Abstract
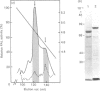
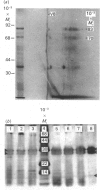
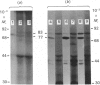
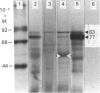
L-Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) purified from suspension-cultured cells of French bean (Phaseolus vulgaris) has been further characterized. A number of techniques, including use of an antiserum and affinity probes, have established that all the antigenic polypeptides represent polymorphic Mr forms of the enzyme. These peptides include an apparently higher-Mr (83,000) form which shows different kinetics of induction from the Mr-77000 forms that have been extensively characterized previously. The larger subunit appeared to be PAL by the following criteria: (a) binding to specific affinity and antibody matrices; (b) peptide mapping; (c) active-site labelling; and (d) amino acid composition. The increased Mr of the larger subunit was not completely attributable to glycosylation, although some sugar residues were detected in this Mr-83000 form but not in the other Mr forms. Mr-83000 subunits were also immunoprecipitated from translations in vitro of mRNA from cells that had been stressed for a long period. They were also detected in leaf tissues that were not yet undergoing an extensive wound response. This form of the enzyme may be constitutive and involved in the low-level accumulation of phenolics in most cell types. By contrast, the Mr-77000 forms of PAL were rapidly induced during elicitor action, wounding or cytokinin-induced xylogenesis as a key regulatory enzyme involved in the synthesis of phenolics under stress conditions or during differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
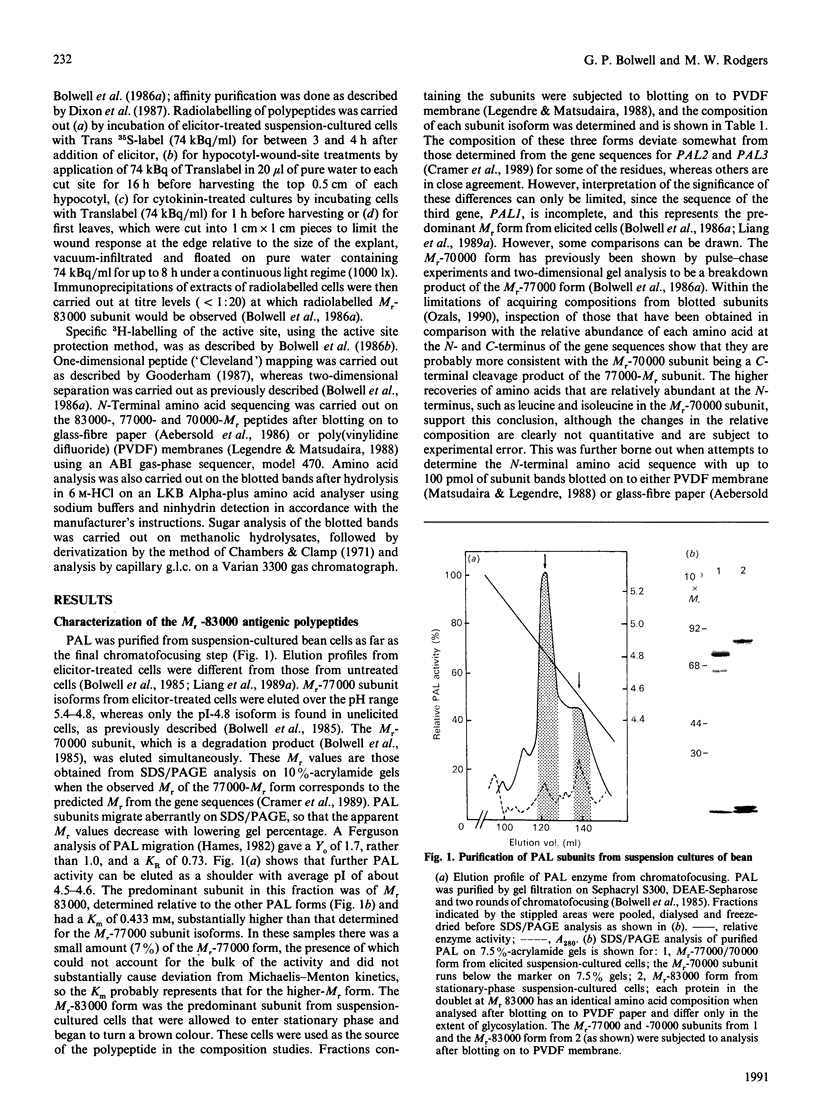
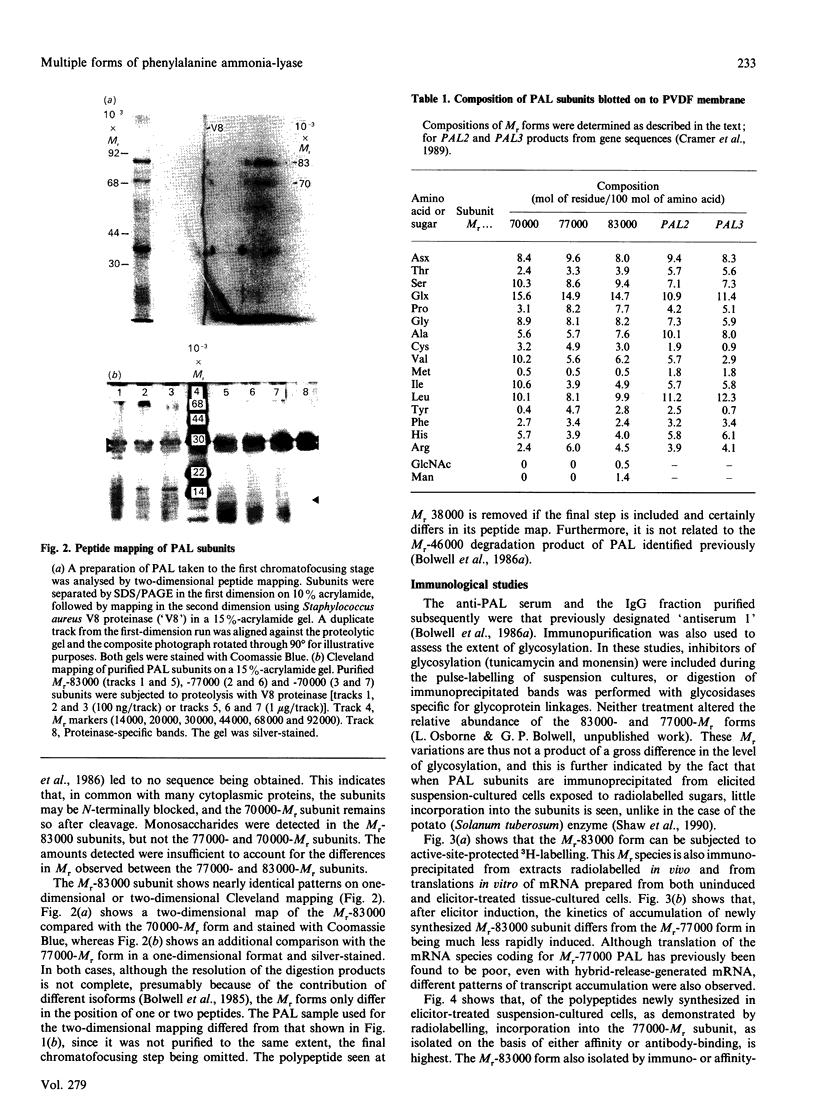
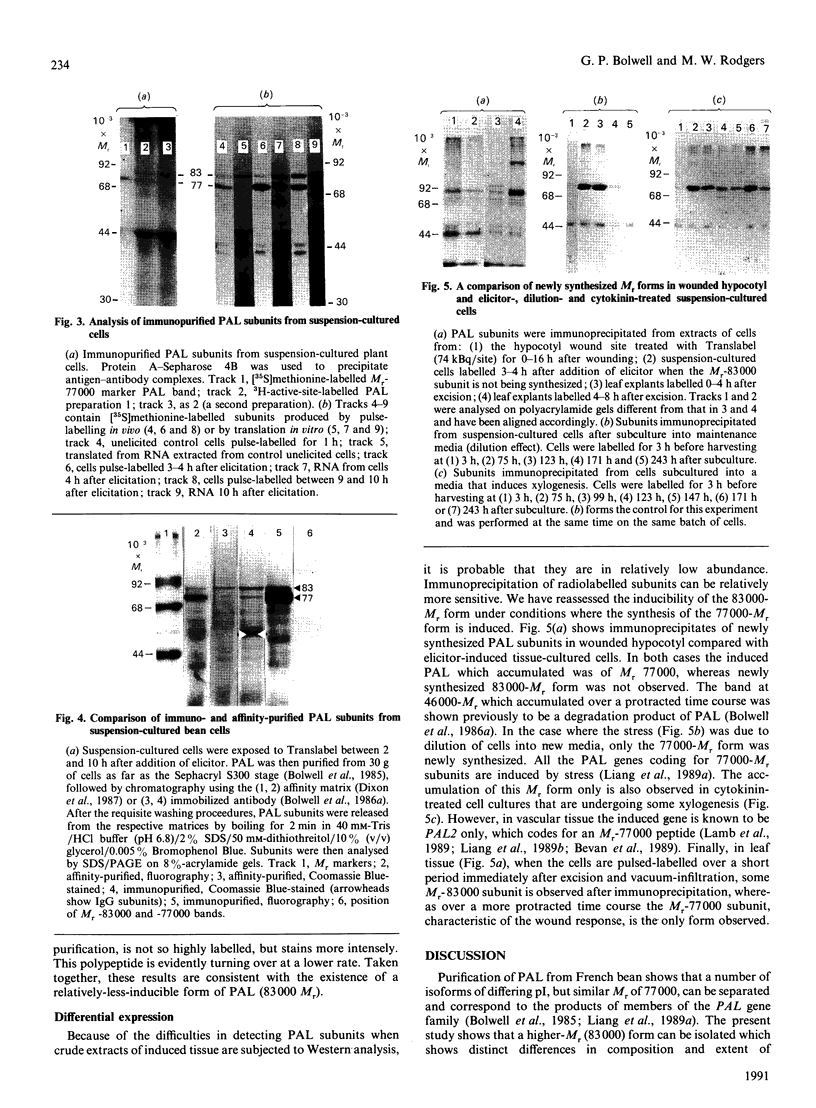
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Induction by growth factors of polysaccharide synthases in bean cell suspension cultures. Biochem J. 1983 Feb 15;210(2):509–515. doi: 10.1042/bj2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Keller B., Schmid J., Lamb C. J. Vascular expression of a bean cell wall glycine-rich protein-beta-glucuronidase gene fusion in transgenic tobacco. EMBO J. 1989 May;8(5):1309–1314. doi: 10.1002/j.1460-2075.1989.tb03510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboi T., Yamada Y. Regulation of the enzyme activities related to lignin synthesis in cell aggregates of tobacco cell culture. Biochim Biophys Acta. 1978 Aug 17;542(2):181–190. doi: 10.1016/0304-4165(78)90014-4. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- LeGendre N., Matsudaira P. Direct protein microsequencing from Immobilon-P Transfer Membrane. Biotechniques. 1988 Feb;6(2):154–159. [PubMed] [Google Scholar]
- Liang X. W., Dron M., Cramer C. L., Dixon R. A., Lamb C. J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989 Aug 25;264(24):14486–14492. [PubMed] [Google Scholar]
- Liang X. W., Dron M., Schmid J., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a phenylalanine ammonia-lyase-beta-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9284–9288. doi: 10.1073/pnas.86.23.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memelink J., Hoge J. H., Schilperoort R. A. Cytokinin stress changes the developmental regulation of several defence-related genes in tobacco. EMBO J. 1987 Dec 1;6(12):3579–3583. doi: 10.1002/j.1460-2075.1987.tb02688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. M., Bolwell G. P., Smith C. Wound-induced phenylalanine ammonia-lyase in potato (Solanum tuberosum) tuber discs. Significance of glycosylation and immunolocalization of enzyme subunits. Biochem J. 1990 Apr 1;267(1):163–170. doi: 10.1042/bj2670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha A. Purification, characterization and induction of L-phenylalanine ammonia-lyase in Phaseolus vulgaris. Eur J Biochem. 1988 Dec 1;178(1):243–248. doi: 10.1111/j.1432-1033.1988.tb14449.x. [DOI] [PubMed] [Google Scholar]