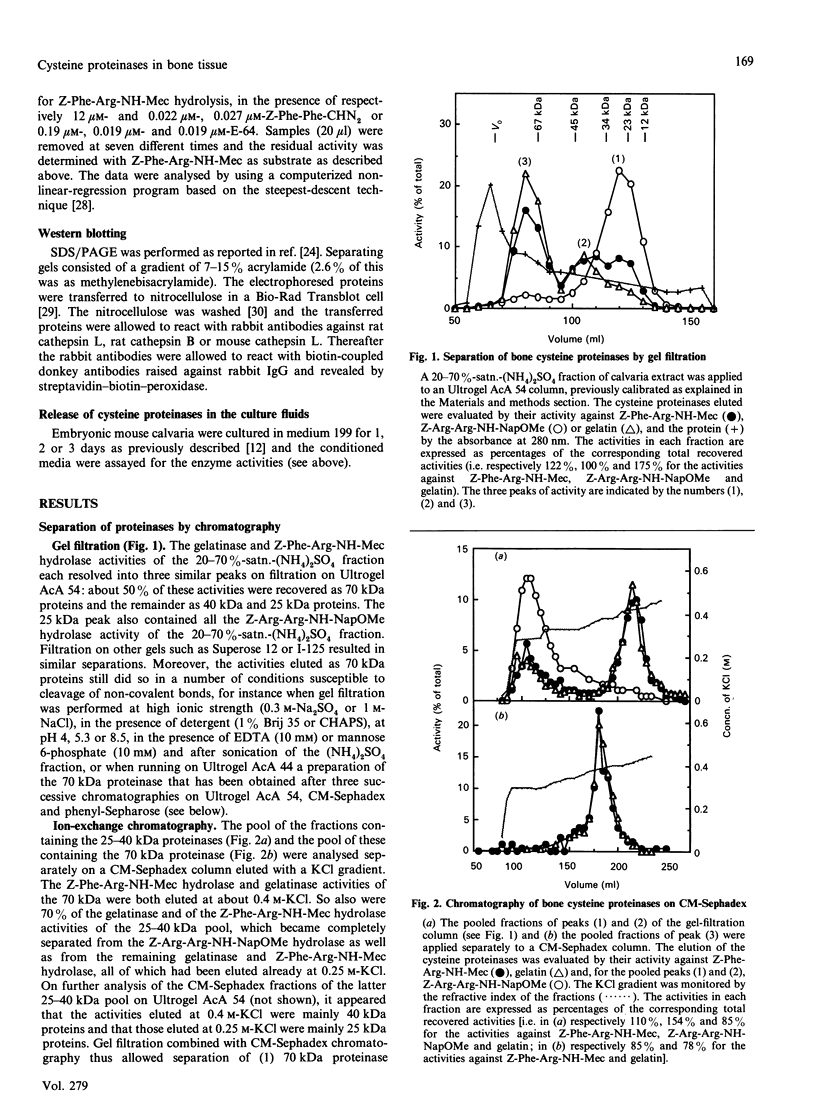
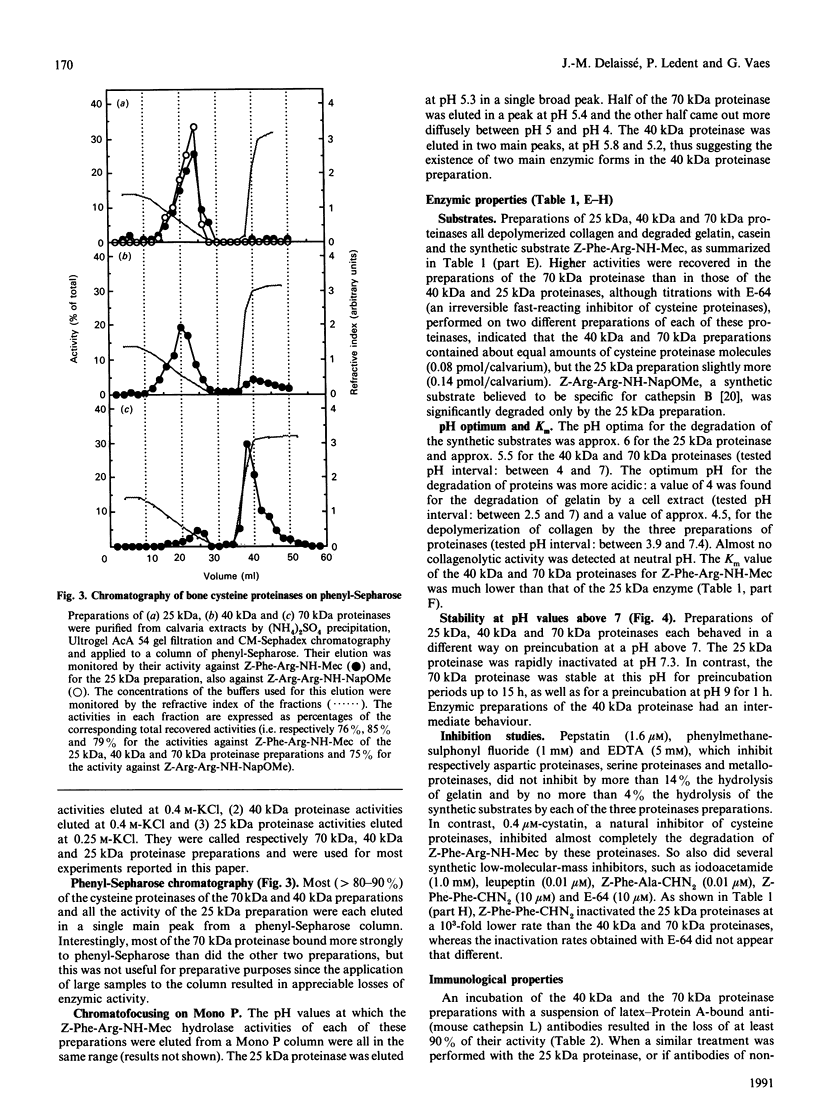
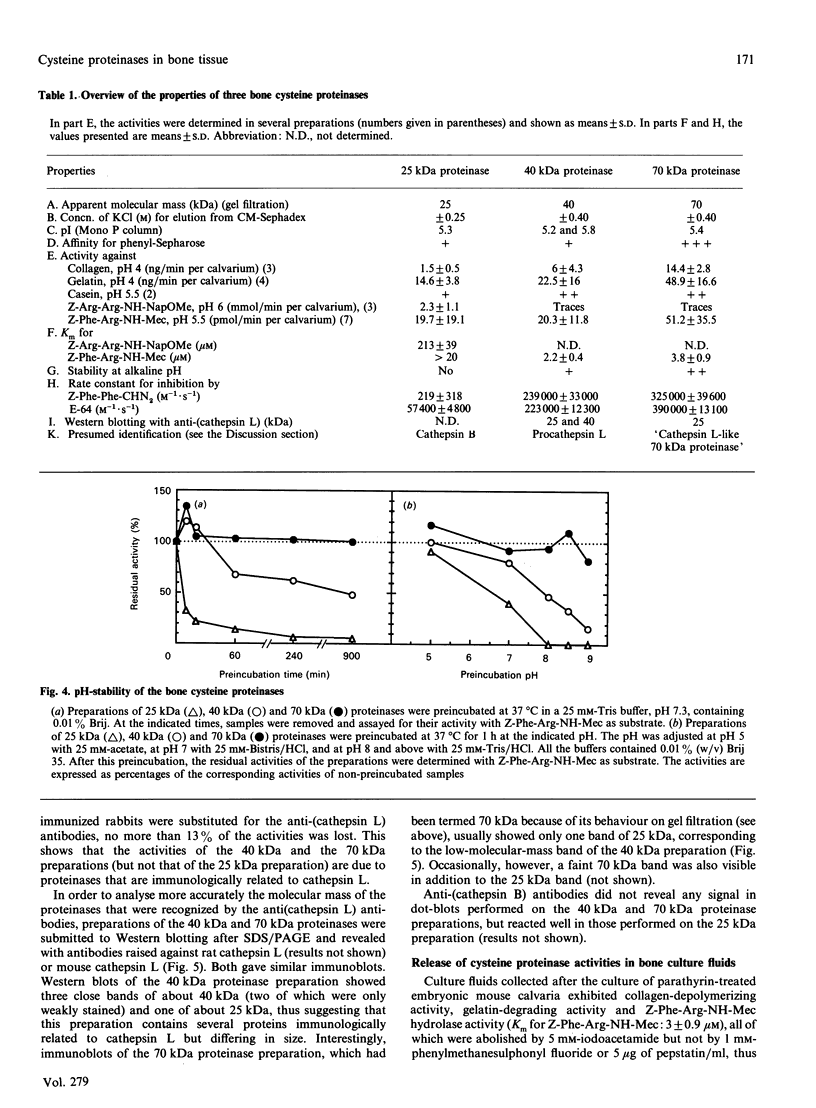
Abstract
The aim of the work was to identify and characterize the cysteine proteinases of bone tissue, as these enzymes appear necessary for bone resorption. Three cysteine-dependent proteolytic activities were separated from a homogenate of mouse calvaria by a fractionation procedure involving (NH4)2SO4 precipitation, gel filtration and ion-exchange chromatography. The first two are typical cathepsins B and L with respect to (1) their reactivity with anti-(cathepsin B) and anti-(cathepsin L) antibodies respectively, (2) their relative rate constants for inhibition by benzyloxycarbonyl-Phe-Phe-CHN2 and L-3-carboxy-trans-2,3-epoxypropionyl-L-leucylamido-(4-guanid ino)butane and (3) their enzymic properties, such as the higher activities of cathepsin L against collagen and gelatin as compared with cathepsin B, and the fact that benzyloxycarbonyl-Arg-Arg 4-methoxy-2-naphthylamide is hydrolysed only by cathepsin B. Cathepsin L was mainly recovered in its precursor form, as indicated by its apparent 40 kDa molecular mass and its relative stability at pH 7.2. The third enzyme is a cathepsin L-like proteinase with an apparent molecular mass of 70 kDa. It is immunoprecipitated by anti-(cathepsin L) antibodies, and appears as the 25 kDa band of mature cathepsin L in Western blots. It further resembles (pro)cathepsin L with regard to its activities against synthetic substrates and proteins such as collagen, and with regard to its response to various inhibitors. However, unlike (pro)cathepsin L, it is eluted as a 70 kDa protein on gel filtration (even in the presence of 1% Brij or 1 M-NaCl), it is stable at pH values as high as 9, and it exhibits stronger affinity for phenyl-Sepharose. It might thus result from a strong complex between mature cathepsin L and another entity that confers stability at alkaline pH and favours hydrophobic interactions. This 70 kDa activity was also detected in mouse muscle and long bones of Ca(2+)-deficient chicks but not in mouse liver, spleen or kidney.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 1989 Jun;224(2):317–324. doi: 10.1002/ar.1092240220. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Brömme D., Steinert A., Friebe S., Fittkau S., Wiederanders B., Kirschke H. The specificity of bovine spleen cathepsin S. A comparison with rat liver cathepsins L and B. Biochem J. 1989 Dec 1;264(2):475–481. doi: 10.1042/bj2640475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Delaisse J. M., Boyde A., Maconnachie E., Ali N. N., Sear C. H., Eeckhout Y., Vaes G., Jones S. J. The effects of inhibitors of cysteine-proteinases and collagenase on the resorptive activity of isolated osteoclasts. Bone. 1987;8(5):305–313. doi: 10.1016/8756-3282(87)90007-x. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984 Dec 14;125(2):441–447. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. Inhibition of bone resorption in culture by inhibitors of thiol proteinases. Biochem J. 1980 Oct 15;192(1):365–368. doi: 10.1042/bj1920365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J., Birkedahl-Hansen H. The influence of dissolved calcium salts on the degradation of hard-tissue collagens by lysosomal cathepsins. Coll Relat Res. 1987 Aug;7(3):185–199. doi: 10.1016/s0174-173x(87)80009-2. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. The nature of the collagenolytic cathepsin of rat liver and its distribution in other rat tissues. Biochem J. 1972 May;127(4):685–692. doi: 10.1042/bj1270685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P., Etherington D. J. Characterisation of cathepsin B and collagenolytic cathepsin from human placenta. Eur J Biochem. 1978 Feb 1;83(1):87–97. doi: 10.1111/j.1432-1033.1978.tb12071.x. [DOI] [PubMed] [Google Scholar]
- Everts V., Beertsen W., Schröder R. Effects of the proteinase inhibitors leupeptin and E-64 on osteoclastic bone resorption. Calcif Tissue Int. 1988 Sep;43(3):172–178. doi: 10.1007/BF02571316. [DOI] [PubMed] [Google Scholar]
- Falanga A., Gordon S. G. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry. 1985 Sep 24;24(20):5558–5567. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- Frisch A., Neufeld E. F. Limited proteolysis of the beta-hexosaminidase precursor in a cell-free system. J Biol Chem. 1981 Aug 10;256(15):8242–8246. [PubMed] [Google Scholar]
- Gieselmann V., Hasilik A., von Figura K. Processing of human cathepsin D in lysosomes in vitro. J Biol Chem. 1985 Mar 10;260(5):3215–3220. [PubMed] [Google Scholar]
- Gohda E., Pitot H. C. Purification and characterization of a new thiol proteinase from rat kidney. Biochim Biophys Acta. 1981 May 14;659(1):114–122. doi: 10.1016/0005-2744(81)90275-8. [DOI] [PubMed] [Google Scholar]
- Gordon S. G., Hasiba U., Cross B. A., Poole M. A., Falanga A. Cysteine proteinase procoagulant from amnion-chorion. Blood. 1985 Dec;66(6):1261–1265. [PubMed] [Google Scholar]
- Gottesman M. M., Cabral F. Purification and characterization of a transformation-dependent protein secreted by cultured murine fibroblasts. Biochemistry. 1981 Mar 17;20(6):1659–1665. doi: 10.1021/bi00509a039. [DOI] [PubMed] [Google Scholar]
- Hirao T., Hara K., Takahashi K. Purification and characterization of cathepsin B from monkey skeletal muscle. J Biochem. 1984 Mar;95(3):871–879. doi: 10.1093/oxfordjournals.jbchem.a134680. [DOI] [PubMed] [Google Scholar]
- Hoflack B., Kornfeld S. Purification and characterization of a cation-dependent mannose 6-phosphate receptor from murine P388D1 macrophages and bovine liver. J Biol Chem. 1985 Oct 5;260(22):12008–12014. [PubMed] [Google Scholar]
- Joseph L. J., Chang L. C., Stamenkovich D., Sukhatme V. P. Complete nucleotide and deduced amino acid sequences of human and murine preprocathepsin L. An abundant transcript induced by transformation of fibroblasts. J Clin Invest. 1988 May;81(5):1621–1629. doi: 10.1172/JCI113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Wiederanders B., Brömme D., Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989 Dec 1;264(2):467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Peeters-Joris C., Vaes G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim Biophys Acta. 1990 Mar 9;1051(3):266–275. doi: 10.1016/0167-4889(90)90132-w. [DOI] [PubMed] [Google Scholar]
- Lenaers-Claeys G., Vaes G. Collagenase, procollagenase and bone resorption. Effects of heparin, parathyroid hormone and calcitonin. Biochim Biophys Acta. 1979 May 16;584(3):375–388. doi: 10.1016/0304-4165(79)90114-4. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Etherington D. J. A comparison of four cathepsins (B, L, N and S) with collagenolytic activity from rabbit spleen. Biochem J. 1988 Dec 1;256(2):433–440. doi: 10.1042/bj2560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Gal S., Gottesman M. M. The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem J. 1987 Dec 1;248(2):449–454. doi: 10.1042/bj2480449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W. Species variants of cathepsin L and their immunological identification. Biochem J. 1986 Nov 15;240(1):285–288. doi: 10.1042/bj2400285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. K., Ellis S. On the substrate specificity of cathepsins B1 and B2 including a new fluorogenic substrate for cathepsin B1. Life Sci. 1975 Oct 15;17(8):1269–1276. doi: 10.1016/0024-3205(75)90137-x. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Mort J. S., Roughley P. J. Cartilage proteoglycan aggregate is degraded more extensively by cathepsin L than by cathepsin B. Biochem J. 1990 Mar 1;266(2):569–573. [PMC free article] [PubMed] [Google Scholar]
- Noel F., Cumps J. The use of a non-linear regression approach for the analysis of the ouabain-K+ interaction with (Na+ + K+)-ATPase from guinea pig and rat hearts. Braz J Med Biol Res. 1989;22(4):433–445. [PubMed] [Google Scholar]
- Obled A., Ouali A., Valin C. Cysteine proteinase content of rat muscle lysosomes. Evidence for an unusual proteinase activity. Biochimie. 1984 Sep-Oct;66(9-10):609–616. doi: 10.1016/0300-9084(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Reilly J. J., Jr, Mason R. W., Chen P., Joseph L. J., Sukhatme V. P., Yee R., Chapman H. A., Jr Synthesis and processing of cathepsin L, an elastase, by human alveolar macrophages. Biochem J. 1989 Jan 15;257(2):493–498. doi: 10.1042/bj2570493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhin J., Wade R. L., Honn K. V., Sloane B. F. Membrane-associated cathepsin L: a role in metastasis of melanomas. Biochem Biophys Res Commun. 1989 Oct 16;164(1):556–561. doi: 10.1016/0006-291x(89)91755-5. [DOI] [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. Cellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclasts. Clin Orthop Relat Res. 1988 Jun;(231):239–271. [PubMed] [Google Scholar]
- Vaes G., Jacques P. Studies on bone enzymes. The assay of acid hydrolases and other enzymes in bone tissue. Biochem J. 1965 Nov;97(2):380–388. doi: 10.1042/bj0970380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederanders B., Kirschke H. Antibodies to rat liver cathepsins: characterization and use for the identification of enzyme precursors. Biomed Biochim Acta. 1986;45(11-12):1421–1431. [PubMed] [Google Scholar]
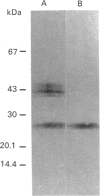
- Yamaguchi N., Chung S. M., Shiroeda O., Koyama K., Imanishi J. Characterization of a cathepsin L-like enzyme secreted from human pancreatic cancer cell line HPC-YP. Cancer Res. 1990 Feb 1;50(3):658–663. [PubMed] [Google Scholar]