Furthermore, calmodulin-activated p-nitrophenyl phosphatase activity was fully inhibited at non-limiting concentrations of MA. 2. Treatment with MA inhibited phosphorylation of the Ca(2+)-ATPase. 3. When the enzyme was treated with 7.2 mM-MA in the presence of 100 microM-Ca2+, Ca(2+)-ATPase activity was decreased by 33%, whereas when the membranes were treated with MA in the presence of 50 microM-VO4(3-), this activity was decreased by only 8%. 4. When membranes were either proteolysed or preincubated with 1 mM-Ca2+, MA quickly inactivated the Ca(2+)-ATPase (k = 1.2 min-1). On the other hand, inactivation of membranes preincubated in the absence of Ca2+ and Mg2+ was slow (k = 0.08 min-1). 5. When the activity was measured in the absence of calmodulin, MA decreased to the same extent the values of KCa (the apparent dissociation constant for Ca2+) and Vmax, but in the presence of calmodulin the treatment decreased Vmax. only. 6. The results are consistent with the idea that MA reacts readily with the Ca(2+)-ATPase when the enzyme is in an E1 conformation, but not an E2 conformation, and that, reciprocally, treatment of the enzyme with MA shifts the enzyme to E1. 7. Provided that Ca2+ is present, ATP, with low apparent affinity (K0.5 = 195 microM), protected against inactivation by MA. However, MA treatment did not change the Km values of either the high-affinity or the low-affinity site for ATP, suggesting that protection results from a shift to a conformation in which the Lys residues are inaccessible to MA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo H. P., Rega A. F., Garrahan P. J. Pre-steady-state phosphorylation of the human red cell Ca2+-ATPase. J Biol Chem. 1988 Nov 25;263(33):17548–17554. [PubMed] [Google Scholar]
- Adamo H. P., Rega A. F., Garrahan P. J. The E2 in equilibrium E1 transition of the Ca2(+)-ATPase from plasma membranes studied by phosphorylation. J Biol Chem. 1990 Mar 5;265(7):3789–3792. [PubMed] [Google Scholar]
- Argüello J. M., Kaplan J. H. N-acetylimidazole inactivates renal Na,K-ATPase by disrupting ATP binding to the catalytic site. Biochemistry. 1990 Jun 19;29(24):5775–5782. doi: 10.1021/bi00476a019. [DOI] [PubMed] [Google Scholar]
- Barrabin H., Garrahan P. J., Rega A. F. Vanadate inhibition of the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1980 Aug 14;600(3):796–804. doi: 10.1016/0005-2736(80)90482-4. [DOI] [PubMed] [Google Scholar]
- Benaim G., Zurini M., Carafoli E. Different conformational states of the purified Ca2+-ATPase of the erythrocyte plasma membrane revealed by controlled trypsin proteolysis. J Biol Chem. 1984 Jul 10;259(13):8471–8477. [PubMed] [Google Scholar]
- Farley R. A., Faller L. D. The amino acid sequence of an active site peptide from the H,K-ATPase of gastric mucosa. J Biol Chem. 1985 Apr 10;260(7):3899–3901. [PubMed] [Google Scholar]
- Farley R. A., Tran C. M., Carilli C. T., Hawke D., Shively J. E. The amino acid sequence of a fluorescein-labeled peptide from the active site of (Na,K)-ATPase. J Biol Chem. 1984 Aug 10;259(15):9532–9535. [PubMed] [Google Scholar]
- Filoteo A. G., Gorski J. P., Penniston J. T. The ATP-binding site of the erythrocyte membrane Ca2+ pump. Amino acid sequence of the fluorescein isothiocyanate-reactive region. J Biol Chem. 1987 May 15;262(14):6526–6530. [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Activation of partial reactions of the Ca2+-ATPase from human red cells by Mg2+ and ATP. Biochim Biophys Acta. 1978 Oct 19;513(1):59–65. doi: 10.1016/0005-2736(78)90111-6. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Tejcka M., Wolf H. U. Calmodulin affinity chromatography yields a functional purified erythrocyte (Ca+ + Mg2+)-dependent adenosine triphosphatase. Biochem J. 1980 Jul 1;189(1):81–88. doi: 10.1042/bj1890081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P., Maeda M., Fischer R., Verma A. K., Krebs J., Penniston J. T., Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem. 1988 Feb 25;263(6):2905–2910. [PubMed] [Google Scholar]
- Jue R., Lambert J. M., Pierce L. R., Traut R. R. Addition of sulfhydryl groups to Escherichia coli ribosomes by protein modification with 2-iminothiolane (methyl 4-mercaptobutyrimidate). Biochemistry. 1978 Dec 12;17(25):5399–5406. doi: 10.1021/bi00618a013. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Kambayashi J., Sakon M., Kosaki G. Lack of tissue specificity of calmodulin: a rapid and high-yield purification method. FEBS Lett. 1981 Apr 20;126(2):203–207. doi: 10.1016/0014-5793(81)80242-6. [DOI] [PubMed] [Google Scholar]
- Krebs J., Vasak M., Scarpa A., Carafoli E. Conformational differences between the E1 and E2 states of the calcium adenosinetriphosphatase of the erythrocyte plasma membrane as revealed by circular dichroism and fluorescence spectroscopy. Biochemistry. 1987 Jun 30;26(13):3921–3926. doi: 10.1021/bi00387a027. [DOI] [PubMed] [Google Scholar]
- Lundahl P. Proteins selectively released from water-extracted human erythrocyte membranes upon citraconylation or maleylation. Electrophoretic analysis and chromatographic fractionation. Biochim Biophys Acta. 1975 Jan 30;379(1):304–316. doi: 10.1016/0005-2795(75)90033-1. [DOI] [PubMed] [Google Scholar]
- Mitchinson C., Wilderspin A. F., Trinnaman B. J., Green N. M. Identification of a labelled peptide after stoicheiometric reaction of fluorescein isothiocyanate with the Ca2+ -dependent adenosine triphosphatase of sarcoplasmic reticulum. FEBS Lett. 1982 Sep 6;146(1):87–92. doi: 10.1016/0014-5793(82)80710-2. [DOI] [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Catalytic and regulatory ATP-binding sites of the red cell Ca2+ pump studied by irreversible modification with fluorescein isothiocyanate. J Biol Chem. 1983 Jan 10;258(1):169–175. [PubMed] [Google Scholar]
- Niggli V., Penniston J. T., Carafoli E. Purification of the (Ca2+-Mg2+)-ATPase from human erythrocyte membranes using a calmodulin affinity column. J Biol Chem. 1979 Oct 25;254(20):9955–9958. [PubMed] [Google Scholar]
- Olorunsogo O. O., Villalobo A., Wang K. K., Roufogalis B. D. The effect of calmodulin on the interaction of carbodiimides with the purified human erythrocyte (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1988 Nov 3;945(1):33–40. doi: 10.1016/0005-2736(88)90359-8. [DOI] [PubMed] [Google Scholar]
- Papp B., Sarkadi B., Enyedi A., Caride A. J., Penniston J. T., Gardos G. Functional domains of the in situ red cell membrane calcium pump revealed by proteolysis and monoclonal antibodies. Possible sites for regulation by calpain and acidic lipids. J Biol Chem. 1989 Mar 15;264(8):4577–4582. [PubMed] [Google Scholar]
- Pedemonte C. H., Kaplan J. H. Carbodiimide inactivation of Na,K-ATPase. A consequence of internal cross-linking and not carboxyl group modification. J Biol Chem. 1986 Mar 15;261(8):3632–3639. [PubMed] [Google Scholar]
- Rega A. F., Richards D. E., Garrahan P. J. Calcium ion-dependent p-nitrophenyl phosphate phosphatase activity and calcium ion-dependent adenosine triphosphatase activity from human erythrocyte membranes. Biochem J. 1973 Sep;136(1):185–194. doi: 10.1042/bj1360185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. D. Effects of pyridoxal phosphate treatment on the (Na + K)-ATPase. J Bioenerg Biomembr. 1984 Jun;16(3):195–207. doi: 10.1007/BF00751049. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Gronda C. M., Fernandez H. N., Gagliardino J. J. Characteristics of a Ca2+-ATPase activity measured in islet homogenates. Biochim Biophys Acta. 1988 Aug 18;943(2):175–182. doi: 10.1016/0005-2736(88)90549-4. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Rega A. F. A study to see whether phosphatidylserine, partial proteolysis and EGTA substitute for calmodulin during activation of the Ca2+-ATPase from red cell membranes by ATP. Biochim Biophys Acta. 1989 Jul 6;996(3):153–159. doi: 10.1016/0167-4838(89)90241-0. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Enyedi A., Gárdos G. Conformational changes of the in situ red cell membrane calcium pump affect its proteolysis. Biochim Biophys Acta. 1987 May 12;899(1):129–133. doi: 10.1016/0005-2736(87)90247-1. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Penniston J. T. Two Ca2+-requiring p-nitrophenylphosphatase activities of the highly purified Ca2+-pumping adenosinetriphosphatase of human erythrocyte membranes, one requiring calmodulin and the other ATP. Biochemistry. 1984 Oct 9;23(21):5010–5015. doi: 10.1021/bi00316a028. [DOI] [PubMed] [Google Scholar]
- Villalobo A., Harris J. W., Roufogalis B. D. Calcium-dependent inhibition of the erythrocyte Ca2+ translocating ATPase by carbodiimides. Biochim Biophys Acta. 1986 Jun 13;858(1):188–194. doi: 10.1016/0005-2736(86)90305-6. [DOI] [PubMed] [Google Scholar]
- Zvaritch E., James P., Vorherr T., Falchetto R., Modyanov N., Carafoli E. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry. 1990 Sep 4;29(35):8070–8076. doi: 10.1021/bi00487a012. [DOI] [PubMed] [Google Scholar]
- de Ancos J. G., Inesi G. Patterns of proteolytic cleavage and carbodiimide derivatization in sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1988 Mar 8;27(5):1793–1803. doi: 10.1021/bi00405a061. [DOI] [PubMed] [Google Scholar]